Abstract
Feeding places for shooting wild boar (ie., bait sites) may cause weed infestations in natural habitats. We examined the vegetation, and the soil seed banks of three current and three—1, 8 and 10 years old—abandoned baits, using a vegetation survey alongside transects and seedling emergence methods. In the case of vegetation, the density and the number of weeds were significantly higher at the current baits. In addition, the abundance of weeds decreased with the time of abandonment, but the number of weeds remained similar. Concerning the seed bank, the species number and the total seed density were highly varied, but due to the frequent disturbances, they were lower at current baits. Only the proportion of weed species was significantly lower at abandoned sites, the abundancy of weed seeds was similar, and did not decrease in time. The youngest bait showed the lowest proportion, while the oldest one showed the highest proportion of weed seeds among the abandoned sites. Generally, long-term persistent seeds were of the highest proportion, except for in the oldest site, indicating a lower level of disturbance. Vegetation regenerates relatively quickly, but the seed banks remain infected for years, which can be a potential source of secondary invasions.
1. Introduction
Feeding wildlife is a widespread management tool used across the world, especially in North America and Central Europe [1]. However, its environmental impacts may be widely diverse [2]. The effects of feeding wildlife on animal populations have been the main subject of study [3], but some researchers have also examined the changes in seedling establishment and regeneration [4]. Only a few publications deal with the herbaceous layer in connection with the effects of wild game feeding [1,5]. In Hungary, supplementary feeding is not so common, because of the increasingly milder winters. However, the use of feeding places for wild boar hunting (at so-called bait sites) is very popular. A bait site is a small clearing established approximately 30 to 50 meters from hunting blinds. In general, corn-cobs or seed are scattered on the ground, but other agricultural and food industry by-products (e.g., molasses, fresh and dried beet slices) are used [6]. The legislation is not strict, thus these sites are often invaded by weeds. Due to the poor quality of the forage and the intensive use of it, ruderal and segetal vegetation may became dominant at these sites. Previous studies showed that bait sites can cause significant degradation in natural habitats. Although, weed infestation typically extends only to the immediate environment of the baits—in general, weed species are present in greater abundance up to eight to ten metres from the centre of the baits—patches of valuable habitat can be destroyed, while open sites, such as forest clearings, are especially vulnerable and represent the most endangered habitats, forest baits have proven to be less susceptible to invasions [7]. What is not yet known, however, is what happens to baits after they are abandoned. As usual, stress-tolerant and nitrophilous species indicate the location of former baits, but the regeneration processes are unknown. There are many studies focusing on the succession of old-fields after the cessation of agricultural cultivation, e.g., [8,9]. In the case of abandoned bait sites, the high proportion of segetal weeds, the frequent soil disturbances and the high anthropogenic impact induce similar conditions to those found in old-fields after abandonment, but the courses of succession may be different. The composition of the soil seed bank is also unknown. The soil seed bank is a major determinant of vegetation dynamics in many plant community types and may play a central role in the recruitment and establishment of species that reproduce by seeds [10]. It may also provide a storage effect [11], allowing for the coexistence of species with different environmental demands and may improve the capacity of invasive species to establish [12]. Moreover, it is well known that weed seeds remain viable in the soil for periods of decades [13,14].
In recent years, due to the effects of viruses affecting animals (ASFV; African Swine Fever Virus) and humans (COVID-19; SARS-CoV-2), the extent of hunting has decreased, and thus more and more bait sites are being abandoned. The issue of identifying the changes that will occur in the vegetation and in the soil seed bank after abandonment of baits is therefore, of growing interest and concern. In the present work, it is assumed that (1) the regeneration of the vegetation will be clearly detectable at abandoned sites, and the extent of weed invasion will decrease with the time of abandonment; (2) the soil seed bank of current baits will be characterized by a high proportion of weed seeds, and this will also decrease with time; (3) the proportion of long-term persistent seeds will also indicate the decreasing level of disturbance, will be the highest at current baits and will decrease with time.
2. Experiments
2.1. Study Site
The area of study is situated in the Mátra Mountains of Hungary. The site under investigation is located in the southern part of the mountain range, in the Turkey oak-sessile oak zone. This habitat is characterized by a variable mixture of Turkey oak (Quercus cerris L.) and sessile oak (Quercus petraea (Matt.) Liebl.). In the tree layer, shadowing trees are absent or very rare [15]. The site is located in the Mátra Landscape Protection Area. Comprehensive forest and wildlife management is carried out in this protected zone [16].
2.2. Sampling Setup
On the basis of preliminary field assessments [7], three current baits that have been in use for several years (C1, C2, C3) were selected and three sites that were abandoned 1, 8 and 10 years ago (A1, A2, A3), respectively, using the space-for-time substitution method [17]. All sites are located in clearings classified as mesic hay and dry molinia meadows according to the MÉTA database (the Hungarian acronym for the Landscape Ecological Vegetation Database & Map of Hungary) [18]. Following the selection, two surveys were conducted in late spring and summer, in May and August 2019. In the course of the surveys, four transects extending from the center of the baits were used to delineate degradation gradients. The transects were arranged in random directions representing replications of the gradient from the most disturbed to the less disturbed stands. Each consisting of 22 × 1 m tangential quadrats in which a vegetation survey was conducted, with the estimation of percentage cover. The centers of the bait sites were determined by the actual placement of the feed, which was clearly visible in each case. At abandoned sites, the stones, often used at bait sites, were used as a guide to find the center. Considering the extent of the bait sites and their degraded vegetation, 22 sampling units were placed in each of the transects.
The soil seed bank experiment was conducted in May 2019. Soil was sampled in 12 10 × 10 × 5 cm plots, in the center of the bait sites, randomly located in a circle with a radius of 2 m. Considering that 90% of the seeds are found the upper 5 cm of the soil [19,20], the soil was sampled to this depth. Soil samples were sieved to remove larger fragments, such as stems, roots, and rocks. Then, samples were transferred into plastic trays and conditioned in a greenhouse for 15 months. Emerging seedlings were regularly counted and removed. Unidentified seedlings were transplanted and grown until they could be identified.
2.3. Data Processing
The seed bank and the vegetation of the current and the abandoned bait sites of various ages were evaluated, based on their species list and abundance (cumulative cover at each sites). To estimate the degree of naturalness of communities, Borhidi’s social behaviour types (SBT) classification was used—and this is, in turn, a version of Ellenberg’s grouping [21] and Grime’s CSR plant functional type system [22] adapted to Pannonian flora. SBTs were derived from species’ behavior and ecological attributes at a given observation level [23]. In this study, two large groups were employed: (1) naturalness indicator species: stress tolerant specialists—S; competitors of natural habitats—C; stress tolerant generalists—G; natural pioneers—NP; disturbance tolerant plants—DT; (2) degradation indicator/weed species: native weed species—W; introduced crops running wild—I; adventitious weeds—A; ruderal competitors of the natural flora—RC; alien competitors and aggressive invaders—AC. To evaluate the changes in the spatial distribution of weeds, the presence of weed species in the quadrats along the transect was compared using p-tests. To assess the effects of a decreasing level of disturbances, a transient (T), short-term persistent (SP), and long-term persistent (LP) seed bank type classification was used, following Thompson [24].
3. Results
3.1. Vegetation Composition
At the current bait sites, 133 species (82 natural, 51 weed) were found, while at abandoned sites, 161 species were found (123 natural, 38 weed). The number of aliens was 12 at current bait sites and 6 at abandoned sites. The cumulative cover (p = 0.0498) and the number (p = 0.0084) of weeds was significantly higher at current bait sites. The number of natural species was lower at current sites (p = 0.0044), but not in cover. However, the proportion of weed species was significantly higher at current sites considering both their density (p = 0.0376) and species number (p = 0.0005). When the SBT categories are considered, only the number of ruderal competitor (RC) species proved to be significantly lower at abandoned sites. The cumulative cover of weeds decreased as the time of abandonment increased (Figure 1a), but the number of weeds remained similar (Figure 1b). The number and the abundance of weeds were higher in all cases in August than in May.
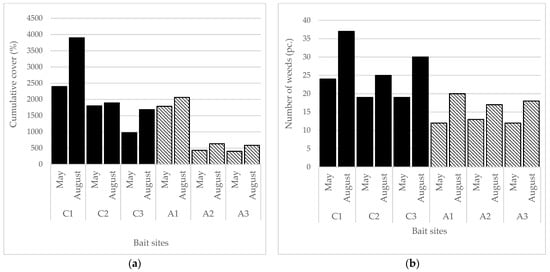
Figure 1.
The abundancy and number of weed species in the vegetation of the current and the abandoned baits, (a) The abundancy of weeds according to the cumulative cover of all weed species in the 88 quadrats at each bait sites; (b) The number of weed species at each bait sites. C1, C2, C3—current bait sites; and abandoned bait sites: A1—abandoned for 1 year, A2—abandoned for 8 years, A3—abandoned for 10 years.
By examining the spatial distribution of the changes along the transect, significant changes can be detected mostly in the center of the baits (Table 1). The highly significant differences are mainly found in the number of the weeds, especially between the youngest and the oldest sites. The cover of weeds differed between the site abandoned for 1 year and the two older sites; and in fact, the latter two proved to be very similar.

Table 1.
Significant differences between the bait sites abandoned for different lenghts of time along the transects (in 1–22 quadrats) according to the cover and the number of weed species.
3.2. Soil Seed Bank Composition
At current bait sites, 46 species were found, 19 natural (41.3%) and 27 weed (58.7%) species, while at abandoned sites 58 species were detected, of which 36 were natural (62.1%) and 22 weed (37.9%) species. The number of aliens was 11 at current bait sites, and 6 at abandoned sites. The species number and the total seed density proved to be highly variable, but in general they were lower at current baits (Figure 2). Only the proportion of weed species was significantly lower at abandoned sites (p = 0.0003), while the abundance of weed seeds was similar to or sometimes even higher than, at current baits. The abundance of weed seeds did not decrease with time, indeed, among the abandoned sites, the most recently abandoned bait had the lowest proportion of weed seeds, and the oldest had the highest (Figure 2a). The number of weed species was also the highest at the oldest abandoned bait (Figure 2b), while the proportion of weeds remained very similar between the abandoned baits of different ages (42.9%, 44.1% and 42.1%).
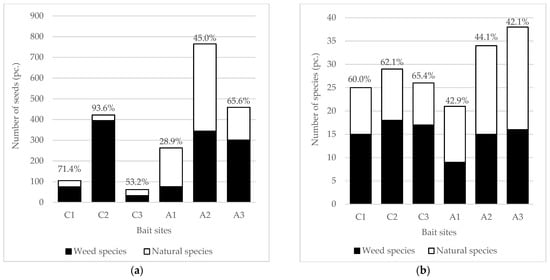
Figure 2.
Seed density and the number of species in the soil seed bank experiment, with the values of weed proportion, (a) The number of weed and natural species seeds in the soil seed bank and the proportion of weeds; (b) The number of weed and natural species in the soil seed banks and the proportion of weeds. C1, C2, C3—current bait sites; and abandoned bait sites: A1—abandoned for 1 year, A2—abandoned for 8 years, A3—abandoned for 10 years.
3.3. Seed Bank Persistence
Long-term persistent seeds were the dominant type found in all cases. At current bait sites, the number and the proportion of long-term persistent species varied between 65.5 and 96.2%. At abandoned sites, it remained quite high (with the maximum value of 94.7% at the site abandoned for 1 year), but decreased considerably with time (Figure 3). At the same time, the proportion of transient and short-term persistent species and their seed density also increased. This is especially noticeable at the oldest abandoned site (A3), where the proportion of seeds, according to their longevity, changed radically.
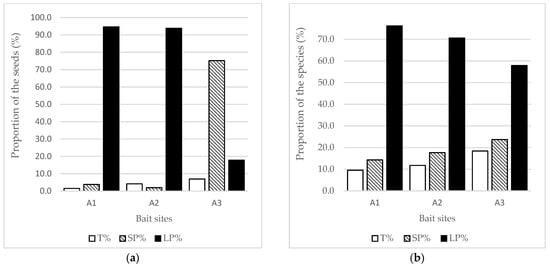
Figure 3.
The proportion of transient (T), short-term persistent (SP) and long-term persistent (LP) seeds and species at abandoned sites, (a) The proportion of the seeds; (b) The proportion of the species. A1: bait abandoned for 1 year; A2: bait abandoned for 8 years; A3: bait abandoned for 10 years.
4. Discussion
4.1. Vegetation Composition
The density and the number of weed species proved to be significantly higher at current baits. As expected, the cover of weeds decreased with the time of abandonment. Significant differences became apparent, especially as far as the center of the baits are concerned, mainly between the most recent and the two oldest sites. The number of weed species, however, did not display a decrease, and was similar at all abandoned sites. Similar results were obtained by Csecserits et al. [25], in their examination of the secondary succession of abandoned sandy agricultural fields in Hungary. Their research also found a decrease in the abundance of species that require some degree of disturbance to establish, but the number of such species did not change in the course of the 5 years studied. Generally, segetal weeds are suppressed by natural species, but some individuals remain present for a long time. Similar changes may well have occurred at the abandoned bait sites. As the addition of contaminated forage ceased, the artificial weed seed fluxes also ended. Presumably, this caused the significant decrease in the number of ruderal competitor (RC) species, and also caused the slight decrease in the number of weed species, as well. Some species, however, may still be present because of the gaps caused by animals, who often return to these sites for a long time after abandonment. These disturbances may promote the germination of remaining weeds [26]. In addition, the soil nutrient enrichment, caused by the accumulated forage, also create conditions favorable to these species [27].
4.2. Soil Seed Bank Composition
The soil seed bank proved to be highly variable and infected after several years. However, significant differences between the current and the abandoned bait sites could not be demonstrated, only that the proportion of weed species was significantly lower at the abandoned sites. The reason for this could be that seed banks are generally less likely to change rapidly and support more species than the aboveground vegetation in invaded communities [28,29]. Contrary to the assumptions made in the early stages of this work, neither the number and the proportion of weed species nor the number and proportion of their seeds decreased with the time of abandonment. Furthermore, the greatest number of weed seeds was detected at the two sites that were abandoned for the longest periods, and the proportion of weed seeds was in fact highest at the oldest site. However, it should be noted that the 1 year-old bait site has a wetter habitat, and thus promotes the germination of the species of the local community. It was not possible to demonstrate an increase in species number and seed density with the increasing level of disturbance, in contrast to other studies [30,31]. The lowest values were found at current bait sites. The reason may be the severe and frequent disturbances, which are concentrated in a relatively small area, and are therefore more severe than those caused by grazing, and which are the focus of most of the other published studies [32,33]. One reason may be that the large amount of accumulated litter (a mixture of the residual forage) can act as a trap for propagules, thus preventing seeds from reaching the soil [34]. Litter can also change physical conditions such as soil temperature and moisture [35], and release allelochemicals, which may be toxic [36], diminishing the amount of viable seeds. What is more, it is proved that at the local scale ground-foraging animals can strongly influence seed availability and the arrival of seeds into regeneration safe sites [37]. The high level of animal and human trampling results in a high degree of soil compaction. This, in turn, may lead to decreased oxygen availability in the soil and allow for the build-up of toxic gases, all of which can reduce the chances of seed survival [38].
4.3. Seed Bank Persistence
The seed bank theory, according to which the proportion of persistent species increases with disturbance [39,40], did, however, prove to be true. The number and seed density of species with a persistent seed bank decreases with the time of abandonment. This may well be related to the decrease in human and animal disturbances, thus displaying a similarity to the changes frequently detected along grazing gradients [32,33].
5. Conclusions
Vegetation proved capable of regenerating relatively quickly. Several studies have shown that the regeneration of abandoned fields is relatively advanced in Eastern Europe. Similarly, rapid secondary succession was found by Jongepierova et al. [41], in the Czech Republic, by Ruprecht [42], in the case of Romanian abandoned fields, and by Csecserits et al. [25], in Hungary. These results are most likely due to the presence of semi-natural grassland patches in the surroundings that serve as propagule sources. In the case of abandoned bait sites, these sources may be taken as a given, because degradation occurs in relatively restricted areas in a matrix of extensive natural habitats. Despite this, complete regeneration will take several years. Additionally, a study was conducted in the Mátra Mountains, which found that old fields returned to resembling natural habitats only 50–110 years after abandonment [43]. Thus, the process of the extinction of weeds lasts for many years. While weed cover may well decreases relatively quickly on the ground, weed seeds in the soil seed bank may remain viable for decades. In this way, the presence of invading weeds may facilitate invasion by other species, and according to the “invasional meltdown hypothesis” [44], the result may be secondary invasions, which many studies have demonstrated [45].
In practical terms, the chronosequence approach was selected instead of monitoring, and mainly because of the different environmental conditions and land-use histories, the predictions may not be so reliable. However, the aim was to identify the prevalent courses of succession at abandoned bait sites. As Csecserits et al. [25], also concluded, the space-for-time substitution method does seem to be effective, as it shows the general trends of successional changes and describes processes that have already occurred nature conservation interventions can therefore rely on the results obtained. Nevertheless, further research is required. Monitoring over the course of a number of years may reveal interannual changes, and it may be possible to detect the effect of other factors such as differences in land-use history and environmental conditions.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/BDEE2021-09422/s1.
Author Contributions
K.R. conceived, designed and performed the experiments; K.R. and S.C. analyzed the data; S.C. contributed analysis tools; K.R. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available on request due to restrictions eg privacy or ethical.
Acknowledgments
We are grateful to Paul Thatcher for English proofreading.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Arnold, J.M.; Gerhardt, P.; Steyaert, S.M.J.G.; Hochbichler, E.; Hacklander, K. Diversionary feeding can reduce red deer habitat selection pressure on vulnerable forest stands, but is not a panacea for red deer damage. For. Ecol. Manag. 2018, 407, 166–173. [Google Scholar] [CrossRef]
- Selva, N.; Berezowska-Cnota, T.; Elguero-Claramunt, I. Unforeseen Effects of Supplementary Feeding: Ungulate Baiting Sites as Hotspots for Ground-Nest Predation. PLoS ONE 2014, 9, e90740. [Google Scholar] [CrossRef] [PubMed]
- Milner, J.M.; Van Beest, F.M.; Schmidt, K.T.; Brook, R.K.; Storaas, T. To feed or not to feed? Evidence of the intended and unintended effects of feeding wild ungulates. J. Wildl. Manag. 2014, 78, 1322–1334. [Google Scholar] [CrossRef]
- Mathisen, K.M.; Rèmy, A.; Skarpe, C. Shoot growth responses at supplementary feeding stations for moose in Norway. Alces 2015, 51, 123–133. [Google Scholar]
- Rinella, M.J.; Dean, R.; Vavra, M.; Parks, C.G. Vegetation responses to supplemental winter feeding of elk in western Wyoming. West. N. Am. Nat. 2012, 72, 78–83. [Google Scholar] [CrossRef]
- Heltai, M.; Sonkoly, K. The role and opportunities of feeding in game management (Review). AWETH 2009, 5, 1–22. [Google Scholar]
- Rusvai, K.; Kispál, L.; Czóbel, S. Assessment of weed invasion at bait sites in the Mátra Landscape Protection Area. Columella J. Agric. Environ. Sci. 2019, 6, 37–44. [Google Scholar] [CrossRef]
- Csecserits, A.; Czúcz, B.; Halassy, M.; Kröel-Dulay, G.; Rédei, T.; Szabó, R.; Szitár, K.; Török, K. Regeneration of sandy old-fields in the forest steppe region of Hungary. Plant Biosyst. 2011, 145, 715–729. [Google Scholar] [CrossRef]
- Boecker, D.; Centeri, C.; Welp, G.; Möseler, B.M. Parallels of secondary grassland succession and soil regeneration in a chronosequence of central-Hungarian old fields. Folia Geobot. 2015, 50, 91–106. [Google Scholar] [CrossRef]
- Thompson, K.; Grime, P.J. Seasonal variation in seed banks of herbaceous species in ten contrasting habitats. J. Ecol. 1979, 67, 893–921. [Google Scholar] [CrossRef]
- Chesson, P.L. The storage effect in stochastic population models. Lect. Notes Biomath. 1984, 54, 76–89. [Google Scholar]
- Gioria, M.; Pyšek, P.; Moravcová, L. Soil seed banks in plant invasions: Promoting species invasiveness and long-term impact on plant community dynamics. Preslia 2012, 84, 327–350. [Google Scholar]
- Lewis, J. Longevity of crop and weed seeds: Survival after 20 years in soil. Weed Res. 1973, 13, 179–191. [Google Scholar] [CrossRef]
- Telewski, F.W.; Zeevaart, J.A.D. 120-yr period from Dr. Beal’s seed viability experiment. Am. J. Bot. 2002, 89, 1285–1288. [Google Scholar] [CrossRef]
- Bölöni, J.; Molnár, Z.; Biró, M.; Horváth, F. Distribution of the (semi-) natural habitats in Hungary II. Woodlands and shrublands. Acta Bot. Hung. 2008, 50, 107–148. [Google Scholar] [CrossRef]
- Katona, K.; Kiss, M.; Bleier, N.; Székely, J.; Nyeste, M.; Kovács, V.; Terhes, A.; Fodor, Á.; Olajos, T.; Rasztovics, E.; et al. Ungulate browsing shapes climate change impacts on forest biodiversity in Hungary. Biodivers. Conserv. 2013, 22, 1167–1180. [Google Scholar] [CrossRef][Green Version]
- Pickett, S.T.A. Space-for-time substitution as an alternative to long-term studies. In Long-Term Studies; Likens, G.E., Ed.; Ecology: Approaches and Alternatives; Springer: New York, NY, USA; Berlin, Germany, 1989; pp. 110–135. [Google Scholar]
- Available online: https://www.novenyzetiterkep.hu/english/node/1090 (accessed on 21 November 2021).
- Koncz, G.; Papp, M.; Török, P.; Kotroczó, Z.; Krakomperger, Z.; Matus, G.; Tóthmérész, B. The role of seed bank in the dynamics of understorey in an oak forest in Hungary. Acta Biol. Hung. 2010, 61, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Jacquemyn, H.; Van Mechelen, C.; Brys, R.; Honnay, O. Management effects on the vegetation and soil seed bank of calcareous grasslands: An 11-year experiment. Biol. Conserv. 2011, 144, 416–422. [Google Scholar] [CrossRef]
- Ellenberg, H. Zeigerwerte der Gefäßpflanzen Mitteleuropas. (Indicator values of vascular plants in Central Europe). Scr. Geobot. 1974, 9, 1–97. [Google Scholar]
- Grime, J.P. Plant. Strategies and Vegetation Processes; Wiley: Chichester, UK; New York, NY, USA, 1979; p. 222. [Google Scholar]
- Borhidi, A. Social behavior types, the naturalness and relative ecological indicator values of the higher plants in the Hungarian Flora. Acta Bot. Hung. 1995, 39, 97–181. [Google Scholar]
- Thompson, K.; Bakker, J.P.; Bekker, R.M. The Soil Seed Banks of North West Europe: Methodology, Density and Longevity; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]
- Csecserits, A.; Szabó, R.; Halassy, M.; Rédei, T. Testing the validity of successional predictions on an old-field chronosequence in Hungary. Community Ecol. 2007, 8, 195–207. [Google Scholar] [CrossRef]
- Kotanen, P.M. Effects of gap area and shape on recoloniza-tion by grassland plants with differing reproductive strategies. Can. J. Bot. 1997, 75, 352–361. [Google Scholar] [CrossRef]
- Bidwell, S.; Attiwill, P.M.; Adams, M.A. Nitrogen availability and weed invasion in a remnant native woodland in urban Melbourne. Austral. Ecol. 2006, 31, 262–270. [Google Scholar] [CrossRef]
- Robertson, S.G.; Hickman, K.R. Aboveground plant community and seed bank composition along an invasion gradient. Plant. Ecol. 2012, 213, 1461–1475. [Google Scholar] [CrossRef]
- Gooden, B.; French, K. Impacts of alien grass invasion in coastal seed banks vary amongst native growth forms and dispersal strategies. Biol. Conserv. 2014, 171, 14–26. [Google Scholar] [CrossRef]
- Hopfensperger, K.N. A review of similarity between seed bank and standing vegetation across ecosystems. Oikos 2007, 116, 1438–1448. [Google Scholar] [CrossRef]
- Bossuyt, B.; Honnay, O. Can the seed bank be used for ecological restoration? An overview of seed bank characteristics in European communities. J. Veg. Sci. 2008, 19, 875–884. [Google Scholar] [CrossRef]
- Matus, G.; Papp, M.; Tothmeresz, B. Impact of management on vegetationdynamics and seed bank formation of inland dune grassland in Hungary. Flora 2005, 200, 296–306. [Google Scholar] [CrossRef]
- Ma, M.; Zhou, X.; Du, G. Role of soil seed bank along a disturbance gradient in an alpine meadow on the Tibet plateau. Flora 2010, 205, 128–134. [Google Scholar] [CrossRef]
- Donath, T.W.; Eckstein, R.L. Effects of bryophytes and grass litter on seedling emergence vary by vertical seed position and seed size. Plant. Ecol. 2010, 207, 257–268. [Google Scholar] [CrossRef]
- Holmgren, M.; Scheffer, M.; Huston, M.A. The interplay of facilitation and competition in plant communities. Ecology 1997, 78, 1966–1975. [Google Scholar] [CrossRef]
- Ruprecht, E.; Józsa, J.; Ölvedi, T.B.; Simon, J. Differential effects of several “litter” types on the germination of dry grassland species. J. Veg. Sci. 2010, 21, 1069–1081. [Google Scholar] [CrossRef]
- Arruda, A.J.; Costa, F.V.; Guerra, T.J.; Junqueira, P.A.; Dayrell, R.L.; Messeder, J.V.; Rodrigues, A.T.S.; Buisson, E.; Silveira, F.A.O. Topsoil disturbance reshapes diaspore interactions with ground-foraging animals in a megadiverse grassland. J. Veg. Sci. 2020, 31, 1039–1052. [Google Scholar] [CrossRef]
- Fenner, M.; Thompson, K. The Ecology of Seeds, 2nd ed.; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Thompson, K. The functional ecology of seed banks. In Seed Ecology; Fenner, M., Ed.; Chapman & Hall: London, UK, 1985; pp. 231–258. [Google Scholar]
- Kiss, R.; Valkó, O.; Tóthmérész, B.; Török, P. Seed bank research in Central-European grassland. An overview In Seed Banks: Types, Roles and Research; Murphy, J., Ed.; Nova Science Publishers: New York, NY, USA, 2016; pp. 1–34. [Google Scholar]
- Jongepierová, I.; Jongepier, J.W.; Klimeš, L. Restoring grassland on arable land: An example of a fast spontaneous succession without weed-dominated stages. Preslia 2004, 76, 361–369. [Google Scholar]
- Ruprecht, E. Successfully recovered grassland: A promising example from Romanian old-fields. Restor. Ecol. 2006, 14, 473–480. [Google Scholar] [CrossRef]
- Molnár, Z. Sár-hegy (D-Mátra–Mátraalja). In The XI. MÉTA-TÚRA Tour Guid Booklet—Manuscript; Bartha, S., Ed.; MTA ÖBKI: Vácrátót, Hungary, 2008; pp. 34–42, (In Hungarian). Available online: https://www.novenyzetiterkep.hu/sites/novenyzetiterkep.hu/files/MT11_Bartha_Molnar_2008_A_XI_META_TURA_Fuzet.pdf (accessed on 16 February 2021).
- Simberloff, D.; Von Holle, B. Positive interactions of nonindigenous species: Invasional meltdown? Biol. Invasions 1999, 1, 21–32. [Google Scholar] [CrossRef]
- Jeschke, J.M.; Aparicio, L.G.; Haider, S.; Heger, T.; Lortie, C.J.; Pyšek, P.; Strayer, D.L. Support for major hypotheses in invasion biology is uneven and declining. NeoBiota 2012, 14, 1–20. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).