Effect of Short-Term Vitamin D Supplementation on Blood Pressure, Arterial Health, and Stress Hormones in Healthy Volunteers †
Abstract
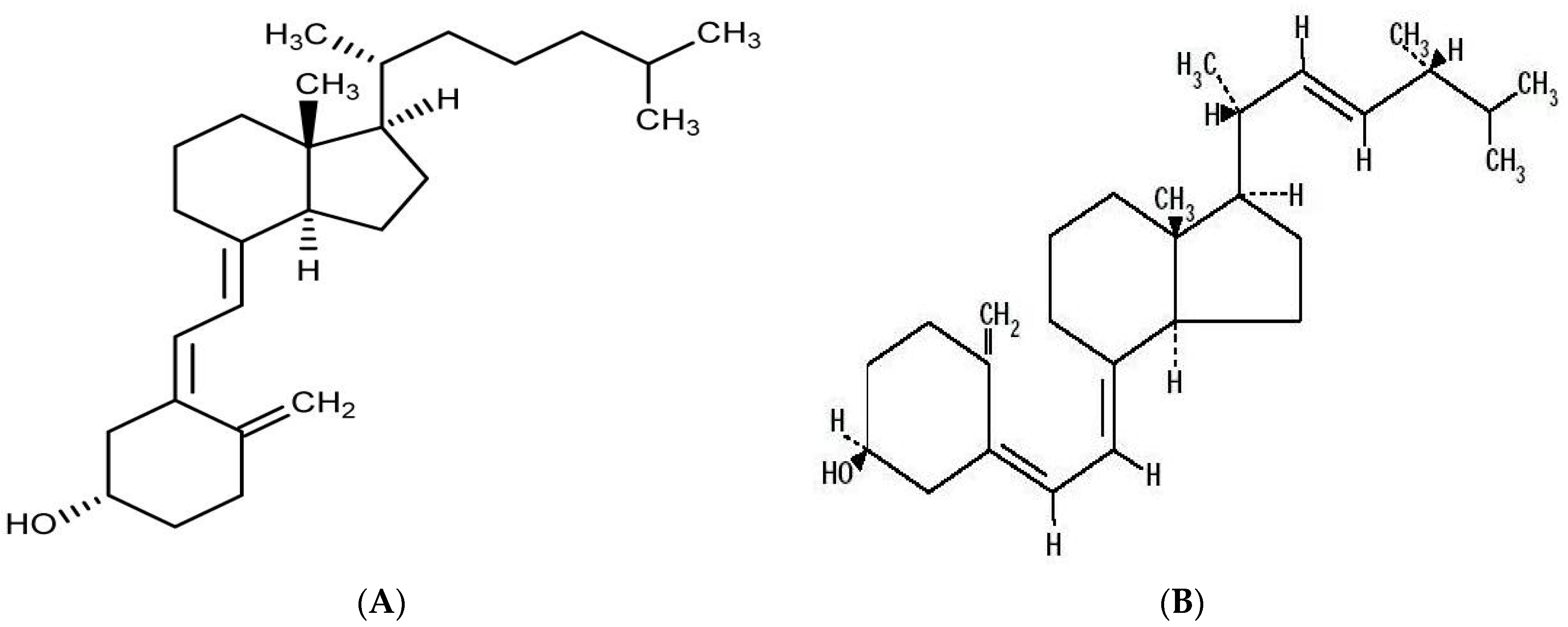
:1. Introduction
2. Methods and Results
2.1. Study Design
2.2. Data Collection
2.3. Data Analysis and Statistics
3. Results
3.1. Sample Characteristics and Diet Intake
3.2. Blood Pressure
3.3. BMI and Pulse Wave Velocity
3.4. Cortisol and Cortisol/Cortisone Ratio
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BP | Blood Pressure |
| BMI | Body Mass Index |
| CVD | Cardiovascular Disease |
| DBP | Diastolic Blood Pressure; |
| ELISA | Enzyme-Linked Immuno Sorbent Assay |
| 25-OH D | 25-hydroxyvitamin D |
| 1.25-(OH)2D | 1.25-dihydroxy-vitamin D |
| PTH | Parathyroid Hormone |
| NO | Nitric Oxide |
| PWV | Pulse Wave Velocity |
| SBP | Systolic Blood Pressure |
| SD | Standard Deviation |
References
- Davies, M.R.; Hruska, K.A. Pathophysiological mechanisms of vascular calcification in end-stage renal disease. Kidney Int. 2001, 60, 472–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pilz, S.; Dobnig, H.; Fischer, J.E.; Wellnitz, B.; Seelhorst, U.; Boehm, B.; März, W. Low vitamin D levels predict stroke in patients referred to coronary angiography. Stroke 2008, 39, 2611–2613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giovannucci, E.; Rimm, E.B.; Liu, Y.; Hollis, B.W. 25-hydroxyvitamin D and risk of myocardial infarction in men: A prospective study. Arch. Intern. Med. 2008, 168, 1174–1180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pilz, S.; Tomaschitz, A.; Ritz, E.; Drechsler, C.; Zittermann, A.; Dekker, J.M.; März, W. Vitamin D status and arterial hypertension: A systematic review. Nat. Rev. Cardiol. 2009, 6, 621–630. [Google Scholar] [CrossRef]
- Ginde, A.A.; Scragg, R.; Schwartz, R.S.; Camargo, C.A. Prospective study of serum 25-hydroxyvitamin D level, cardiovascular disease mortality, and all-cause mortality in older US adults. J. Am. Geriatr. Soc. 2009, 57, 1595–1603. [Google Scholar] [CrossRef]
- Holick, M.F. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin. Proc. 2006, 81, 353–373. [Google Scholar] [CrossRef] [Green Version]
- Al-Dujaili, E.A.; Munir, N.; Iniesta, R.R. Effect of vitamin D supplementation on cardiovascular disease risk factors and exercise performance in healthy participants: A randomized placebo-controlled preliminary study. Ther. Adv. Endocrinol. Metab. 2016, 7, 153–165. [Google Scholar] [CrossRef] [Green Version]
- Zittermann, A.; Gummert, J.F. Nonclassical vitamin D actions. Nutrients 2010, 2, 408–425. [Google Scholar] [CrossRef] [Green Version]
- Binkley, N.; Ramamurthy, R.; Krueger, D. Low vitamin D status: Definition, prevalence, consequences, and correction. Endocrinol. Metab. Clin. N. Am. 2010, 39, 287–301. [Google Scholar] [CrossRef] [Green Version]
- Dawson-Hughes, B.; Heaney, R.P.; Holick, M.F.; Lips, P.; Meunier, P.J.; Vieth, R. Estimates of optimal vitamin D status. Osteoporos. Int. 2005, 16, 713–716. [Google Scholar] [CrossRef]
- Alam, M.S.; Czajkowsky, D.M.; Islam, M.A.; Rahman, M.A. The role of vitamin D in reducing SARS-CoV-2 infection: An update. Int. Immunopharmacol. 2021, 97, 107686. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Boutouyrie, P.; Asmar, R.; Gautier, I.; Laloux, B.; Guize, L. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. HypGrtension 2001, 37, 1236–1241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, Y.; Stallmann-Jorgensen, I.S.; Pollock, N.K.; Harris, R.A.; Keeton, D.; Huang, Y.; Li, K.; Bassali, R.; Guo, D.; Thomas, J.; et al. A 16-w randomized clinical trial of 2000 international units daily vitamin D3 supplementation in black youth: 25-hydroxyvitamin D, adiposity, and arterial stiffness. J. Clin. Endocrinol. Metab. 2010, 95, 4584–4591. [Google Scholar] [CrossRef] [Green Version]
- Mancia, G.; De Backer, G.; Dominiczak, A.; Cifkova, R.; Fagard, R.; Germano, G.; Grassi, G.; Heagerty, A.M. Guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J. Hypertens. 2007, 25, 1105–1187. [Google Scholar] [CrossRef] [PubMed]
- Avolio, A. Arterial stiffness. Pulse 2013, 1, 14–28. [Google Scholar] [CrossRef] [PubMed]
- Blacher, J.; Asmar, R.; Djane, S.; London, G.M.; Safar, M.E. Aortic pulse wave velocity as a marker of cardiovascular risk in hypertensive patients. Hypertension 1999, 33, 1111–1117. [Google Scholar] [CrossRef] [Green Version]
- Sugden, J.; Davies, J.; Witham, M.; Morris, A.D.; Struthers, A.D. Vitamin D improves endothelial function in patients with Type 2 diabetes mellitus and low vitamin D levels. Diabet. Med. 2008, 25, 320–325. [Google Scholar] [CrossRef]
- Van den Berghe, G.; Van Roosbroeck, D.; Vanhove, P.; Wouters, P.J.; De Pourcq, L.; Bouillon, R. Bone turnover in prolonged critical illness: Effect of vitamin D. J. Clin. Endocrinol. Metab. 2003, 88, 4623–4632. [Google Scholar] [CrossRef]
- Cannata-Andía, J.B.; Rodríguez-García, M.; Carrillo-López, N.; Diaz-Lopez, B. Vascular calcifications: Pathogenesis, management, and impact on clinical outcomes. J. Am. Soc. Nephrol. 2006, 17 (Suppl. 3), S267–S273. [Google Scholar] [CrossRef] [Green Version]
- Scragg, R.; Sowers, M.; Bell, C. Serum 25-hydroxyvitamin D, ethnicity, and blood pressure in the Third National Health and Nutrition Examination Survey. Am. J. Hypertens. 2007, 20, 713–719. [Google Scholar] [CrossRef]
- Kota, S.K.; Kota, S.K.; Jammula, S.; Tripathy, P.R.; Panda, S.; Modi, K.D. Renin–angiotensin system activity in vitamin D deficient, obese individuals with hypertension: An urban Indian study. Indian J. Endocrinol. Metab. 2011, 15 (Suppl. 4), S395–S401. [Google Scholar] [CrossRef] [PubMed]
- Snijder, M.B.; van Dam, R.M.; Visser, M.; Seidill, J.C. Adiposity in relation to vitamin D status and parathyroid hormone levels: A population-based study in older men and women. J. Clin. Endocrinol. Metab. 2005, 90, 4119–4123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Querfeld, U.; Hoffmann, M.M.; Klaus, G.; Eifinger, F.; Ackerschott, M.; Michalk, D.; Kern, P.A. Antagonistic effects of vitamin D and parathyroid hormone on lipoprotein lipase in cultured adipocytes. J. Am. Soc. Nephrol. 1999, 10, 2158–2164. [Google Scholar] [CrossRef] [PubMed]
- Connell, J.M.; Davies, E. The new biology of aldosterone. J. Endocrinol. 2005, 186, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Hammer, F.; Stewart, P.M. Cortisol metabolism in hypertension. Best Pract. Res. Clin. Endocrinol. Metab. 2006, 20, 337–353. [Google Scholar] [CrossRef] [PubMed]
- Whitworth, J.A.; Williamson, P.M.; Mangos, G.; Kelly, J.J. Cardiovascular consequences of cortisol excess. Vasc. Health Risk Manag. 2005, 1, 291. [Google Scholar] [CrossRef] [Green Version]
- Stewart, J.; Manmathan, G.; Wilkinson, P. Primary prevention of cardiovascular disease: A review of contemporary guidance and literature. JRSM Cardiovasc. Dis. 2017, 6, 2048004016687211. [Google Scholar] [CrossRef] [Green Version]
- Mancia, G.; Fagard, R.; Narkiewicz, K.; Rendon, J.; Zanchetti, A.; Böhm, M.; Christiaens, T.; Cifkova, R.; De Backer, G.; Dominiczak, A.; et al. 2013 ESH/ESC practice guidelines for the management of arterial hypertension: ESH-ESC the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur. Heart J. 2013, 34, 2159–2219. [Google Scholar] [CrossRef] [Green Version]
- Walker, B.R.; Best, R.; Shackleton, C.H.; Padfield, P.L.; Edwards, C.R. Increased vasoconstrictor sensitivity to glucocorticoids in essential hypertension. Hypertension 1996, 27, 190–196. [Google Scholar] [CrossRef]
- Geer, E.B.; Islam, J.; Buettner, C. Mechanisms of glucocorticoid-induced insulin resistance: Focus on adipose tissue function and lipid metabolism. Endocrinol. Metab. Clin. N. Am. 2014, 43, 75–102. [Google Scholar] [CrossRef] [Green Version]
- Morton, N.M.; Seckl, J.R. 11β-hydroxysteroid dehydrogenase type 1 and obesity. Front. Horm. Res. 2008, 36, 146–164. [Google Scholar] [CrossRef] [PubMed]
- Jackson, L.V.; Thalange, N.K.; Cole, T.J. Blood pressure centiles for Great Britain. Arch. Dis. Child. 2007, 92, 298–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hickson, S.S.; Butlin, M.; Broad, J.; Avolio, A.P.; Wilkinson, I.B.; McEniery, C.M. Validity and repeatability of the Vicorder apparatus: A comparison with the SphygmoCor device. Hypertens. Res. 2009, 32, 1079–1085. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Cockcroft, J.; Van Bortel, L.; Boutouyrie, P.; Giannattasio, C.; Hayoz, D.; Pannier, B.; Vlachopoulos, C.; Wilkinson, I.; Struijker-Boudier, H. Expert consensus document on arterial stiffness: Methodological issues and clinical applications. Eur. Heart J. 2006, 27, 2588–2605. [Google Scholar] [CrossRef] [Green Version]
- Al-Dujaili, E.A. Development and validation of a simple and direct ELISA method for the determination of conjugated (glucuronide) and non-conjugated testosterone excretion in urine. Clin. Chim. Acta 2006, 364, 172–179. [Google Scholar] [CrossRef]
- Al-Dujaili, E.A.; Baghdadi, H.H.; Howie, F.; Mason, J.I. Validation and application of a highly specific and sensitive ELISA for the estimation of cortisone in saliva, urine and in vitro cell-culture media by using a novel antibody. Steroids 2012, 77, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Aardal, E.; Holm, A.-C. Cortisol in saliva-reference ranges and relation to cortisol in serum. Eur. J. Clin. Chem. Clin. Biochem. 1995, 33, 927–932. [Google Scholar] [CrossRef] [Green Version]
- Boutouyrie, P.; Vermeersch, S. Reference Values for Arterial Stiffness’ Collaboration. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘establishing normal and reference values’. Eur. Heart J. 2010, 31, 2338–2350. [Google Scholar] [CrossRef]
- Pfeifer, M.; Begerow, B.; Minne, H.W.; Nachtigall, D.; Hansen, C. Effects of a short-term vitamin D3 and calcium supplementation on blood pressure and parathyroid hormone levels in elderly women. J. Clin. Endocrinol. Metab. 2001, 86, 1633–1637. [Google Scholar] [CrossRef]
- Gallagher, J.; Riggs, B.L.; Eisman, J.; Hamstra, A.; Arnaud, S.B.; DeLuca, H.F. Intestinal calcium absorption and serum vitamin D metabolites in normal subjects and osteoporotic patients: Effect of age and dietary calcium. J. Clin. Investig. 1979, 64, 729–736. [Google Scholar] [CrossRef] [Green Version]
- Margolis, R.N.; Christakos, S. The nuclear receptor superfamily of steroid hormones and vitamin D gene regulation: An update. Ann. N. Y. Acad. Sci. 2010, 1192, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C. Vitamin D regulation of the renin–angiotensin system. J. Cell Biochem. 2003, 88, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Al-Dujaili, E.A.S.; Good, G.; Tsang, C. Consumption of Pomegranate Juice Attenuates Exercise-Induced Oxidative Stress, Blood Pressure and Urinary Cortisol/Cortisone Ratio in Human Adults. EC Nutr. 2016, 4, 982–995. [Google Scholar]
- Palermo, M.; Shackleton, C.H.; Mantero, F.; Stewart, P.M. Urinary free cortisone and the assessment of 11β-hydroxysteroid dehydrogenase activity in man. Clin. Endocrinol. 1996, 45, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Avenell, A.; Cook, J.A.; MacLennan, G.S.; MacPherson, G. Vitamin D supplementation and type 2 diabetes: A substudy of a randomised placebo-controlled trial in older people (RECORD trial, ISRCTN 51647438). Age Ageing 2009, 38, 606–609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Razzaque, M.S. The dualistic role of vitamin D in vascular calcifications. Kidney Int. 2011, 79, 708–714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richart, T.; Li, Y.; Staessen, J.A. Renal versus extrarenal activation of vitamin D in relation to atherosclerosis, arterial stiffening, and hypertension. Am. J. Hypertens. 2007, 20, 1007–1015. [Google Scholar] [CrossRef] [Green Version]
- Zittermann, A.; Frisch, S.; Berthold, H.K.; Gotting, C.; Kuhn, J.; Kleesiek, K.; Stehle, P.; Koertke, H.; Koerfer, R. Vitamin D supplementation enhances the beneficial effects of weight loss on cardiovascular disease risk markers. Am. J. Clin. Nutr. 2009, 89, 1321–1327. [Google Scholar] [CrossRef]
- Romero-Ortuno, R.; Cogan, L.; Browne, J.; Healy, M.; Casey, M.C.; Cunningham, C.; Walsh, J.B.; Kenny, R.A. Seasonal variation of serum vitamin D and the effect of vitamin D supplementation in Irish community-dwelling older people. Age Ageing 2011, 40, 168–174. [Google Scholar] [CrossRef]
- Al-Dujaili, E.; Giudice, V.; Fyfe, L. Effects of vitamin D supplementation on blood pressure, glucocorticoids and cardiovascular risk markers in healthy subjects. Endocr. Abstr. Biosci. 2013, 31, 184. [Google Scholar] [CrossRef]
- Quinkler, M.; Stewart, P.M. Hypertension and the cortisol-cortisone shuttle. J. Clin. Endocrinol. Metab. 2003, 88, 2384–2392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brock, K.; Huang, W.-Y.; Fraser, D.; Ke, L.; Tseng, M.; Stolzenberg-Solomon, R.; Peters, U.; Ahn, J.; Purdue, M.; Mason, R.S.; et al. Low vitamin D status is associated with physical inactivity, obesity and low vitamin D intake in a large US sample of healthy middle-aged men and women. J. Steroid Biochem. Mol. Biol. 2010, 121, 462–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aardal-Eriksson, E.; Karlberg, B.E.; Holm, A.-C. Salivary cortisol-an alternative to serum cortisol determinations in dynamic function tests. Clin. Chem. Lab. Med. 1998, 36, 215–222. [Google Scholar] [CrossRef] [PubMed]
| Week Number | Activities |
|---|---|
| Week 0: Supplement-free | 5-d wash-out period for all volunteersCompleting 2-d diet diary |
| 1 d before starting supplementation | Collection of three saliva samples and 24-h urine sample (Baseline data) |
| Week 1: Supplementation starts (Vitamin D3 or placebo) | First meeting (day 1); measure BP, PWV, weight, and height. |
| Week 2: Supplementation continues (Vitamin D3 or placebo). | Completing 2-d diet diary |
| 1 d before supplementation terminates | Collection of three saliva samples and 24-h urine sample (Intervention data) |
| Last day of supplementation | Second meeting to measure BP, PWV, weight, and height. Urine sample collected |
| Variable | Vit D (Mean ± SD) | Placebo (Mean ± SD) |
|---|---|---|
| Males/females | 8/12 | 4/6 |
| Age | 27.5 ± 9.6 | 26.9 ± 8.8 |
| Weight (kg) | 66.5 ± 13.1 | 67.1 ± 12.5 |
| BMI | 24.1 ± 3.3 | 24.5 ± 2.9 |
| SBP (mm Hg) | 121.7 ± 8.1 | 123.7 ± 7.8 |
| DBP (mm Hg) | 71.4 ± 5.48 | 71.6 ± 5.5 |
| PWV (m/s) | 6.5 ± 0.8 | 6.5 ± 0.7 |
| Baseline (Mean ± SD) | Intervention (Mean ± SD) | Difference | p Value | |
|---|---|---|---|---|
| Total energy intake (kcal/d) | 1417.1 ± 301.6 | 1352.6 ± 256.5 | 65.4 | 0.28 |
| Fat (g) | 52.3 ± 17.9 | 49.1 ± 18.0 | 3.2 | 0.66 |
| Protein (g) | 48.2 ± 14.8 | 45.3 ± 12.0 | 2.9 | 0.53 |
| Carbohydrates (g) | 189.1 ± 44.1 | 182.9 ± 25.1 | −6.2 | 0.07 |
| Vitamin D3 (μg/d) | 1.93 ± 1.11 | 21.99 ± 1.06 | 20.06 | <0.0001 |
| Vitamin D3 Arm | Baseline (Mean ± SD) | Intervention (Mean ± SD) | Difference (Mean ± SD) | p-Value |
|---|---|---|---|---|
| Systolic (mmHg) | 121.7 ± 8.1 | 116.4 ± 7.2 | 5.3 ± 6.46 | 0.032 |
| Diastolic (mmHg) | 71.4 ± 5.48 | 68.1 ± 6.17 | 3.3 ± 4.46 | 0.002 |
| PWV (m/s) | 6.51 ± 0.8 | 6.03 ± 0.6 | 0.48 ± 0.31 | 0.007 |
| BMI (Kg/m2) | 24.1 ± 3.3 | 23.81 ± 3.23 | 0.29 ± 0.12 | 0.161 |
| Placebo arm | ||||
| Systolic (mmHg) | 123.7 ± 7.8 | 122.9 ± 8.1 | 0.8 ± 0.76 | 0.432 |
| Diastolic (mmHg) | 71.6 ± 5.5 | 71.3 ± 6.1 | 0.3 ± 0.46 | 0.752 |
| PWV (m/s) | 6.5 ± 0.7 | 6.46 ± 0.9 | 0.04 ± 0.4 | 0.542 |
| BMI (Kg/m2) | 24.5 ± 2.9 | 24.35 ± 3.1 | 0.15 ± 0.3 | 0.338 |
| Pomegranate Group | Cortisol (ng/mL) | Sig. | Cortisone (ng/mL) | Sig. | |
|---|---|---|---|---|---|
| Mean ± SD | p Value | Mean ± SD | p Value | ||
| Morning | Day 0 | 6.17 ± 1.7 | 7.5 ± 2.8 | ||
| Day 14 | 6.71 ± 2.1 | 0.321 | 8.72 ± 2.9 | 0.05 | |
| Noon | Day 0 | 4.11 ± 1.5 | 4.13 ± 1.4 | ||
| Day 14 | 4.26 ± 1.7 | 0.568 | 6.26 ± 2.9 | 0.044 | |
| Evening | Day 0 | 2.89 ± 1.8 | 3.74 ± 1.3 | ||
| Day 14 | 3.32 ± 1.5 | 0.508 | 5.28 ± 2.8 | 0.048 | |
| Overall | Day 0 | 4.54 ± 1.5 | 5.33 ± 2.6 | ||
| Overall | Day 14 | 4.76 ± 1.6 | 0.556 | 6.98 ± 3.3 | 0.042 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abu Hajleh, M.N.; Al-Dujaili, E.A.S. Effect of Short-Term Vitamin D Supplementation on Blood Pressure, Arterial Health, and Stress Hormones in Healthy Volunteers. Biol. Life Sci. Forum 2022, 12, 15. https://doi.org/10.3390/IECN2022-12398
Abu Hajleh MN, Al-Dujaili EAS. Effect of Short-Term Vitamin D Supplementation on Blood Pressure, Arterial Health, and Stress Hormones in Healthy Volunteers. Biology and Life Sciences Forum. 2022; 12(1):15. https://doi.org/10.3390/IECN2022-12398
Chicago/Turabian StyleAbu Hajleh, Maha N., and Emad A. S. Al-Dujaili. 2022. "Effect of Short-Term Vitamin D Supplementation on Blood Pressure, Arterial Health, and Stress Hormones in Healthy Volunteers" Biology and Life Sciences Forum 12, no. 1: 15. https://doi.org/10.3390/IECN2022-12398
APA StyleAbu Hajleh, M. N., & Al-Dujaili, E. A. S. (2022). Effect of Short-Term Vitamin D Supplementation on Blood Pressure, Arterial Health, and Stress Hormones in Healthy Volunteers. Biology and Life Sciences Forum, 12(1), 15. https://doi.org/10.3390/IECN2022-12398







