Immunonutritional Benefits of Chenopodium quinoa’s Ingredients Preventing Obesity-Derived Metabolic Imbalances †
Abstract
1. Introduction
2. Materials and Methods
2.1. Ingredients
2.2. Experimental Model and Subject
2.3. Cell Preparation for Flow Cytometry and the Gating Strategy
2.4. Statistical Analyses
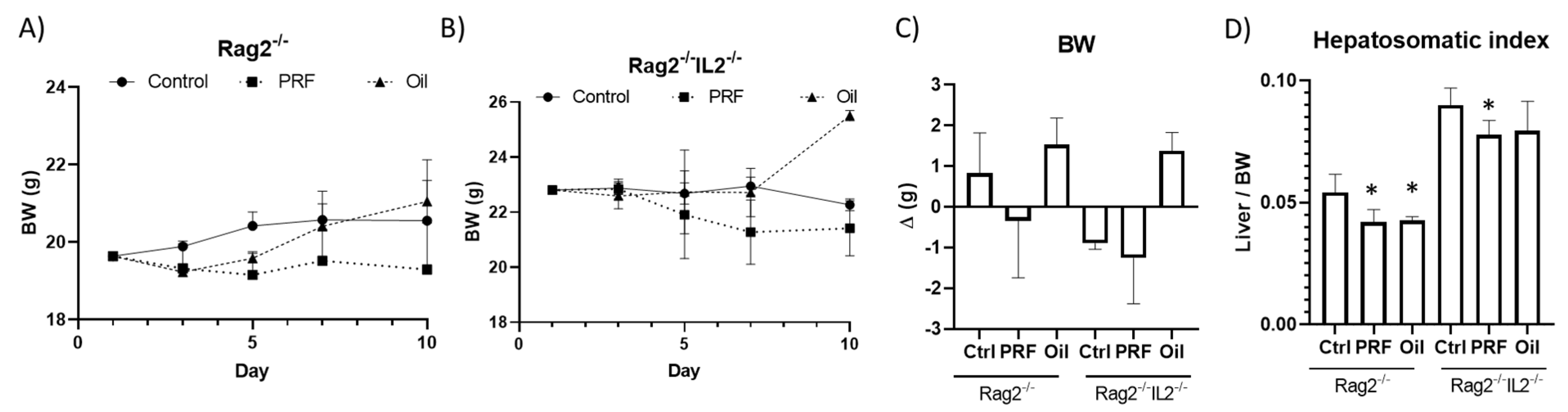
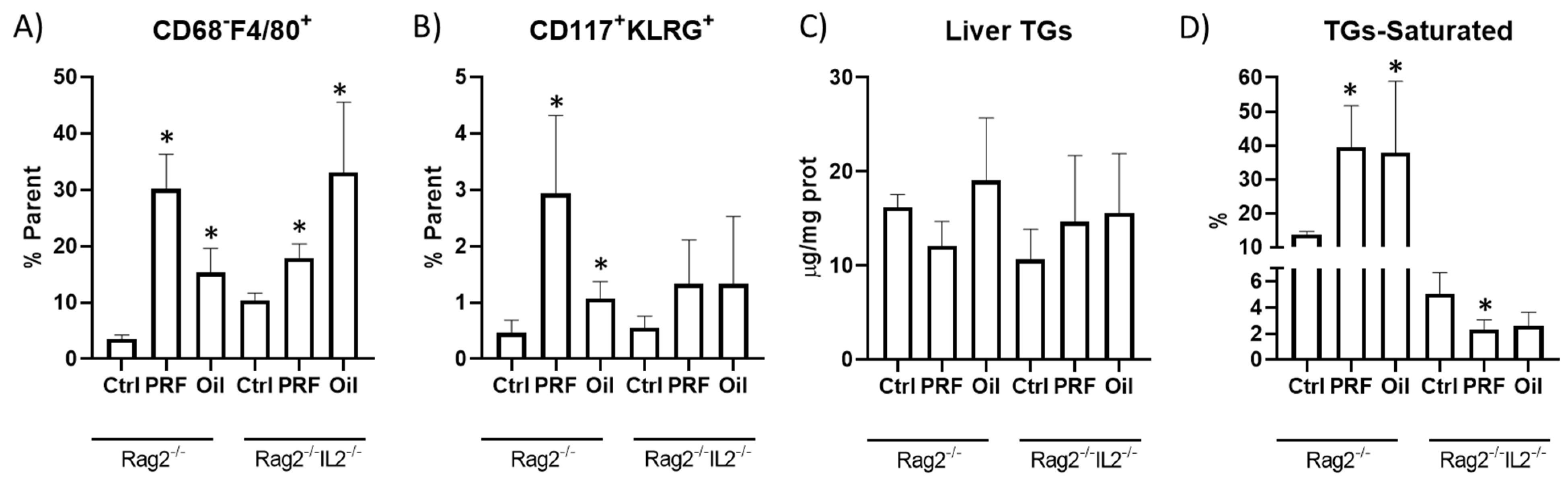
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mao, K.; Baptista, A.P.; Tamoutounour, S.; Zhuang, L.; Bouladoux, N.; Martins, A.J.; Huang, Y.; Gerner, M.Y.; Belkaid, Y.; Germain, R.N. Innate and adaptive lymphocytes sequentially shape the gut microbiota and lipid metabolism. Nature 2018, 554, 255–259. [Google Scholar] [CrossRef]
- Sasaki, T.; Moro, K.; Kubota, T.; Kubota, N.; Kato, T.; Ohno, H.; Nakae, S.; Saito, H.; Koyasu, S. Innate Lymphoid Cells in the Induction of Obesity. Cell Rep. 2019, 28, 202–217. [Google Scholar] [CrossRef] [PubMed]
- Schuppan, D.; Schattenberg, J.M. Non-alcoholic steatohepatitis: Pathogenesis and novel therapeutic approaches. J. Gastroenterol. Hepatol. 2013, 28, 68–76. [Google Scholar] [CrossRef]
- Cox, N.; Crozet, L.; Holtman, I.R.; Loyher, P.L.; Lazarov, T.; White, J.B.; Mass, E.; Stanley, E.R.; Elemento, O.; Glass, C.K.; et al. Diet-regulated production of PDGFcc by macrophages controls energy storage. Science 2021, 373, eabe9383. [Google Scholar] [CrossRef] [PubMed]
- Selma-Gracia, R.; Haros, C.M.; Laparra Llopis, J.M. Inclusion of Salvia hispanica L. and Chenopodium quinoa into bread formulations improves metabolic imbalances derived from a high-fat intake in hyperglycaemic mice. Food Funct. 2020, 11, 7994–8002. [Google Scholar] [CrossRef]
- Selma-Gracia, R.; Megušar, P.; Haros, C.M.; Laparra, J.M. Immunonutritional Bioactives from Chenopodium quinoa and Salvia hispanica L. Flour Positively Modulate Insulin Resistance and Preserve Alterations in Peripheral Myeloid Population. Nutrients 2021, 13, 1537. [Google Scholar] [CrossRef] [PubMed]
- Ballester-Sánchez, J.; Gil, J.V.; Fernández-Espinar, M.T.; Haros, C.M. Quinoa wet-milling: Effect of steeping conditions on starch recovery and quality. Food Hydrocoll. 2019, 89, 837–843. [Google Scholar] [CrossRef]
- Bal, S.M.; Golebski, K.; Spits, G. Plasticity of innate lymphoid cell subsets. Nat. Rev. Immunol. 2020, 20, 552–565. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcos Pasero, H.; Bojarczuk, A.; Haros, C.M.; Laparra Llopis, J.M. Immunonutritional Benefits of Chenopodium quinoa’s Ingredients Preventing Obesity-Derived Metabolic Imbalances. Biol. Life Sci. Forum 2022, 17, 20. https://doi.org/10.3390/blsf2022017020
Marcos Pasero H, Bojarczuk A, Haros CM, Laparra Llopis JM. Immunonutritional Benefits of Chenopodium quinoa’s Ingredients Preventing Obesity-Derived Metabolic Imbalances. Biology and Life Sciences Forum. 2022; 17(1):20. https://doi.org/10.3390/blsf2022017020
Chicago/Turabian StyleMarcos Pasero, Helena, Adrianna Bojarczuk, Claudia Monika Haros, and Jose Moisés Laparra Llopis. 2022. "Immunonutritional Benefits of Chenopodium quinoa’s Ingredients Preventing Obesity-Derived Metabolic Imbalances" Biology and Life Sciences Forum 17, no. 1: 20. https://doi.org/10.3390/blsf2022017020
APA StyleMarcos Pasero, H., Bojarczuk, A., Haros, C. M., & Laparra Llopis, J. M. (2022). Immunonutritional Benefits of Chenopodium quinoa’s Ingredients Preventing Obesity-Derived Metabolic Imbalances. Biology and Life Sciences Forum, 17(1), 20. https://doi.org/10.3390/blsf2022017020






