Molecular Encapsulation of Hydrolyzed Chia Seed Oil by Ultrasonically Treated Amylose Inclusion Complexes †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chia Seed Oil Enzymatic Hydrolysis
2.3. Formation of Untreated and Ultrasonically Treated Inclusion Complexes
2.4. Attenuated Total Reflectance Fourier-Transform Infrared Spectroscopy (ATR-FTIR)
2.5. X-ray Diffraction (XRD)
2.6. Differential Scanning Calorimetry (DSC)
2.7. Statistical Analysis
3. Results and Discussion
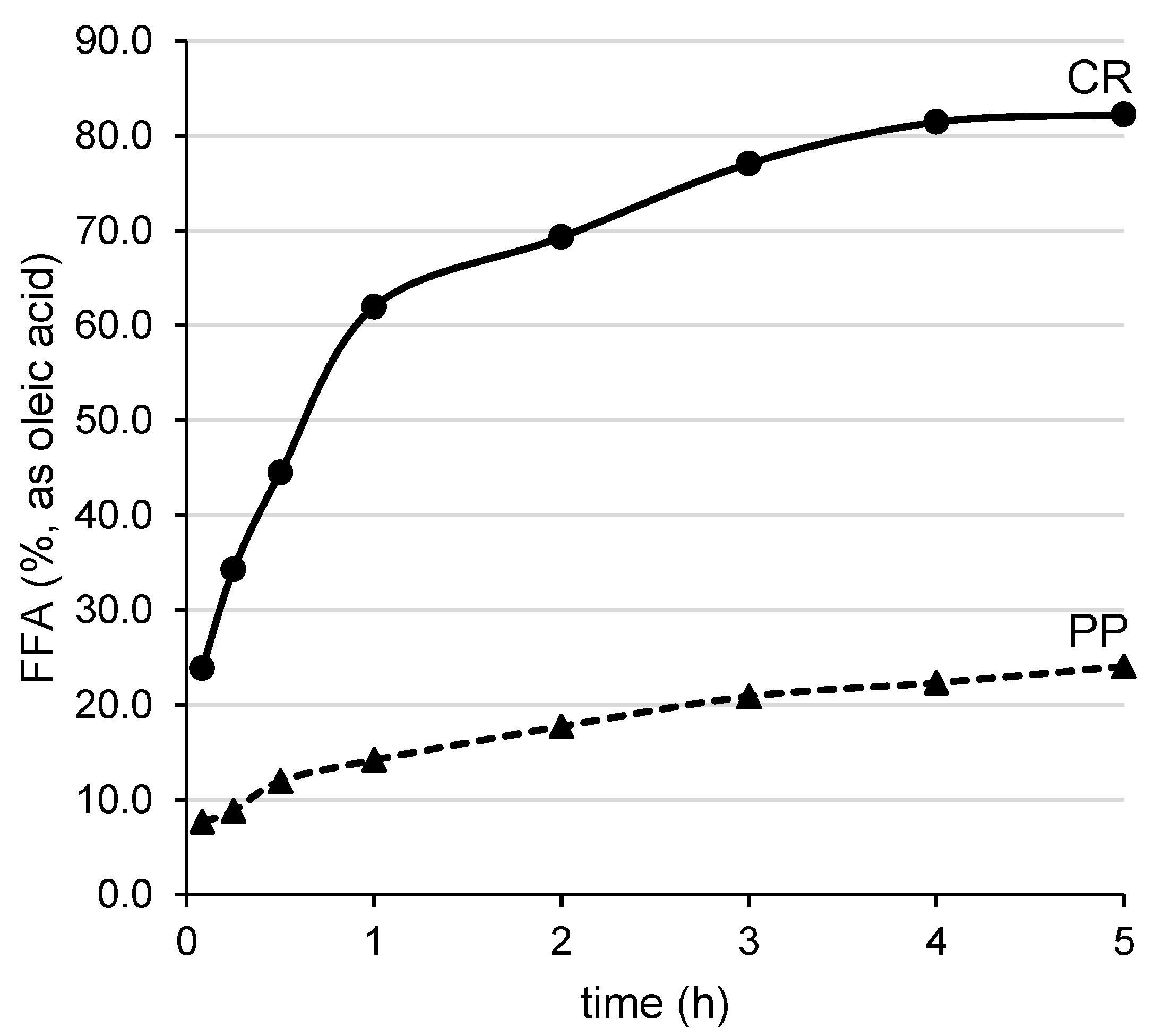
3.1. Enzymatic Hydrolysis of Chia Seed Oil
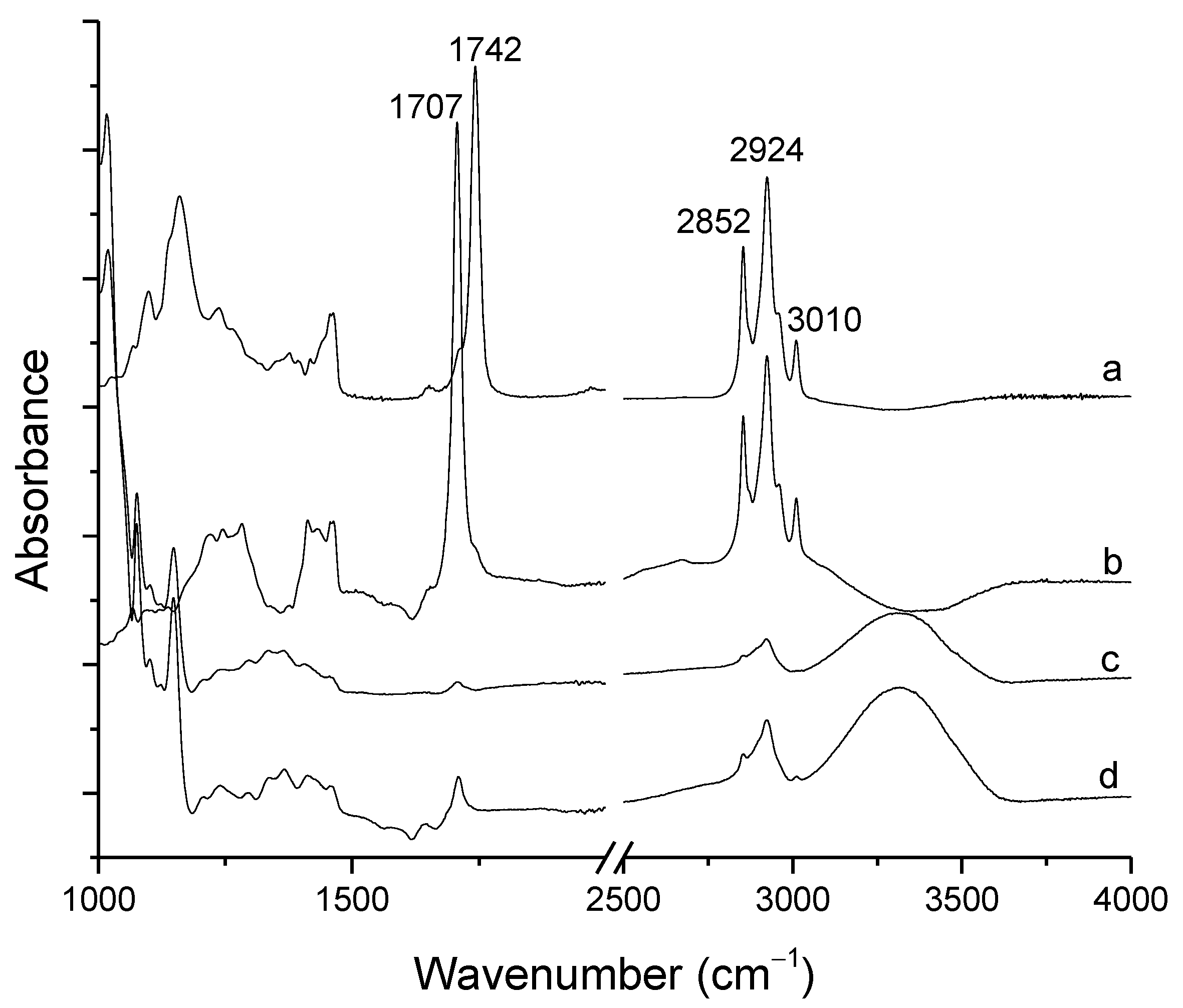
3.2. Attenuated Total Reflectance Fourier-Transform Infrared Spectroscopy (ATR-FTIR)
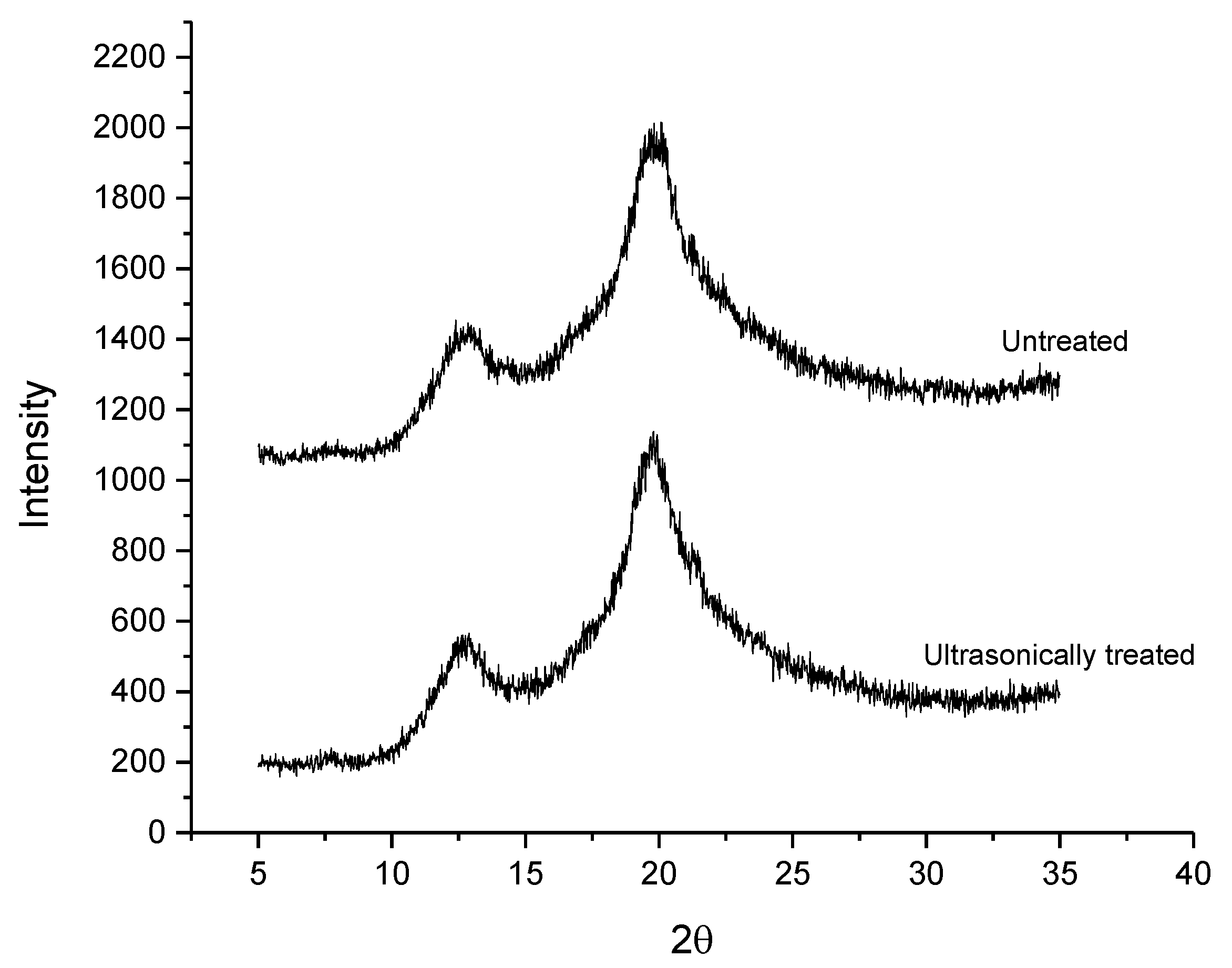
3.3. X-ray Diffraction
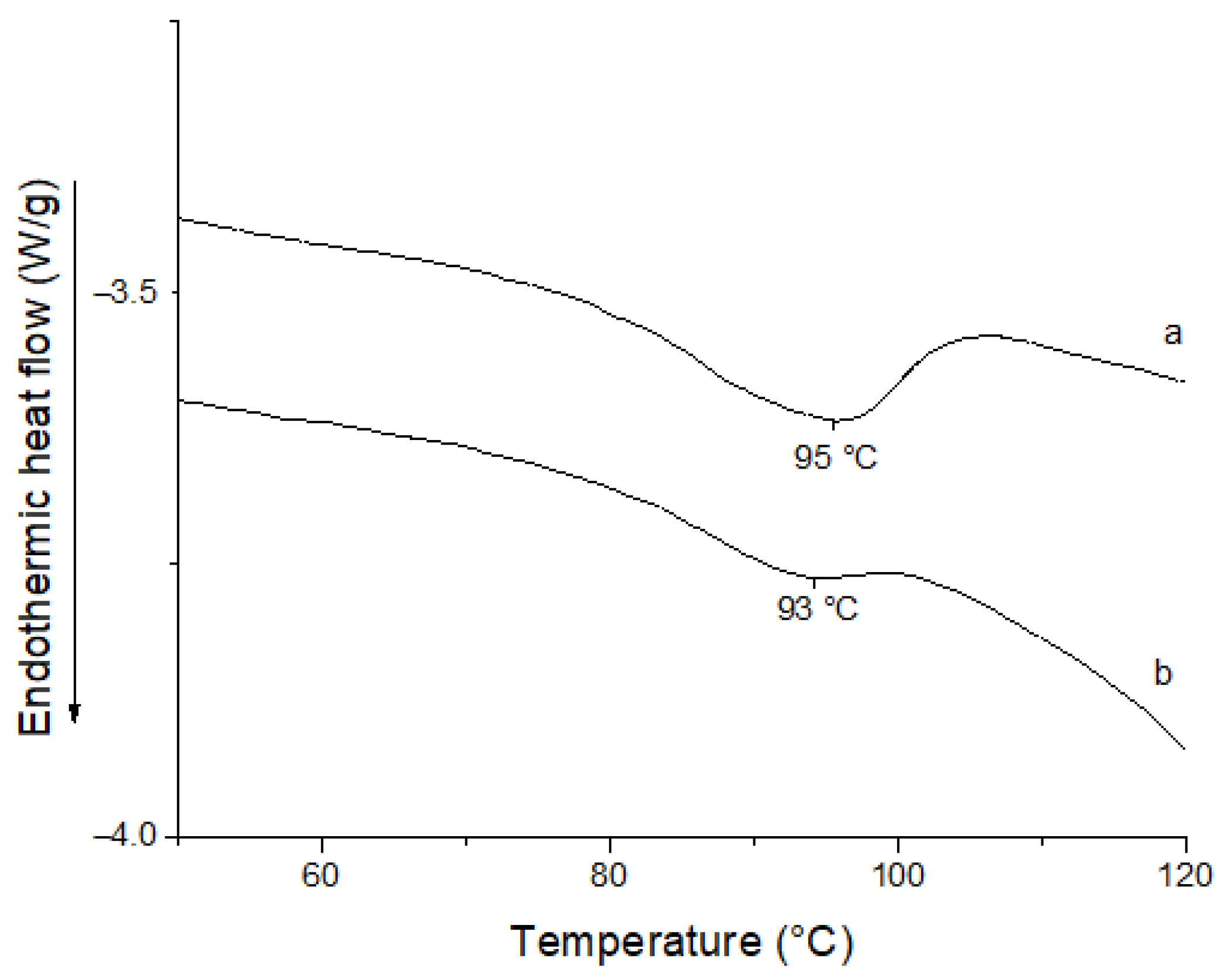
3.4. Differential Scanning Calorimetry
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Di Marco, A.E.; Ixtaina, V.Y.; Tomás, M.C. Analytical and technological aspects of amylose inclusion complexes for potential applications in functional foods. Food Biosci. 2022, 47, 101625. [Google Scholar] [CrossRef]
- Chao, C.; Yu, J.; Wang, S.; Copeland, L.; Wang, S. Mechanisms Underlying the Formation of Complexes between Maize Starch and Lipids. J. Agric. Food Chem. 2018, 66, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Liu, P.; Gao, W.; Wu, Z.; Yu, B.; Wang, R.; Cui, B.; Qiu, L.; Sun, C. Preparation of starch-lipid complex by ultrasonication and its film forming capacity. Food Hydrocoll. 2020, 99, 105340. [Google Scholar] [CrossRef]
- Di Marco, A.E.; Ixtaina, V.Y.; Tomás, M.C. Inclusion complexes of high amylose corn starch with essential fatty acids from chia seed oil as potential delivery systems in food. Food Hydrocoll. 2020, 108, 106030. [Google Scholar] [CrossRef]
- Mendes, A.A.; Oliveira, P.C.; De Castro, H.F. Properties and biotechnological applications of porcine pancreatic lipase. J. Mol. Catal. B Enzym. 2012, 78, 119–134. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Marco, A.E.; Ixtaina, V.Y.; Tomás, M.C. Molecular Encapsulation of Hydrolyzed Chia Seed Oil by Ultrasonically Treated Amylose Inclusion Complexes. Biol. Life Sci. Forum 2022, 17, 24. https://doi.org/10.3390/blsf2022017024
Di Marco AE, Ixtaina VY, Tomás MC. Molecular Encapsulation of Hydrolyzed Chia Seed Oil by Ultrasonically Treated Amylose Inclusion Complexes. Biology and Life Sciences Forum. 2022; 17(1):24. https://doi.org/10.3390/blsf2022017024
Chicago/Turabian StyleDi Marco, Andrea E., Vanesa Y. Ixtaina, and Mabel C. Tomás. 2022. "Molecular Encapsulation of Hydrolyzed Chia Seed Oil by Ultrasonically Treated Amylose Inclusion Complexes" Biology and Life Sciences Forum 17, no. 1: 24. https://doi.org/10.3390/blsf2022017024
APA StyleDi Marco, A. E., Ixtaina, V. Y., & Tomás, M. C. (2022). Molecular Encapsulation of Hydrolyzed Chia Seed Oil by Ultrasonically Treated Amylose Inclusion Complexes. Biology and Life Sciences Forum, 17(1), 24. https://doi.org/10.3390/blsf2022017024





