Optimization of Pigment Extraction from Quinoa Flour Fermented by Monascus purpureus Supplemented with Sodium Chloride †
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Strain
2.2. Inoculation of M. purpureus in Quinoa Grains
2.3. Hydroethanol Extraction of Pigments and Spectrophotometric Analyses
2.4. Obtaining Yields
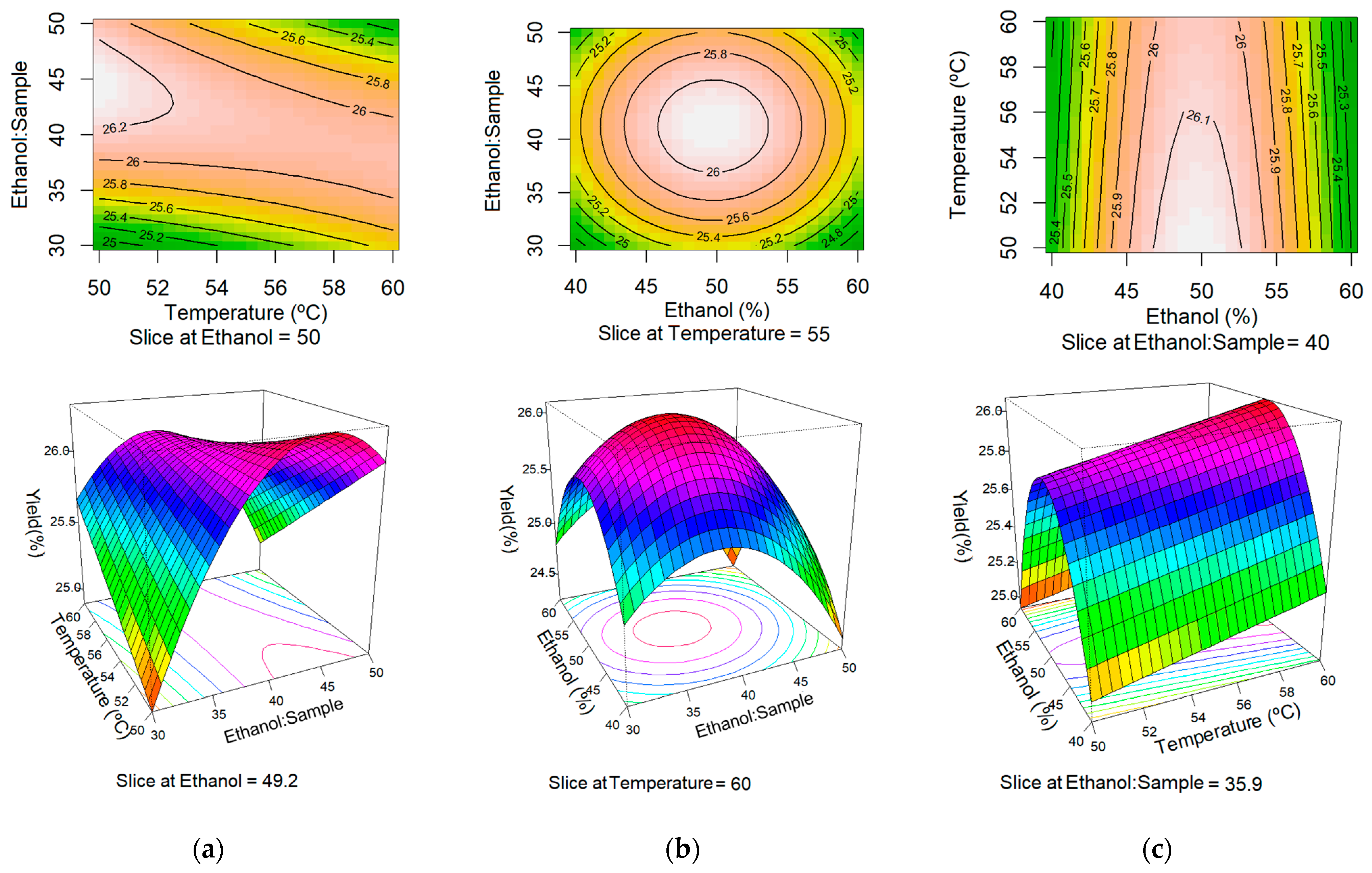
2.5. Response Surface Methodology
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Da Costa, J.P.; Vendruscolo, F. Production of red pigments by Monascus ruber CCT 3802 using lactose as a substrate. Biocatal. Agric. Biotechnol. 2017, 11, 50–55. [Google Scholar] [CrossRef]
- Silbir, S.; Goksungur, Y. Natural red pigment production by Monascus purpureus in submerged fermentation systems using a food industry waste: Brewer’s spent grain. Foods 2019, 8, 161. [Google Scholar] [CrossRef] [PubMed]
- Shetty, A.V.; Dave, N.; Murugesan, G.; Pai, S.; Pugazhendhi, A.; Varadavenkatesan, T.; Vinayagam, R.; Selvaraj, R. Production and extraction of red pigment by solid-state fermentation of broken rice using Monascus sanguineus NFCCI 2453. Biocatal. Agric. Biotechnol. 2021, 33, 101964. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, X.; Wu, Z.; Wang, Z. Investigation of relationship between lipid and Monascus pigment accumulation by extractive fermentation. J. Biotechnol. 2015, 212, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.L.; Wu, X.P.; Chen, B.; Deng, S.S.; Chen, Z.E.; Huang, Y.Y.; Jin, S.S. Comparative analysis of genetic polymorphisms among Monascus strains by ISSR and RAPD markers. J. Sci. Food Agric. 2017, 97, 636–640. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Shao, Y.; Zhou, Y.; Chen, W.; Chen, F. Monascus pigments. In Industrial Biotechnology of Vitamins, Biopigments, and Antioxidants; Vandamme, E.J., Revuelta, J.L., Eds.; Wiley-VCH: Weinheim, Germany, 2016; pp. 497–535. [Google Scholar]
- Zhang, C.; Zhang, N.; Chen, M.; Wang, H.; Shi, J.; Wang, B.; Sol, B.; Wang, C. Metabolomics analysis of the effect of glutamic acid on Monacolin K synthesis in Monascus purpureus. Front. Microbiol. 2020, 11, 610471. [Google Scholar] [CrossRef] [PubMed]
- Zhen, Z.; Xiong, X.; Liu, Y.; Zhang, J.; Wang, S.; Li, L.; Gao, M. NaCl inhibits citrinin and stimulates Monascus pigments and monacolin K production. Toxins 2019, 11, 118. [Google Scholar] [CrossRef] [PubMed]
- Bhavsar, S.; Dudhagara, P.; Tank, S. R software package based statistical optimization of process components to simultaneously enhance the bacterial growth, laccase production and textile dye decolorization with cytotoxicity study. PloS ONE 2018, 13. [Google Scholar] [CrossRef] [PubMed]
| Run Order | Ethanol (%) | Temperature (°C) | Ethanol: Sample (mL:g) | Yield (%) | Abs 400 nm | Abs 470 nm | Abs 500 nm | Density (g/mL) |
|---|---|---|---|---|---|---|---|---|
| 1 | 60 | 50 | 40 | 25.0 ± 0.10 | 0.260 ± 0.0081 | 0.151 ± 0.0046 | 0.194 ± 0.0061 | 0.894 ± 0.0056 |
| 2 | 50 | 55 | 40 | 26.2 ± 0.26 | 0.270 ± 0.0068 | 0.155 ± 0.0044 | 0.198 ± 0.0055 | 0.922 ± 0.0209 |
| 3 | 40 | 50 | 40 | 25.4 ± 0.00 | 0.241 ± 0.0032 | 0.136 ± 0.0031 | 0.169 ± 0.0036 | 0.909 ± 0.0214 |
| 4 | 40 | 55 | 50 | 24.8 ± 0.47 | 0.199 ± 0.0078 | 0.112 ± 0.0052 | 0.140 ± 0.0058 | 0.935 ± 0.0101 |
| 5 | 40 | 60 | 40 | 25.6 ± 0.14 | 0.246 ± 0.0070 | 0.141 ± 0.0050 | 0.177 ± 0.0065 | 0.936 ± 0.0074 |
| 6 | 50 | 55 | 40 | 26.2 ± 0.26 | 0.270 ± 0.0068 | 0.155 ± 0.0044 | 0.198 ± 0.0056 | 0.922 ± 0.0209 |
| 7 | 60 | 55 | 50 | 25.1 ± 0.28 | 0.221 ± 0.0085 | 0.127 ± 0.0055 | 0.163 ± 0.0070 | 0.901 ± 0.0044 |
| 8 | 50 | 50 | 50 | 26.1 ± 0.40 | 0.203 ± 0.0069 | 0.114 ± 0.0044 | 0.144 ± 0.0064 | 0.924 ± 0.0057 |
| 9 | 60 | 55 | 30 | 24.4 ± 0.53 | 0.362 ± 0.0200 | 0.212 ± 0.0130 | 0.275 ± 0.0167 | 0.900 ± 0.0112 |
| 10 | 40 | 55 | 30 | 24.2 ± 0.04 | 0.309 ± 0.0095 | 0.177 ± 0.0059 | 0.222 ± 0.0079 | 0.935 ± 0.0156 |
| 11 | 50 | 60 | 50 | 24.8 ± 0.25 | 0.216 ± 0.0100 | 0.122 ± 0.0060 | 0.156 ± 0.0076 | 0.913 ± 0.0033 |
| 12 | 50 | 50 | 30 | 25.1 ± 0.16 | 0.325 ± 0.0053 | 0.186 ± 0.0030 | 0.237 ± 0.0031 | 0.927 ± 0.0031 |
| 13 | 60 | 60 | 40 | 25.1 ± 0.22 | 0.273 ± 0.0067 | 0.161 ± 0.0044 | 0.209 ± 0.0059 | 0.893 ± 0.0134 |
| 14 | 50 | 60 | 30 | 25.7 ± 0.16 | 0.341 ± 0.0029 | 0.199 ± 0.0015 | 0.256 ± 0.0023 | 0.916 ± 0.0086 |
| Mean | Std. Error | p_Value | |
|---|---|---|---|
| Intercept | −24.68 | 5.425 | <0.0001 |
| Temperature (°C) | 0.3437 | 0.07828 | <0.0001 |
| Ethanol (%) | 0.8219 | 0.1058 | <0.0001 |
| Ethanol:sample ratio (v/v) | 1.044 | 0.1357 | <0.0001 |
| Ethanol2 | −0.008265 | 0.001056 | <0.0001 |
| Ethanol:sample ratio2 | −0.006747 | 0.001056 | <0.0001 |
| Temperature × ethanol:sample | −0.008864 | 0.001927 | <0.0001 |
| Goodness of fit | |||
| Multiple R-squared | 0.769 | ||
| Adjusted R-squared | 0.729 | ||
| Residuals | 0.111 |
| Mean | Std. Error | p_Value | |
|---|---|---|---|
| Intercept | 0.0016 | 0.00019 | <0.0001 |
| (Abs 400 + Abs 470 +Abs 500) | 0.0088 | 0.00019 | <0.0001 |
| Goodness of fit | |||
| Multiple R-squared | 0.964 | ||
| Adjusted R-squared | 0.964 | ||
| Residuals | 0.00075 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quispe-Rivera, E.; Tucta-Huillca, F.; Silva-Jaimes, M.; Gonzales-Barron, U.; Cadavez, V. Optimization of Pigment Extraction from Quinoa Flour Fermented by Monascus purpureus Supplemented with Sodium Chloride. Biol. Life Sci. Forum 2022, 18, 67. https://doi.org/10.3390/Foods2022-13021
Quispe-Rivera E, Tucta-Huillca F, Silva-Jaimes M, Gonzales-Barron U, Cadavez V. Optimization of Pigment Extraction from Quinoa Flour Fermented by Monascus purpureus Supplemented with Sodium Chloride. Biology and Life Sciences Forum. 2022; 18(1):67. https://doi.org/10.3390/Foods2022-13021
Chicago/Turabian StyleQuispe-Rivera, Evelyn, Franz Tucta-Huillca, Marcial Silva-Jaimes, Ursula Gonzales-Barron, and Vasco Cadavez. 2022. "Optimization of Pigment Extraction from Quinoa Flour Fermented by Monascus purpureus Supplemented with Sodium Chloride" Biology and Life Sciences Forum 18, no. 1: 67. https://doi.org/10.3390/Foods2022-13021
APA StyleQuispe-Rivera, E., Tucta-Huillca, F., Silva-Jaimes, M., Gonzales-Barron, U., & Cadavez, V. (2022). Optimization of Pigment Extraction from Quinoa Flour Fermented by Monascus purpureus Supplemented with Sodium Chloride. Biology and Life Sciences Forum, 18(1), 67. https://doi.org/10.3390/Foods2022-13021









