Bioactivity of Monoterpene Alcohols as an Indicator of Biopesticidal Essential Oils against the Root Knot Nematode Meloidogyne ethiopica †
Abstract
:1. Introduction
2. Materials and Methods
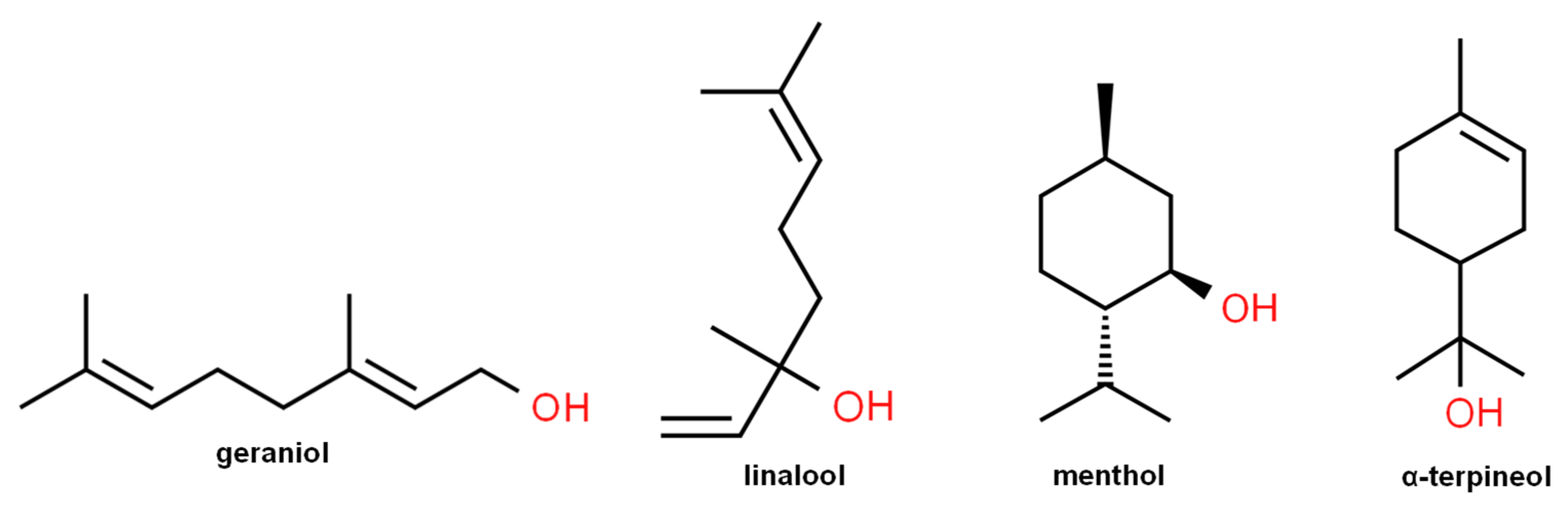
2.1. Chemicals
2.2. Meloidogne ethiopica Juveniles
2.3. Direct Contact Bioassays
2.4. Data Treatment
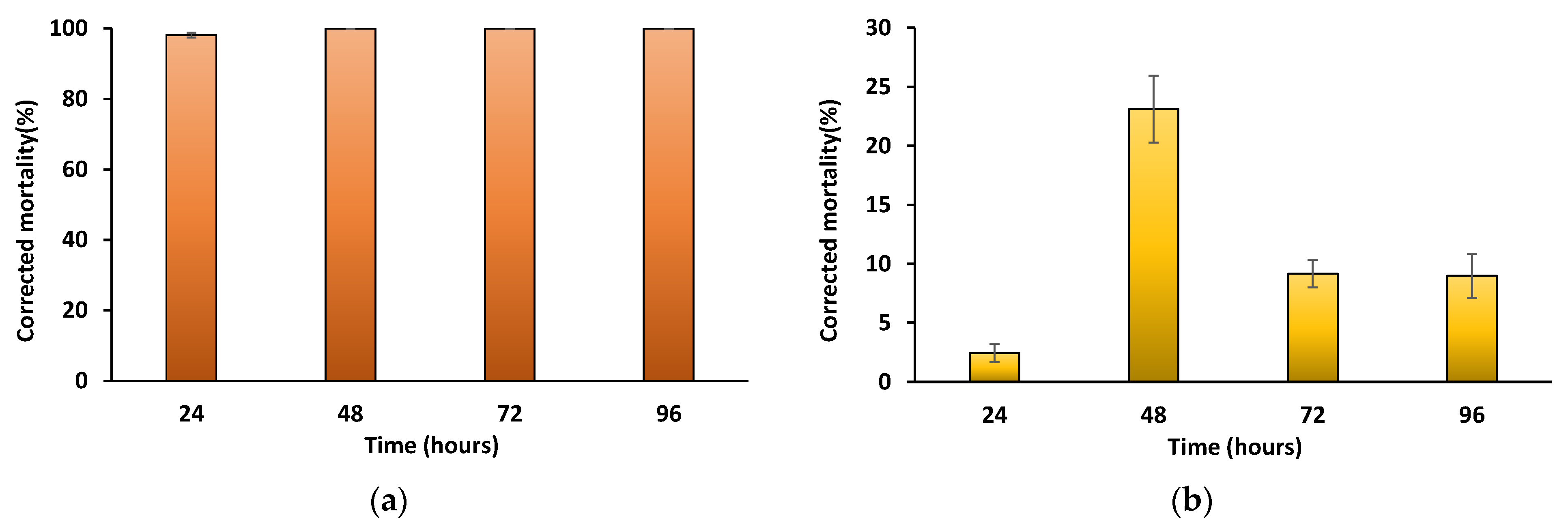
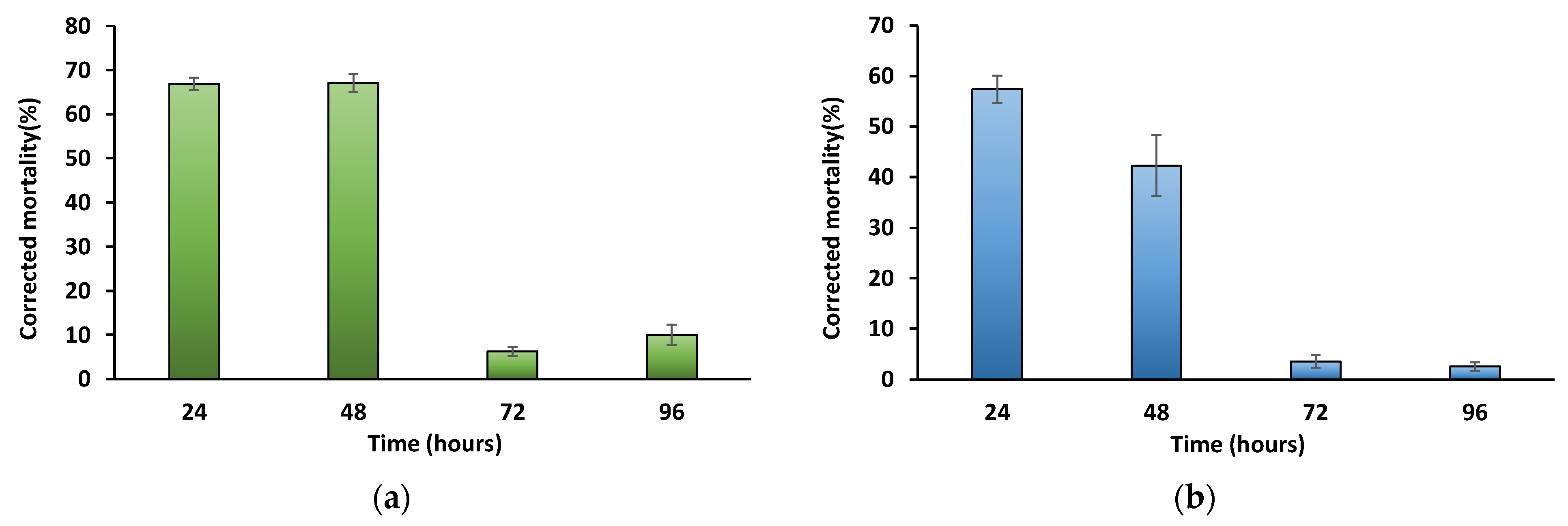
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jones, J.T.; Haegeman, A.; Danchin, E.G.J.; Gaur, H.S.; Helder, J.; Jones, M.G.K.; Kikuchi, T.; Manzanilla-López, R.; Palomares-Rius, J.E.; Wesemael, W.M.L.; et al. Top 10 plant-parasitic nematodes in molecular plant pathology. Mol. Plant Pathol. 2013, 14, 946–961. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, A.G. Taxonomy of Meloidogyne (Nematodea: Heteroderidae) with descriptions of four new species*. Trans. Zool. Soc. London 1968, 31, 263–401. [Google Scholar] [CrossRef]
- Whitehead, A.G. The Distribution of Root-Knot Nematodes (Meloidogyne Spp.) in Tropical Africa. Nematologica 1969, 15, 315–333. [Google Scholar] [CrossRef]
- Carneiro, R.; Randig, O.; Almeida, M.R.; Gomes, A.C. Additional information on Meloidogyne ethiopica Whitehead, 1968 (Tylenchida: Meloidogynidae), a root-knot nematode parasitising kiwi fruit and grape-vine from Brazil and Chile. Nematology 2004, 6, 109–123. [Google Scholar] [CrossRef]
- Aydınlı, G.; Mennan, S.; Devran, Z.; Širca, S.; Urek, G. First Report of the Root-Knot Nematode Meloidogyne ethiopica on Tomato and Cucumber in Turkey. Plant Dis. 2013, 97, 1262. [Google Scholar] [CrossRef] [PubMed]
- Širca, S.; Urek, G.; Karssen, G. First Report of the Root-Knot Nematode Meloidogyne ethiopica on Tomato in Slovenia. Plant Dis. 2004, 88, 680. [Google Scholar] [CrossRef] [PubMed]
- Maleita, C.M.; Simões, M.J.; Egas, C.; Curtis, R.H.C.; de, O. Abrantes, I.M. Biometrical, Biochemical, and Molecular Diagnosis of Portuguese Meloidogyne hispanica Isolates. Plant Dis. 2012, 96, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Conceição, I.L.; Tzortzakakis, E.A.; Gomes, P.; Abrantes, I.; da Cunha, M.J. Detection of the root-knot nematode Meloidogyne ethiopica in Greece. Eur. J. Plant Pathol. 2012, 134, 451–457. [Google Scholar] [CrossRef]
- Gerič Stare, B.; Strajnar, P.; Susič, N.; Urek, G.; Širca, S. Reported populations of Meloidogyne ethiopica in Europe identified as Meloidogyne luci. Plant Dis. 2017, 101, 1627–1632. [Google Scholar] [CrossRef] [PubMed]
- Forghani, F.; Hajihassani, A. Recent Advances in the Development of Environmentally Benign Treatments to Control Root-Knot Nematodes. Front. Plant Sci. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Faria, J.M.S.; Rodrigues, A.M. Essential Oils as Potential Biopesticides in the Control of the Genus Meloidogyne: A Review. Biol. Life Sci. Forum 2021, 3, 26. [Google Scholar] [CrossRef]
- Faria, J.M.S.; Rodrigues, A.M.; Sena, I.; Moiteiro, C.; Bennett, R.N.; Mota, M.; Figueiredo, A.C. Bioactivity of Ruta graveolens and Satureja Montana Essential Oils on Solanum tuberosum Hairy Roots and Solanum tuberosum Hairy Roots with Meloidogyne chitwoodi Co-cultures. J. Agric. Food Chem. 2016, 64, 7452–7458. [Google Scholar] [CrossRef] [PubMed]
- Masyita, A.; Mustika Sari, R.; Dwi Astuti, A.; Yasir, B.; Rahma Rumata, N.; Emran, T.B.; Nainu, F.; Simal-Gandara, J. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem. X 2022, 13, 100217. [Google Scholar] [CrossRef] [PubMed]
- Faria, J.M.S.; Sena, I.; Ribeiro, B.; Rodrigues, A.M.; Maleita, C.M.N.; Abrantes, I.; Bennett, R.; Mota, M.; Figueiredo, A.C.d.S. First report on Meloidogyne chitwoodi hatching inhibition activity of essential oils and essential oils fractions. J. Pest Sci. 2016, 89, 207–217. [Google Scholar] [CrossRef]
- Faria, J.M.S.; Barbosa, P.; Bennett, R.N.; Mota, M.; Figueiredo, A.C. Bioactivity against Bursaphelenchus xylophilus: Nematotoxics from essential oils, essential oils fractions and decoction waters. Phytochemistry 2013, 94, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Faria, J.M.S.; Barbosa, P.; Vieira, P.; Vicente, C.S.L.; Figueiredo, A.C.; Mota, M. Phytochemicals as Biopesticides against the Pinewood Nematode Bursaphelenchus xylophilus: A Review on Essential Oils and Their Volatiles. Plants 2021, 10, 2614. [Google Scholar] [CrossRef] [PubMed]
- Faria, J.M.S.; Barbosa, P.; Teixeira, D.M.; Mota, M. A Review on the Nematicidal Activity of Volatile Allelochemicals against the Pinewood Nematode. Environ. Sci. Proc. 2020, 3, 1. [Google Scholar] [CrossRef]
- Echeverrigaray, S.; Zacaria, J.; Beltrão, R. Nematicidal activity of monoterpenoids against the root-knot nematode meloidogyne incognita. Phytopathology 2010, 100, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Nasiou, E.; Giannakou, I.O. Effect of geraniol, a plant-based alcohol monoterpene oil, against Meloidogyne javanica. Eur. J. Plant Pathol. 2018, 152, 701–710. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faria, J.M.S.; Rusinque, L.; Vicente, C.S.L.; Inácio, M.L. Bioactivity of Monoterpene Alcohols as an Indicator of Biopesticidal Essential Oils against the Root Knot Nematode Meloidogyne ethiopica . Biol. Life Sci. Forum 2022, 16, 15. https://doi.org/10.3390/IECHo2022-12485
Faria JMS, Rusinque L, Vicente CSL, Inácio ML. Bioactivity of Monoterpene Alcohols as an Indicator of Biopesticidal Essential Oils against the Root Knot Nematode Meloidogyne ethiopica . Biology and Life Sciences Forum. 2022; 16(1):15. https://doi.org/10.3390/IECHo2022-12485
Chicago/Turabian StyleFaria, Jorge M. S., Leidy Rusinque, Cláudia S. L. Vicente, and Maria L. Inácio. 2022. "Bioactivity of Monoterpene Alcohols as an Indicator of Biopesticidal Essential Oils against the Root Knot Nematode Meloidogyne ethiopica " Biology and Life Sciences Forum 16, no. 1: 15. https://doi.org/10.3390/IECHo2022-12485
APA StyleFaria, J. M. S., Rusinque, L., Vicente, C. S. L., & Inácio, M. L. (2022). Bioactivity of Monoterpene Alcohols as an Indicator of Biopesticidal Essential Oils against the Root Knot Nematode Meloidogyne ethiopica . Biology and Life Sciences Forum, 16(1), 15. https://doi.org/10.3390/IECHo2022-12485








