Silvicultural Practices as Main Drivers of the Spread of Tree of Heaven (Ailanthus altissima (Mill.) Swingle) †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Study Design
3. Results
3.1. The Impacts of Selective Cuttings and Clear-Cuttings on the Spread of A. altissima (i)
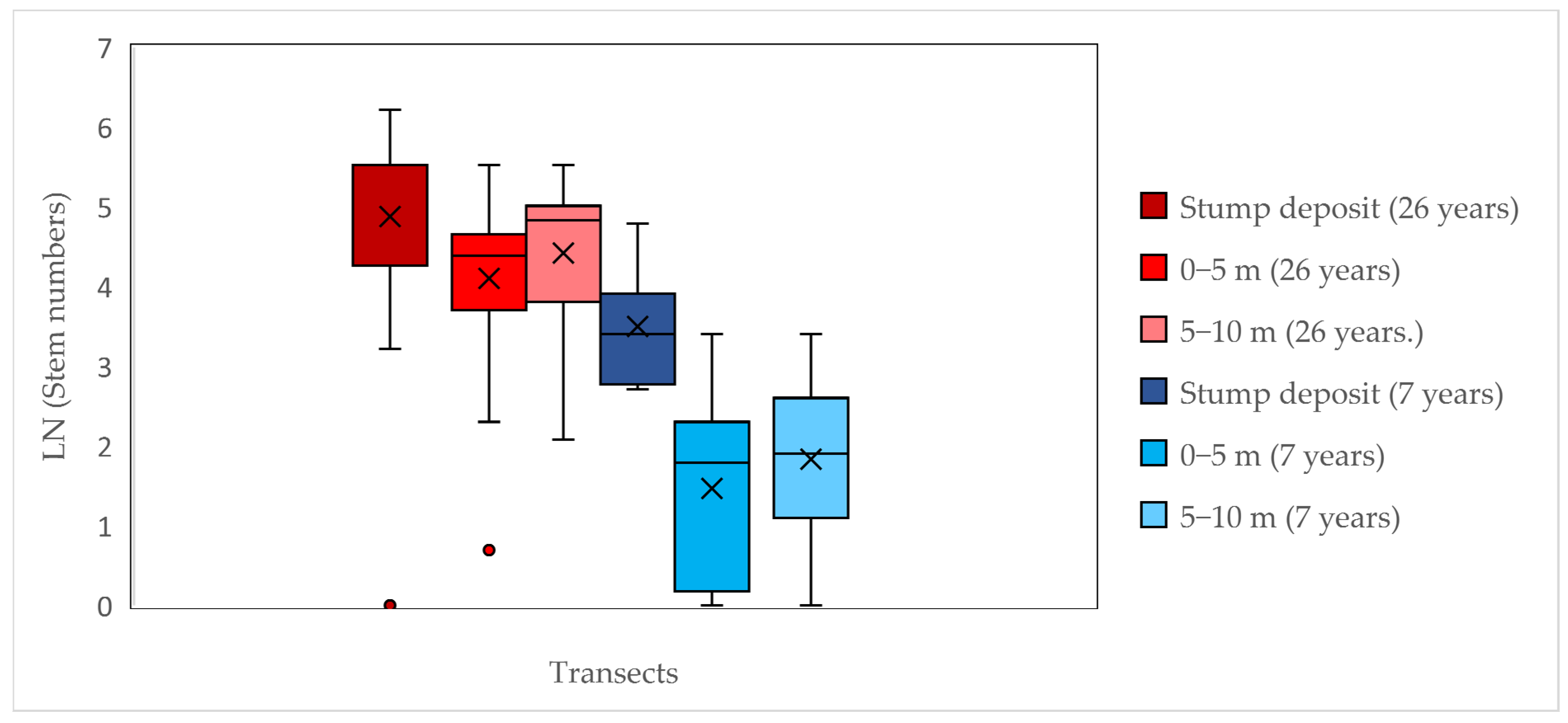
3.2. The Impacts of Stump Deposits on the Spread of A. altissima (ii, iii)
4. Discussion
4.1. Selective Cuttings and Clear-Cuttings as Main Drivers of the Spread of A. altissima (i)
4.2. Stump Deposits as Main Drivers of the Spread of A. altissima (i, ii)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kowarik, I.; Säumel, I. Biological Flora of Central Europe: Ailanthus altissima (Mill.) Swingle. Perspect. Plant Ecol. Evol. Syst. 2007, 8, 207–237. [Google Scholar] [CrossRef]
- Goeze Liste Der Seit Dem 16. Jahrhundert Bis Auf Die Gegenwart in Die Gärten Und Parks Europas Eingeführten Bäume Un Sträucher. Mitteilungen Dtsch. Dendrol. Ges. 1916, 25, 129–201. [Google Scholar]
- Korda, M. A mirigyes bálványfa (Ailanthus altissima (MILL.) SWINGLE) elterjedésének és elterjesztésének története Magyarországon. In A Magyarországon Inváziós Növényfajok Elterjedésének és Elterjesztésének Története I; Bartha, D., Ed.; Soproni Egyetem EMK Növénytani Tanszék: Sopron, Hungary, 2018; Volume XIX, pp. 111–194. [Google Scholar]
- Wickert, K.L.; O’Neal, E.S.; Davis, D.D.; Kasson, M.T. Seed Production, Viability, and Reproductive Limits of the Invasive Ailanthus altissima (Tree-of-Heaven) within Invaded Environments. Forests 2017, 8, 226. [Google Scholar] [CrossRef] [Green Version]
- Feret, P.P. Early Flowering in Ailanthus. For. Sci. 1973, 19, 237–239. [Google Scholar] [CrossRef]
- Kota, N.L.; Landenberger, R.E.; McGraw, J.B. Germination and Early Growth of Ailanthus and Tulip Poplar in Three Levels of Forest Disturbance. Biol. Invasions 2007, 9, 197–211. [Google Scholar] [CrossRef]
- Rebbeck, J.; Jolliff, J. How Long Do Seeds of the Invasive Tree, Ailanthus Altissima Remain Viable? For. Ecol. Manag. 2018, 429, 175–179. [Google Scholar] [CrossRef]
- Burch, P.L.; Zedaker, S.M. Removing the Invasive Tree Ailanthus Altissima and Restoring Natural Cover. J. Arboric. 2003, 29, 18–24. [Google Scholar]
- Knapp, L.; Canham, C. Invasion of an Old-Growth Forest in New York by Ailanthus Altissima: Sapling Growth and Recruitment in Canopy Gaps. J. Torrey Bot. Soc. 2000, 127, 307–315. [Google Scholar] [CrossRef]
- Fotiadis, G.; Kyriazopoulos, A.; Fraggakis, I. The Behaviour of Ailanthus Altissima Weed and Its Effects on Natural Ecosystems. J. Environ. Biol. Acad. Environ. Biol. India 2011, 32, 801–806. [Google Scholar]
- Martin, P.H.; Canham, C.D. Dispersal and Recruitment Limitation in Native versus Exotic Tree Species: Life-History Strategies and Janzen-Connell Effects. Oikos 2010, 119, 807–824. [Google Scholar] [CrossRef]
- Call, L.J.; Nilsen, E. Analysis of Spatial Patterns and Spatial Association between the Invasive Tree-of-Heaven (Ailanthus altissima) and the Native Black Locust (Robinia pseudoacacia). Am. Midl. Nat. 2003, 150, 1–14. [Google Scholar] [CrossRef]
- Walker, G.; Robertson, M.; Gaertner, M.; Gallien, L.; Richardson, D. The Potential Range of Ailanthus altissima (Tree of Heaven) in South Africa: The Roles of Climate, Land Use and Disturbance. Biol. Invasions 2017, 19, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Radtke, A.; Ambraß, S.; Zerbe, S.; Tonon, G.; Fontana, V.; Ammer, C. Traditional Coppice Forest Management Drives the Invasion of Ailanthus altissima and Robinia pseudoacacia into Deciduous Forests. For. Ecol. Manag. 2013, 291, 308–317. [Google Scholar] [CrossRef]
- Rebbeck, J.; Hutchinson, T.; Iverson, L.; Yaussy, D.; Fox, T. Distribution and Demographics of Ailanthus Altissima in an Oak Forest Landscape Managed with Timber Harvesting and Prescribed Fire. For. Ecol. Manag. 2017, 401, 233–241. [Google Scholar] [CrossRef]
- Carter, W.; Fredericksen, T. Tree Seedling and Sapling Density and Deer Browsing Incidence on Recently Logged and Mature Non-Industrial Private Forestlands in Virginia, USA. For. Ecol. Manag. 2007, 242, 671–677. [Google Scholar] [CrossRef]
- Walmsley, J.D.; Godbold, D.L. Stump Harvesting for Bioenergy—A Review of the Environmental Impacts. For. Int. J. For. Res. 2010, 83, 17–38. [Google Scholar] [CrossRef] [Green Version]
- Erdélyi, A.; Hartdégen, J.; Molnár, Á.P.; Hajagos, G.; Vadász, C. A Mirigyes Bálványfa (Ailanthus altissima (Mill) Swingle) Finomléptékű Elterjedésének Vizsgálata Archív És Recens Adatok Alapján a Peszéri-Erdőben. Tájökológiai Lapok 2019, 17, 75–84. [Google Scholar]
- Faragó, S. A bálványfa. In Erdészeti Kutatások; Lengyel, G., Ed.; Mezőgazdasági Könyés Folyóiratkiadó Vállalat: Budapest, Hungary, 1964; Volume 60, pp. 87–110. [Google Scholar]
- Maschek, O.; Halmschlager, E. Effects of Verticillium nonalfalfae on Ailanthus altissima and Associated Indigenous and Invasive Tree Species in Eastern Austria. Eur. J. For. Res. 2018, 137. [Google Scholar] [CrossRef]

| Stand ID (National Forestry Database) | Assessed Area (ha)/Stand Area (ha) | Forestry Intervention | Stem Numbers/1 ha (dbh > 5 cm) | Prevalence (%) Before | Prevalence (%) After | Stem Numbers Before/1 ha (dbh < 5 cm) | Stem Numbers After/1 ha (dbh < 5 cm) | Stem Number Growth (dbh < 5 cm) |
|---|---|---|---|---|---|---|---|---|
| Kunpeszér 3 E | 4.1/8 | Clear-cutting | 50 | 59 | 97 | 175 | 12,000 | ×68.5 |
| Kunpeszér 4 G | 5.9/5.9 | Clear-cutting | 110 | 77 | 99 | 1600 | 4000 | ×2.5 |
| Kunpeszér 8 C | 2.3/2.3 | Clear-cutting | 250 | 100 | 100 | 5000 | 42,000 | ×8.4 |
| Kunpeszér 14 A | 1/2.9 | Clear-cutting | 3 | 17 | 98 | 15 | 4000 | ×266.6 |
| Kunpeszér 26 I | 2.4/2.4 | Selective cutting | 9 | 59 | 69 | 80 | 265 | ×3.3 |
| Kunpeszér 27 B | 1.2/2.2 | Selective cutting | 3 | 54 | 82 | 60 | 235 | ×3.9 |
| Kunpeszér 27 E | 2.7/3.6 | Selective cutting | 64 | 30 | 100 | 200 | 38,500 | ×192.5 |
| Kunpeszér 6 B | 1.9/1.9 | Control | 127 | 98 | 100 | 1700 | 1200 | ×0.7 |
| Kunpeszér 11 B | 0.6/0.6 | Control | 157 | 87 | 100 | 2800 | 6000 | ×2.1 |
| Kunpeszér 10 C | 1.7/1.7 | Control | 40 | 44 | 65 | 320 | 330 | ×1 |
| Kunpeszér 23 D | 1.7/1.7 | Control | 7 | 62 | 50 | 510 | 1100 | ×2.1 |
| Kunpeszér 23 E | 0.6/7.2 | Control | 28 | 84 | 92 | 1500 | 5000 | ×3.3 |
| Stand ID (National Forestry Database) | Assessed Area (ha)/Stand Area (ha) | Stand Age (Years) | Prevalence (%) | Stem Numbers/1 ha (dbh > 5 cm) | Stem Numbers/1 ha (dbh < 5 cm) |
|---|---|---|---|---|---|
| Kunpeszér 9 B | 2.4/2.4 | 4 | 90 | 7 | 2300 |
| Kunpeszér 25 C | 0.8/0.8 | 4 | 63 | 0 | 100 |
| Kunpeszér 26 B | 1.8/1.8 | 4 | 67 | 0 | 228 |
| Kunpeszér 5 D | 0.8/0.8 | 5 | 87 | 28 | 700 |
| Kunpeszér 7 K | 2.2/2.2 | 5 | 100 | 53 | 2000 |
| Kunpeszér 19 B | 1.3/1.3 | 7 | 66 | 3 | 270 |
| Kunpeszér 20 J | 1.9/1.9 | 7 | 88 | 168 | 4250 |
| Kunpeszér 11 J | 6.7/6.7 | 14 | 94 | 250 | 2000 |
| Kunpeszér 11 F | 4.5/4.5 | 16 | 90 | 530 | 11,700 |
| Kunpeszér 11 L | 1.6/1.6 | 17 | 90 | 523 | 6500 |
| Kunpeszér 27 F | 1.9/2.7 | 22 | 87 | 93 | 2700 |
| Kunpeszér 14 B | 3.2/12 | 26 | 73 | 155 | 2000 |
| Kunpeszér 20 D | 1/1 | 26 | 100 | 331 | 8100 |
| Stands | Distance Pairs | dbh > 10 cm | 10 cm > dbh > 5 cm | dbh < 5 cm | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| W | z | p | W | z | p | W | z | p | ||
| 26 years old stand | sd/0−5 m | 15.5 | 3.48 | ≤0.05 | 2.5 | 4.65 | ≤0.05 | 40 | 3.58 | ≤0.05 |
| sd/5−10 m | 6 | 3.8 | ≤0.05 | 8 | 4.53 | ≤0.05 | 55 | 3.37 | <0.05 | |
| 0−5 m/5−10 m | 17 | 1.72 | 0.09 | 79.5 | 0.91 | 0.26 | 52 | −2.98 | <0.05 | |
| 7 years old stand | sd/0−5 m | - | - | - | 0 | 3.92 | ≤0.05 | 0 | 3.92 | ≤0.05 |
| sd/5−10 m | - | - | - | 0 | 3.92 | ≤0.05 | 0 | 3.92 | ≤0.05 | |
| 0−5 m/5−10 m | - | - | - | - | - | - | 66 | −1.45 | 0.14 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erdélyi, A.; Hartdégen, J.; Malatinszky, Á.; Vadász, C. Silvicultural Practices as Main Drivers of the Spread of Tree of Heaven (Ailanthus altissima (Mill.) Swingle). Biol. Life Sci. Forum 2021, 2, 17. https://doi.org/10.3390/BDEE2021-09467
Erdélyi A, Hartdégen J, Malatinszky Á, Vadász C. Silvicultural Practices as Main Drivers of the Spread of Tree of Heaven (Ailanthus altissima (Mill.) Swingle). Biology and Life Sciences Forum. 2021; 2(1):17. https://doi.org/10.3390/BDEE2021-09467
Chicago/Turabian StyleErdélyi, Arnold, Judit Hartdégen, Ákos Malatinszky, and Csaba Vadász. 2021. "Silvicultural Practices as Main Drivers of the Spread of Tree of Heaven (Ailanthus altissima (Mill.) Swingle)" Biology and Life Sciences Forum 2, no. 1: 17. https://doi.org/10.3390/BDEE2021-09467
APA StyleErdélyi, A., Hartdégen, J., Malatinszky, Á., & Vadász, C. (2021). Silvicultural Practices as Main Drivers of the Spread of Tree of Heaven (Ailanthus altissima (Mill.) Swingle). Biology and Life Sciences Forum, 2(1), 17. https://doi.org/10.3390/BDEE2021-09467






