Abstract
Background: Evidence suggests that turmeric or curcumin intake can improve antioxidant defense, blood pressure, ageing and gut microbiota. The effects of turmeric concentrate (curcumin) intake on cardiovascular risk factors and exercise-induced oxidative stress were investigated. Methods: A randomized placebo-controlled study was performed to assess the effects of turmeric extract in healthy volunteers before and after a 30-minute exercise bout. Participants (n = 22) were given either 500 mg turmeric concentrate (Curcumin C3, Jarrow Formulas, Los Angeles, CA, USA) or placebo supplements. Anthropometry, systolic and diastolic blood pressure (SBP and DBP), pulse wave velocity (PWV), biomarkers of oxidative stress, perceived exertion and lipid peroxidation were assessed. Results: There were no significant differences in all baseline parameters between the placebo and the curcumin groups (p > 0.05). In the curcumin group, blood pressure response to exercise following curcumin intake was blunted, and the increase was not significant compared to basal values. In the last run, there was a significant difference (before–after) between curcumin and placebo groups (Δ in SBP: 7.3 ± 6.8 vs. 13.8 ± 6.3 mmHg, p = 0.007, and Δ in DBP: 2.3 ± 6.9 V 8.0 ± 6.8 mmHg, p = 0.012). Final PWV scores were reduced significantly in the curcumin group (7.2 ± 0.97 to 6.7 ± 0.77 m/s, p = 0.033), and this reduction was significant compared to the control (Δ of 0.56 vs. 0.21 m/s, p = 0.04). A significant increase was observed in urinary antioxidant power (p = 0.031) and total polyphenol levels (p = 0.022) post curcumin intervention, and those in the placebo did not show significant changes. The increase in exercise-induced MDA levels was blunted only in the curcumin group, and the before–after difference was significant compared to the control (Δ of −0.81 vs. +0.205 μmole/day, p = 0.032). The distance ran by the participants taking curcumin was significantly longer (p = 0.005), and compared to the placebo, the before–after difference was significant (Δ of −0.69 vs. +0.28 km, p = 0.014). Conclusion: Our study suggests that turmeric concentrate intake can reduce blood pressure and improve antioxidant, anti-inflammatory status and arterial compliance. Curcumin may improve exercise performance and ameliorate oxidative stress. Larger studies are warranted to validate these findings and test other cardiovascular risk factors.
1. Introduction
Regular physical exercise is known to convey several health benefits and is regarded to be a good practice to combat many metabolic diseases including cardiovascular disease (CVD), cancer, obesity, and diabetes [1,2,3]. However, strenuous, and prolonged exercise generates inflammatory cytokines and reactive oxygen species (ROS) [4,5]. It has been reported that the negative effects associated with eccentric exercise such as inflammation and DOMS are caused by a large increase in inflammatory cytokines in the working muscle, plasma and brain generated because of oxidative stress which stimulates the production of free radicals [5,6]. Retamoso et al. [7] found that eccentric exercise may cause delayed onset muscle soreness (DOMS), causing discomfort of skeletal muscles. MDA is the most used biomarker of oxidative stress in several conditions such as cancer, chronic obstructive pulmonary disease, and cardiovascular diseases [8]. It is vital to monitor both SBP and DBP throughout exercise. In addition, pulse wave velocity (PWV) is regarded the gold standard measurement of arterial stiffness and it is generally assessed by using carotid–femoral or brachial–ankle approaches [9]. PWV rises with stiffening of the aorta and consequently causes an earlier return of reflected pressure waves from the periphery to the aorta which enhances aortic SBP and reduces DBP [10]. In CVD and hypertensive patients, the incidence of oxidative stress can be determined in biological fluids by the thiobarbitoric acid (TBARS) assay [8,11]. These patients also display impaired antioxidant defense, and in the heart, the production of ROS exceeds the capacity of the antioxidant defense mechanism to buffering the ROS, resulting in cardiac dysfunction, ischemia-reperfusion injury, hypertrophy, cell death and heart failure [11].
Curcumin is a natural polyphenol derived from the rhizome of Curcuma longa [12,13] that has antioxidative, anti-inflammatory, cardiovascular, mental wellbeing and ageing protective effects and interacts with gut microbiota and several other actions [14,15,16]. Curcumin and turmeric extract have been shown to improve oxidative stress markers [17] by acting on cytokine/ROS-mediated inflammatory pathways to reduce the expression of NFkB/cyclooxygenase-2, enhancing antioxidant activity [18] and inhibiting the production of prostaglandin and NF-kB signaling [19]. In addition, curcumin may have musculo-protective effects against exercise-induced muscle damage (EIMD) by inhibiting free radical formation in injured skeletal muscles [18,20]. The aim of this short preliminary study was to investigate the effects of turmeric extract (rich in curcumin) intake on exercise-induced oxidative stress, blood pressure, PWV and lipid peroxidation in human volunteers.
2. Methods and Results
Study Design: We used a randomized placebo-controlled parallel study to investigate the efficacy of curcumin supplementation on exercise-induced oxidative stress and blood pressure over a period of 11 days (Figure 1). The study was granted ethical approval by the Ethics Committee of Queen Margaret University (QMU); code, 11010149-Honors/Curcumin/DNBS/QMU Ethical Committee.
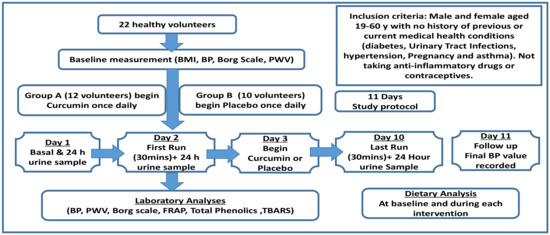
Figure 1.
Study protocol demonstrating the trial arrangements and measurements performed for all participants.
Supplement: Each participant in both groups was supplied with eight capsules, either turmeric concentrate (curcumin C3 complex (500 mg; Jarrow Formulas, Los Angeles, CA, USA) or placebo capsules made by filling empty gelatin capsules with corn flour.
Anthropometry and Physiological Measurements: Measurements of Body Mass Index (BMI) were facilitated by recording the weight and height of each subject on day 1 of the study, allowing us to calculate their BMI using the equation: [BMI = weight (kg)/height (m)2]. Arterial compliance measured by pulse wave velocity (PWV) was performed between the carotid and femoral artery (PWVcf) by means of a validated Vicorder™ device (Skidmore Medical Limited, Bristol, UK). Blood pressure (BP) was measured, and the average of three SBP and DBP readings were calculated. A Borg rating of perceived exertion scale (1–10) was used to measure each subject’s level of exertion and intensity during each of the 30-minute runs [21].
Urinary Biomarkers assays: Urinary Polyphenols Levels, Urinary FRAP excretion and MDA concentration were measured.
3. Discussion
This short study has highlighted some of the beneficial effects of curcumin supplements in healthy volunteers. The present study confirms the antioxidant and anti-inflammatory properties of curcumin as evidenced in the significant increase in antioxidant concentrations (FRAP) and total polyphenol levels in the urine samples of the curcumin group [16,22] (Table 1). Studies have confirmed that polyphenols possess antioxidant and free radical scavenging properties, which reduce low-density lipoprotein (LDL) oxidation [23]. We believe that the increase in antioxidant and polyphenol concentrations might have provided a protective mechanism against vascular dysfunction by neutralizing free radicals and reducing BP during exercise [24] (Table 2 and Table 3).

Table 1.
Total polyphenol concentration of 24-h urine samples measured in GAE/day. Data presented as the mean ± SD. Significance of data was measured against the basal concentration of each group (p value ≤ 0.05).

Table 2.
SBP and DBP readings recorded for all participants at basal, first run and last run before and after the exercise. It also shows the Δ change (mmHg) in SBP and DBP between pre-exercise and post exercise. Significance levels: * < 0.05; ** < 0.01; *** < 0.001; NS > 0.05.

Table 3.
Mean of the antioxidant concentrations of urine samples obtained from the FRAP assay. Data presented as the mean ± SD. Significant difference was measured against the basal concentration of each group (p ≤ 0.05).
This supports the data of published studies that showed curcumin intake was able to reduce BP [25]. Other CVD parameters that may be of relevance: Arterial stiffness compliance as measured by PWVcf was significantly reduced after curcumin intake, indicating its cardiovascular beneficial effects [26]. Turmeric extract intake attenuated the exercise-induced increase in lipid peroxidation (Table 4). In addition, the Borg score of perceived exertion was lowered, and thus the curcumin group felt they were able to run at a greater intensity during the last run compared to their first run [27] (Table 5). The limitations in this present study were that of short duration with a low number of participants, and the study implemented a parallel design due to the notion that both crossover and parallel designs offer advantages and disadvantages (Table 6). Finally, the influence of oral bioavailability of turmeric and curcumin should be considered in relation to gut microbiota of individuals [28].

Table 4.
TBARS as a determinant of lipid peroxidation was assessed by the estimation of MDA levels (μmole/day). Data are presented as the mean ± SD. Significant differences were measured against the basal concentration of each group.

Table 5.
Mean exercise and perceived exertion parameters of participants before and after the intervention. Data presented as the mean ± SD. Significant difference for the last run was measured against the first run (p ≤ 0.05).

Table 6.
Baseline demographics of all participants in the curcumin and placebo groups. Data presented as the mean ± SD.
4. Conclusions
Our study has demonstrated that curcumin possesses antioxidant and anti-inflammatory properties which have proven to lower blood pressure and improve CVD risk factors, exercise performance and ameliorate oxidative stress. Larger studies investigating the effects of turmeric extract or curcumin on oxidative stress, exercise performance and other cardiovascular parameters are warranted.
Author Contributions
Conceptualization and supervision, E.A.S.A.-D.; methodology and formal analysis, E.A.S.A.-D. and M.N.A.H.; writing and revision, M.N.A.H. and E.A.S.A.-D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Queen Margaret University (code Honors/11010149/Curcumin/BSc-NUT/DNBS/QMU).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Study data are available from the authors upon request.
Acknowledgments
The authors would like to thank Anum Mizher of the Queen Margaret University for her technical support and the University of Edinburgh for the estimation of steroid hormones. We are also grateful for all the volunteers who participated in this research study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Warburton, D.E.R.; Nicol, C.W.; Bredin, S.S.D. Health benefits of physical activity: The evidence. Can. Med. Assoc. J. 2006, 174, 801–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinckard, K.; Baskin, K.K.; Stanford, K.I. Effects of Exercise to Improve Cardiovascular Health. Front. Cardiovasc. Med. 2019, 6, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.; Min, K.; Talbert, E.E.; Kavazis, A.N.; Smuder, A.J.; Willis, W.T.; Powers, S.K. Exercise Protects Cardiac Mitochondria against Ischemia–Reperfusion Injury. Med. Sci. Sports Exerc. 2012, 44, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Morton, J.P.; Kayani, A.C.; McArdle, A.; Drust, B. The Exercise-Induced Stress Response of Skeletal Muscle, with Specific Emphasis on Humans. Sports Med. 2009, 39, 643–662. [Google Scholar] [CrossRef] [PubMed]
- Nikolaidis, M.G.; Kyparos, A.; Spanou, C.; Paschalis, V.; Theodorou, A.A.; Vrabas, I.S. Redox biology of exercise: An integrative and comparative consideration of some overlooked issues. J. Exp. Biol. 2012, 215, 1615–1625. [Google Scholar] [CrossRef] [Green Version]
- Davis, J.M.; Murphy, E.A.; Carmichael, M.D.; Zielinski, M.R.; Groschwitz, C.M.; Brown, A.S.; Gangemi, J.D.; Ghaffar, A.; Mayer, E.P. Curcumin effects on inflammation and performance recovery following eccentric exercise-induced muscle damage. Am. J. Physiol. Integr. Comp. Physiol. 2007, 292, R2168–R2173. [Google Scholar] [CrossRef] [Green Version]
- Retamoso, L.T.; Silveira, M.E.; Lima, F.D.; Busanello, G.L.; Bresciani, G.; Ribeiro, L.; Chagas, P.M.; Nogueira, C.W.; Braga, A.C.M.; Furian, A.F.; et al. Increased xanthine oxidase-related ROS production and TRPV1 synthesis preceding DOMS post-eccentric exercise in rats. Life Sci. 2016, 152, 52–59. [Google Scholar] [CrossRef]
- Khoubnasabjafari, M.; Ansarin, K.; Jouyban, A. Reliability of malondialdehyde as a biomarker of oxidative stress in psychological disorders. BioImpacts 2015, 5, 123–127. [Google Scholar]
- Kohara, K. Central blood pressure, arterial stiffness and the heart in hypertensive patients. Hypertens. Res. 2009, 32, 1056–1058. [Google Scholar] [CrossRef]
- Lee, R.; Margaritis, M.; Channon, K.; Antoniades, C. Evaluating Oxidative Stress in Human Cardiovascular Disease: Methodological Aspects and Considerations. Curr. Med. Chem. 2012, 19, 2504–2520. [Google Scholar] [CrossRef] [Green Version]
- D’Oria, R.; Schipani, R.; Leonardini, A.; Natalicchio, A.; Perrini, S.; Cignarelli, A.; Laviola, L.; Giorgino, F. The Role of Oxidative Stress in Cardiac Disease: From Physiological Response to Injury Factor. Oxidative Med. Cell. Longev. 2020, 2020, 5732956. [Google Scholar] [CrossRef] [PubMed]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its’ Effects on Human Health. Foods 2017, 6, 10–92. [Google Scholar] [CrossRef] [PubMed]
- Amro, B.I.; Hajleh, M.N.A.; Afifi, F. Evidence-Based Potential of some Edible, Medicinal and Aromatic Plants as Safe Cosmetics and Cosmeceuticals. Trop. J. Nat. Prod. Res. 2021, 5, 16–48. [Google Scholar] [CrossRef]
- Boarescu, P.M.; Boarescu, I.; Bocșan, I.C.; Gheban, D.; Bulboacă, A.E.; Nicula, C.; Pop, R.M.; Râjnoveanu, R.-M.; Bolboacă, S.D. Antioxidant and Anti-Inflammatory Effects of Curcumin Nanoparticles on Drug-Induced Acute Myocardial Infarction in Diabetic Rats. Antioxidants 2019, 8, 504. [Google Scholar] [CrossRef] [Green Version]
- Kuszewski, J.C.; Howe, P.R.C.; Wong, R.H.X. An Exploratory Analysis of Changes in Mental Wellbeing Following Curcumin and Fish Oil Supplementation in Middle-Aged and Older Adults. Nutrients 2020, 12, 2902. [Google Scholar] [CrossRef]
- Scazzocchio, B.; Minghetti, L.; D’Archivio, M. Interaction between Gut Microbiota and Curcumin: A New Key of Understanding for the Health Effects of Curcumin. Nutrients 2020, 12, 2499. [Google Scholar] [CrossRef] [PubMed]
- Menon, V.P.; Sudheer, A.R. Antioxidant and anti-inflammatory properties of curcumin. The molecular targets and therapeutic uses of curcumin in health and disease. Adv. Exp. Med. Biol. 2007, 595, 105–125. [Google Scholar]
- Fang, W.; Nasir, Y. The effect of curcumin supplementation on recovery following exercise-induced muscle damage and delayed-onset muscle soreness: A systematic review and meta-analysis of randomized controlled trials. Phytother. Res. 2021, 35, 1768–1781. [Google Scholar] [CrossRef]
- Moriyuki, K.; Sekiguchi, F.; Matsubara, K.; Nishikawa, H.; Kawabata, A. Curcumin inhibits the proteinase-activated receptor-2–triggered prostaglandin E2 production by suppressing cyclooxygenase-2 upregulation and Akt-dependent activation of nuclear factor-κB in human lung epithelial cells. J. Pharmacol. Sci. 2010, 114, 225–229. [Google Scholar] [CrossRef]
- Jäger, R.; Caldwell, A.R.; Sanders, E.; Mitchell, J.B.; Rogers, J.; Purpura, M.; Oliver, J.M. Curcumin reduces muscle damage and soreness following muscle-damaging exercise. FASEB J. 2017, 31, lb766. [Google Scholar] [CrossRef]
- Williams, N. The Borg Rating of Perceived Exertion (RPE) scale. Occup. Med. 2017, 67, 404–405. [Google Scholar] [CrossRef] [Green Version]
- Blanton, C.; Gordon, B. Effect of Morning vs. Evening Turmeric Consumption on Urine Oxidative Stress Biomarkers in Obese, Middle-Aged Adults: A Feasibility Study. Int. J. Environ. Res. Public Health 2020, 17, 4088. [Google Scholar] [CrossRef] [PubMed]
- Sahebkar, A.; Serban, M.-C.; Ursoniu, S.; Banach, M. Effect of curcuminoids on oxidative stress: A systematic review and meta-analysis of randomized controlled trials. J. Funct. Foods 2015, 18, 898–909. [Google Scholar] [CrossRef]
- Neves, M.R.C.A.T.D.P.M.F.; Cunha, M.R.; De Paula, T. Effects of Nutrients and Exercises to Attenuate Oxidative Stress and Prevent Cardiovascular Disease. Curr. Pharm. Des. 2019, 24, 4800–4806. [Google Scholar] [CrossRef]
- Qin, S.; Huang, L.; Gong, J.; Shen, S.; Huang, J.; Ren, H.; Hu, H. Efficacy and safety of turmeric and curcumin in lowering blood lipid levels in patients with cardiovascular risk factors: A meta-analysis of randomized controlled trials. Nutr. J. 2017, 16, 68. [Google Scholar] [CrossRef]
- Santos-Parker, J.R.; Strahler, T.R.; Bassett, C.J.; Bispham, N.Z.; Chonchol, M.B.; Seals, D.R. Curcumin supplementation improves vascular endothelial function in healthy middle-aged and older adults by increasing nitric oxide bioavailability and reducing oxidative stress. Aging 2017, 9, 187–208. [Google Scholar] [CrossRef] [Green Version]
- Tabrizi, R.; Vakili, S.; Akbari, M.; Mirhosseini, N.; Lankarani, K.B.; Rahimi, M.; Mobini, M.; Jafarnejad, S.; Vahedpoor, Z.; Asemi, Z. The effects of curcumin-containing supplements on biomarkers of inflammation and oxidative stress: A systematic review and meta-analysis of randomized controlled trials. Phytother. Res. 2019, 33, 253–262. [Google Scholar] [CrossRef]
- Stohs, S.J.; Chen, O.; Ray, S.D.; Ji, J.; Bucci, L.R.; Preuss, H.G. Highly Bioavailable Forms of Curcumin and Promising Avenues for Curcumin-Based Research and Application: A Review. Molecules 2020, 25, 1397. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).