Abstract
The aim of the work was the enzymatic synthesis of flavours and fragrances, antioxidants and antimicrobials with the use of benzyl alcohol and its selected derivatives via transesterification with vinyl acetate. The conversion yields of esters were dependent on the structure of alcohols and ranged from about 17% (4-hydroxybenzyl acetate) to approximately 90% (benzyl acetate). The performed reactions allowed the creation of esters with pleasant odours, such as benzyl, 4-methoxybenzyl, and piperonyl acetates. Furthermore, vanillyl acetate was proven to be the best antioxidant with the IC50 value of 0.83 ± 0.04 mM. In the reference to antimicrobial activity the largest inhibition zone diameters were determined for 2-hydroxybenzyl acetate and the values obtained against E. coli PCM 2057 were 19.0 ± 1.0 mm and 20.3 ± 0.6 in the case of S. aureus PCM 2054.
1. Introduction
The “green” alternative to traditional chemical synthesis can be enzyme-based processes. Phenolic compounds are known to be biologically active substances but in many cases, their enzymatic modification by esterification or transesterification allows for obtaining esters with new features, such as changed flavour properties, enhanced solubility in lipophilic media or enhanced antimicrobial activity [1].
The aim of the work was the enzymatic synthesis of flavours and fragrances, antioxidants and antimicrobials with the use of benzyl alcohol and its selected derivatives, namely 2-hydroxybenzyl, 4-hydroxybenzyl, 4-methoxybenzyl (anisyl), 4-hydroxy-3-methoxybenzyl (vanillyl), 4-nitrobenzyl, and 3,4-(methylenedioxy)benzyl (piperonyl) alcohols via transesterification with vinyl acetate.
2. Materials and Methods
2.1. Materials
Chemicals used in the study were purchased from Sigma-Aldrich (Poznań, Poland) and Avantor Performance Materials Poland S.A. (Gliwice, Poland). Candida antarctica lipase B (CALB) was acquired from Sigma-Aldrich (product number: L4777). Culture media and their ingredients were purchased from Graso Biotech (Starogard Gdański, Poland) and BTL Sp. z o. o. (Łódź, Poland).
2.2. Microorganisms
For antimicrobial activity the following bacteria were used: Escherichia coli PCM 2057 and Staphylococcus aureus PCM 2054 from the Polish Collection of Microorganisms of the Institute of Immunology and Experimental Therapy, Polish Academy of Sciences (Wrocław, Poland).
2.3. Enzymatic Synthesis of Esters
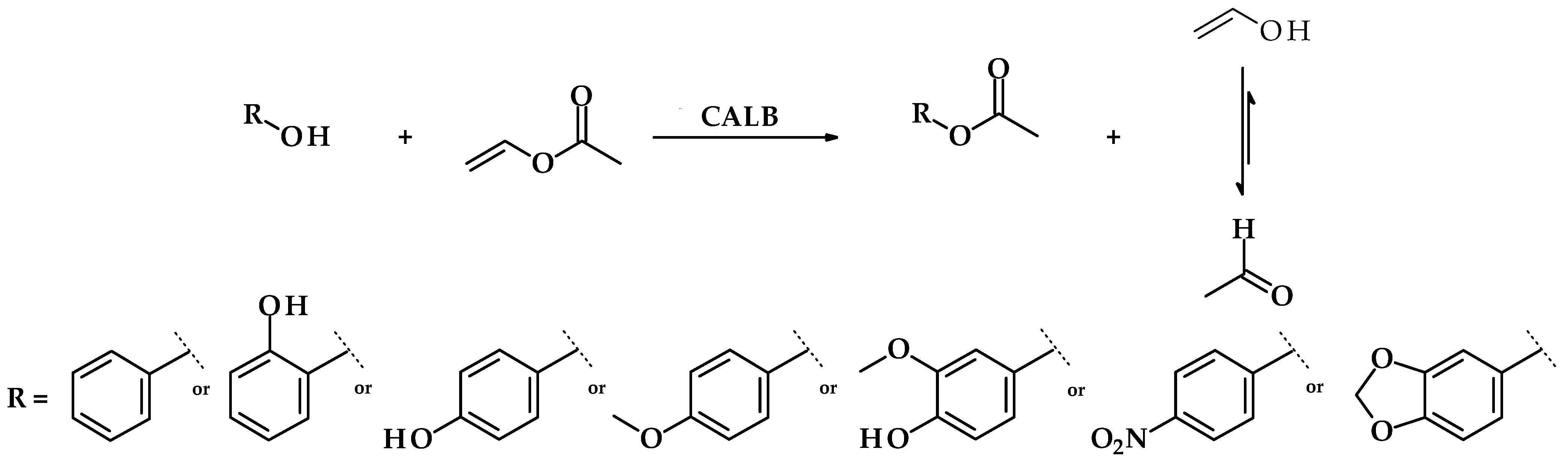
The syntheses of acetate esters of benzyl alcohol and its derivatives were carried out according to Figure 1. Briefly, the reactions were performed in 25 mL of isooctane and 2 mmoles of chosen alcohol and vinyl acetate. Candida antarctica lipase B (10% w/w by the mass of substrates) was used as a biocatalyst. The reactions were carried out at 37 °C at 250 rpm on a rotary shaker for 72 h. Tautomerization of vinyl alcohol to acetaldehyde results in shifting the equilibrium state towards the formation of the esters.
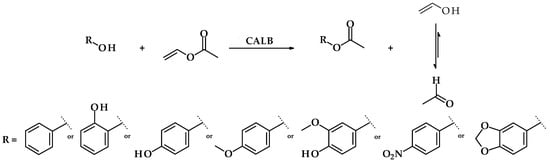
Figure 1.
Reaction scheme for benzyl alcohol derivatives esters syntheses via transesterification with vinyl acetate and Candida antarctica lipase B as a biocatalyst.
2.4. Column Chromatography and 1H NMR of the Synthesized Esters
Column chromatography was used for esters purification. Chloroform was as a mobile phase, while silica gel 60 (0.040–0.063 mm; 230–400 mesh) was used as a stationary phase. 1H NMR was employed for confirmation of the esters’ structures. Spectra were recorded on Bruker AVANCE 300 MHz spectrometer (Bruker, Billerica, MA, USA) with CDCl3 as a solvent. Proton chemical shifts are reported below in ppm (δ) and were relative to tetramethylsilane (TMS) as an internal standard.
Benzyl acetate: 1H NMR (300 MHz, CDCl3): δ 2.10 (3H, s), 5.11 (2H, s), 7.28–7.44 (5H, m).
2-Hydroxybenzyl acetate: 1H NMR (300 MHz, CDCl3): δ 2.14 (3H, s), 5.15 (2H, s), 6.88–7.02 (2H, m), 7.24–7.37 (2H, m), 7.78 (1H, s).
4-Hydroxybenzyl acetate: 1H NMR (300 MHz, CDCl3): δ 2.11 (3H, s), 5.06 (2H, s), 5.16 (1H, s), 6.78–6.91 (2H, m), 7.20–7.37 (2H, m).
4-Methoxybenzyl acetate: 1H NMR (300 MHz, CDCl3): δ 2.10 (3H, s), 3.83 (3H, s), 5.07 (2H, s), 6.86–6.97 (2H, m), 7.27–7.38 (2H, m).
Vanillyl acetate: 1H NMR (300 MHz, CDCl3): δ 2.11 (3H, s), 3.92 (3H, s), 5.05 (2H, s), 5.72 (1H, s), 6.89–6.91 (3H, m).
4-Nitrobenzyl acetate: 1H NMR (300 MHz, CDCl3): δ 2.16 (3H, s), 5.20 (2H, s), 7.47–7.57 (2H, m), 8.18–8.28 (2H, m).
Piperonyl acetate: 1H NMR (300 MHz, CDCl3): δ 2.08 (3H, s), 5.00 (2H, s), 5.96 (2H, s), 6.76–6.88 (3H, m).
2.5. Evaluation of Antioxidant Activity
DPPH· (2,2-diphenyl-1-picrylhydrazyl) assay was applied for evaluation of the antioxidant activity of the obtained esters. Briefly, stock solutions of the obtained esters (concentration = 10 mM) in methanol and 0.004% methanolic solution of DPPH· were prepared. The antioxidant activities of tested compounds were determined after 60 minutes with the use of a Rayleigh UV-1601 spectrophotometer (BRAIC, Beijing, China) at 517 nm. Moreover, the IC50 parameters, i.e., the concentration required for a 50% reduction in the DPPH· were calculated.
2.6. Evaluation of Antimicrobial Activity
In the reference to antimicrobial activity, the disc diffusion method was used. Esters were dissolved in ethanol and, on 6 mm discs, tested esters were applied (5 mg of the compound was on the disc). Bacterial 0.5 McFarland density suspensions were spread over the surface of the Mueller–Hinton agar plates and 6 mm discs soaked with chosen ester were placed. Agar plates were incubated for 16–18 h at 37 °C. Subsequently, after incubation inhibition, zone diameters were measured.
2.7. Statistical Analysis
Statistical analysis was performed using Statistica 13.3 software (TIBCO Software Inc., Palo Alto, CA, USA). A one-way analysis of variance (ANOVA) and Tukey’s post-hoc test were applied.
3. Results and Discussion
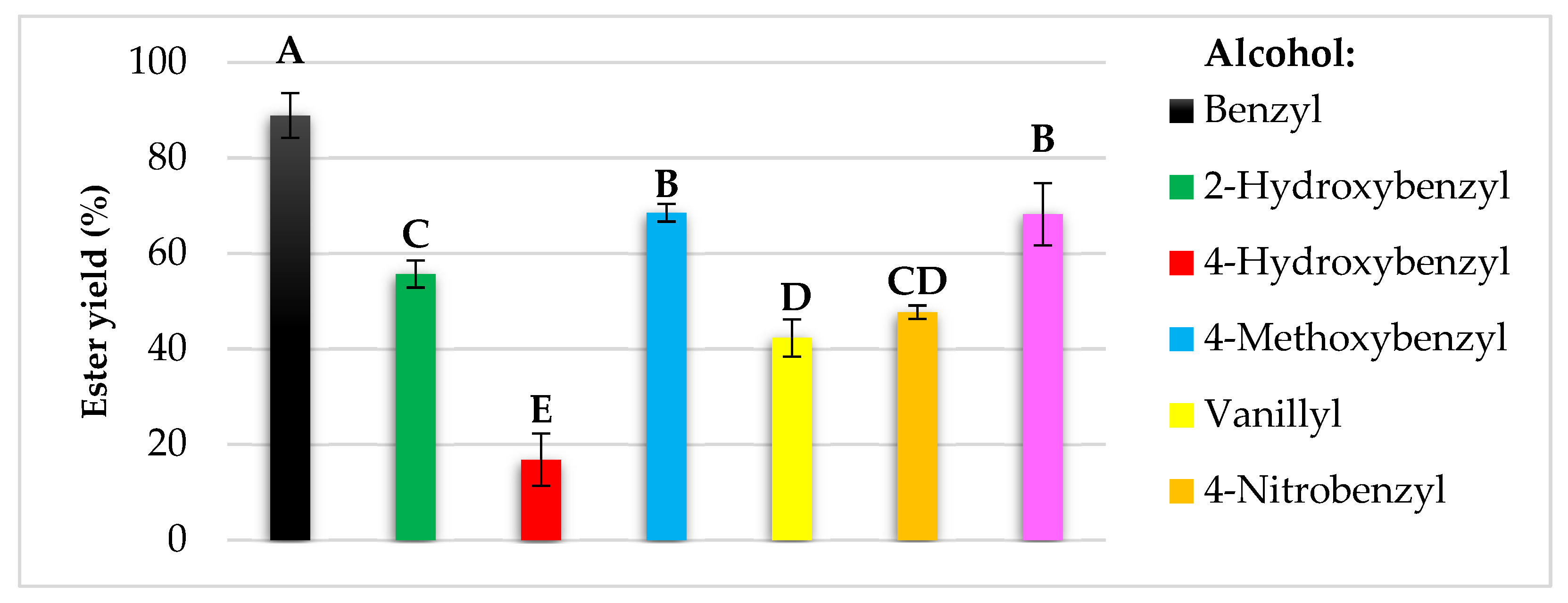
Modification of natural chemical compounds is a common method in the search for new functional compounds that can be used in various industries [2]. In the current research, benzyl alcohol and its six derivatives were enzymatically converted into acetate esters via transesterification of vinyl acetate. The use of C. antarctica lipase B allowed for the obtaining of seven esters and their yields ranged from 17 to 90% (Figure 2).
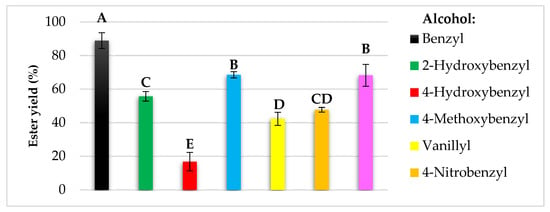
Figure 2.
The yields of enzymatic syntheses of esters of benzyl alcohol and its derivatives. The bars present the means ± SD of isolated yields. Yield: 100 × (actual quantity received/ideal quantity calculated). Means with the same capital letter (A–E) did not differ significantly (α = 0.05).
It was shown that conversion yields were dependent on the structure of the alcohol used, as well as their solubility in the reaction medium, i.e., isooctane. The lowest conversion was achieved for 4-hydroxybenzyl alcohol, followed by vanillyl and 4-nitrobenzyl alcohols with 42 and 48% yields, respectively. In the case of 2-hydroxybenzyl, piperonyl, and 4-metoxybenzyl alcohols yields exceeded the value of 50%, and the highest conversion was observed for non-substituted benzyl alcohol with approximately 90%.
There are known studies regarding the influence of the solvent, structure of the substrates, and enzymes on the result of the enzymatic reactions. Lee et al. [3] approached this topic in an interesting way. The authors optimized the enzymatic synthesis of naringin oleate. In the first stage of their study, lipases originated from C. antarctica, Thermomyces lanuginosus, and Rhizomucor miehei were compared, and T. lanuginosus enzyme showed the highest conversion. Optimization of other factors also influenced the final conversion rate, and the selection of solvent affected the conversion the most. Among eight tested solvents with different partition coefficients (logP) and dielectric constants, acetonitrile proved to be the best medium, and the conversion was 93% [3]. According to Baek et al. [4], it is obligatory to choose the appropriate solvent for enzymatic reactions with a suitable logP value. This is also associated with the impact of the solvent on the enzyme activity and the solubility of substrates, whereas in the study of Baek et al. [4], the synthesis of octyl formate was achieved with high conversion (>80%) when solvents with logP values greater than ~1.5 were applied.
Among the synthesized compounds, three of them, namely benzyl, 4-methoxybenzyl, and piperonyl acetates, had flavouring properties and are included in the lists of flavouring substances compiled by the Council of Europe, the Joint FAO/WHO Expert Committee on Food Additives, and the Flavor and Extract Manufacturers Association (Table 1). Esters of short carboxylic acids, such as acetates or butyrates have often fruity and floral fragrances and occur in a wide range of food products. The conventional, i.e., chemical synthesis of flavour esters is frequently associated with low yield and intense reaction conditions. In addition, other features, such as time inefficiency may be overcome by developing and intensifying enzymatic approaches [5]. The odour and flavour of benzyl acetate are described as sweet and fruity with noticeable jasmine notes. In the case of 4-methoxybenzyl acetate, apart from sweet notes, the following ones can be noted: vanilla, cherry, and coumarin-like. The latter is also with a fruity base but jammy in the taste and strawberry jam in the smell (Table 1).

Table 1.
Flavouring esters permitted for use in food with benzyl alcohol moiety.
The current study was continued in order to evaluate the antioxidant activities of the synthesized acetate esters with the use of the DPPH radical method. Table 2 presents antioxidant activities and IC50 values of the obtained esters. The radical scavenging capacities of phenolic compounds are dependent on the presence, amount, and arrangement of substituents in the aromatic ring. It is generally known that the hydroxyl group is the key substituent in the phenolics referring to the antioxidant activity [6]. Hence, the following esters, 2-hydroxybenzyl, 4-hydroxybenzyl, and vanillyl acetates, had IC50 values lower than 100 mM and the activity greater than 5%.

Table 2.
Antioxidant activities of the obtained esters determined by means of DPPH· assay.
Moreover, it can be observed that substituents such as nitro, methoxyl, or methylenedioxy groups did not reduce the DPPH radical. Interestingly, the hydroxyl group in the ortho position contributed to the reduction in the radical to a greater extent than the para hydroxyl group. Vanillyl ester proved to be the best antioxidant and the IC50 value was 0.83 ± 0.04 mM. Similarly, in other studies in the research of a wide group of phenolics, it has been observed that antioxidant properties are highly dependent on the number of phenolic hydroxyl groups, as well as their position. Furthermore, the presence of electron-donating methoxyl groups also influences the antioxidant activity of phenolic compounds [6,7].
In the current work, the antibacterial activity of the synthesized esters against E. coli and S. aureus was also evaluated (Table 3). Five of the seven synthesized esters did not exhibit any antibacterial effect in the disc diffusion method, and the inhibition zone diameters did not exceed 9 mm. According to the proposed ester sensitivity, i.e., inhibition zone, <10 mm means ester is not effective; 10–16 mm—weak activity; 16–20 mm—medium activity; and >20 mm—strong activity. Vanillyl acetate was able to inhibit the growth of the tested bacteria in a weak manner with the diameters of 10.7 ± 1.5 and 13.7 ± 0.6 mm for E. coli and S. aureus, respectively. The highest activity was observed for 2-hydroxybenzyl acetate with moderate action against E. coli (19.0 ± 1.0) and with strong activity toward S. aureus (20.3 ± 0.6).

Table 3.
Comparison of antimicrobial activity of the obtained esters.
In nature, 2-hydroxybenzyl alcohol occurs mainly in the form of β-glucoside (salicin). It can be found in the bark and leaves of such plants as willows (Salix, hence the name), poplars (Populus), or meadowsweet (Filipendula ulmaria) [8]. Both salicin, 2-hydroxybenzyl alcohol, and other derivatives of this alcohol have analgesic, anti-inflammatory, and antipyretic properties [9]. Moreover, the antimicrobial effect of 2-hydroxybenzyl alcohol has also been confirmed. Masika et al. [10] isolated two compounds from the bark of Salix capensis, namely catechol and 2-hydroxybenzyl alcohol. Both compounds exhibited moderate antibacterial activity with MIC = 250 µg/mL against Bacillus subtilis, S. aureus, and E. coli and 62.5 µg/mL toward Pseudomonas aeruginosa.
4. Conclusion
The presented herein study showed the possibility of modifying benzyl alcohol and its derivatives to acetate esters via enzymatic transesterification of vinyl acetate. The synthesized esters can be categorized into three groups, i.e., flavouring substances (benzyl, 4-methoxy, and piperonyl acetates), antioxidant agents (vanillyl acetate) and antimicrobial compounds (2-hydroxybenzyl acetate). The described preliminary research should be extended in order to deepen the knowledge about the activity of the compounds obtained and compare them with their precursors (benzyl and other alcohols). Then, the antimicrobial activity against a larger amount of food spoilage microorganisms and the antioxidant activity using other known assays should be investigated, as well as in convenience foods.
Author Contributions
Conceptualization, B.Z.; methodology, B.Z., K.J. and K.W.; formal analysis, B.Z.; investigation, B.Z.; writing—original draft preparation, B.Z.; writing—review and editing, B.Z., K.J., K.W. and A.F.; visualization, B.Z.; supervision, B.Z. and A.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Białecka-Florjańczyk, E.; Fabiszewska, A.; Zieniuk, B. Phenolic Acids Derivatives—Biotechnological Methods of Synthesis and Bioactivity. Curr. Pharm. Biotechnol. 2018, 19, 1098–1113. [Google Scholar] [CrossRef] [PubMed]
- Zieniuk, B.; Białecka-Florjańczyk, E.; Wierzchowska, K.; Fabiszewska, A. Recent advances in the enzymatic synthesis of lipophilic antioxidant and antimicrobial compounds. World J. Microbiol. Biotechnol. 2021, 38, 11. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, K.; Son, J.; Lee, H.; Song, J.H.; Lee, T.; Jeon, H.; Kim, H.S.; Park, S.J.; Yoo, H.Y.; et al. Improved Productivity of Naringin Oleate with Flavonoid and Fatty Acid by Efficient Enzymatic Esterification. Antioxidants 2022, 11, 242. [Google Scholar] [CrossRef] [PubMed]
- Baek, Y.; Lee, J.; Son, J.; Lee, T.; Sobhan, A.; Lee, J.; Koo, S.-M.; Shin, W.H.; Oh, J.-M.; Park, C. Enzymatic Synthesis of Formate Ester through Immobilized Lipase and Its Reuse. Polymers 2020, 12, 1802. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, K.S.; Rathod, V.K. Process Intensification of Enzymatic Synthesis of Flavor Esters: A Review. Chem. Rec. 2022, 22, e202100213. [Google Scholar] [CrossRef] [PubMed]
- Parcheta, M.; Świsłocka, R.; Orzechowska, S.; Akimowicz, M.; Choińska, R.; Lewandowski, W. Recent Developments in Effective Antioxidants: The Structure and Antioxidant Properties. Materials 2021, 14, 1984. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; He, Y.; Lu, F. The structure-antioxidant activity relationship of dehydrodiferulates. Food Chem. 2018, 269, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Kulasekaran, S.; Cerezo-Medina, S.; Harflett, C.; Lomax, C.; de Jong, F.; Rendour, A.; Ruvo, G.; Hanley, S.J.; Beale, M.H.; Ward, J.L. A willow UDP-glycosyltransferase involved in salicinoid biosynthesis. J. Exp. Bot. 2021, 72, 1634–1648. [Google Scholar] [CrossRef]
- Kim, C.S.; Subedi, L.; Park, K.J.; Kim, S.Y.; Choi, S.U.; Kim, K.H.; Lee, K.R. Salicin derivatives from Salix glandulosa and their biological activities. Fitoterapia 2015, 106, 147–152. [Google Scholar] [CrossRef]
- Masika, P.J.; Sultana, N.; Afolayan, A.J.; Houghton, P.J. Isolation of two antibacterial compounds from the bark of Salix capensis. S. Afr. J. Bot. 2005, 71, 441–443. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).