Abstract
Fusarium wilt, caused by Fusarium oxysporum f. sp. cubense Tropical Race 4 (Foc TR4), is one of the most severe banana diseases in the world. In this study, banana plants treated with endophytic bacteria Kocuria rhizophila showed increased PO enzyme activity, reaching the highest activity 72 h after inoculation in the shoots (0.1640 ± 0.0335 μmol/min) and 24 h after inoculation in the roots (0.0129 ± 0.0024 μmol/min). PPO enzyme activity increased significantly 24 h after inoculation in roots (0.0131 ± 0.0026 μmol/min) and 6 h after inoculation in shoots (0.0201 ± 0.0065 μmol/min). PAL enzyme activity on roots (1.776 μmol/min) and shoots (1.2170 μmol/min) inoculated with endophytic bacteria showed the highest value at 24 h. The highest total phenolic content in shoots treated with endophytic bacteria was at 72 h in roots (41.15384 mg GAE/g samples) and shoots (39.6102 mg GAE/g samples).
1. Introduction
The banana plant (Musa spp.) is a plant that can live in tropical and subtropical climates. This plant originates in Indomalayan realm and has many benefits, so the demand for bananas is very high. Based on the latest data from FAO 2019, banana exports were recorded at 20.2 million tons worldwide [1]. Unfortunately, banana production was declining due to fusarium wilt disease caused by Fusarium oxysporum f. sp. cubense Tropical Race 4 (Foc TR4). The use of endophytic bacteria as an alternative for controlling fusarium wilt has not been widely studied and could be an environmentally friendly alternative for controlling fusarium wilt. The endophytic bacteria Kocuria rhizophila, which has been isolated and investigated in previous research (unpublished data), was further analyzed at the biochemical level for its ability to induce systemic resistance.
2. Materials and Method
2.1. In Vitro Triple Culture
In vitro triple culture of banana, endophyte, and fusarium was performed based on the previous method [2]. Banana plantlets were obtained from a local commercial plant tissue culture laboratory. Banana plantlets that were at least seven days old after subculture were removed from the tube to be inoculated with an endophytic bacteria culture, using an insectarium needle, inserted perpendicularly to the pseudostem axis at a position about 0.5 cm above the root. Plantlets were subcultured to fresh MS0 medium and co-cultivated for seven days. On the seventh day after co-cultivation (7 hpi), the banana plantlets were injured again with insectarium needles before being infected with Foc TR4. A piece of Potato Dextrose Agar (PDA), covered with 5–7 days culture of Foc TR4 mycelium, was placed near the point of injury. As a control for the effect of endophytic bacteria on banana plantlets, banana plantlets that were not pre-inoculated with endophytic bacterial isolates and not challenged with Foc TR4 were used.
2.2. Endophyte Bacteria Inoculation and Sampling Time Points
Samples used for biochemical assay were banana plantlets inoculated with endophyte bacteria and Foc TR4 (in vitro triple culture assay) with specific time points and harvested at 0, 6, 24, and 72 h of endophyte post-inoculation (0 hpi, 6 hpi, 24 hpi, 72 hpi, respectively). Controls used banana plantlets infected with Foc TR4 (in vitro triple culture assay) with specific time points and harvested at 0, 6, 24, and 72 h with composite triplicates sampling from three banana plantlets for roots and three banana plantlets for shoots, for PO and PPO assay.
2.3. Crude Enzyme Extraction
Crude enzyme extraction was carried out using the previously described method [3]. At this stage, crude enzyme extraction will be carried out for biochemical assay, including the peroxidase (PO), polyphenol oxidase (PPO), and phenylalanine ammonia-lyase (PAL) enzyme, and analysis of total phenolic content.
2.4. Peroxidase (PO) Enzyme Activity Assay
PO enzyme activity assay was carried out using the previously described method [3]. A total of 750 μL of 0.1 M phosphate buffer solution (pH 6.5), 100 μL of 0.05 M pyrogallol solution in buffer, and 240 μL of 0.08% hydrogen peroxide solution were prepared and then resuspended until homogeneous. Then, the mixture was added with 20 μL of the enzyme extract sample and the absorbance was measured at a wavelength of 430 nm with a microplate reader Infinite 200 PRO Nanoquant Microplate Reader (Tecan) (Switzerland) for 3 min by recording the absorbance every 30 s (0, 30, 60, 90, 120, 150, and 180 s) and averaged at the end. The activity was expressed as μmol/min.
2.5. Polyphenol Oxidase (PPO) Enzyme Activity Assay
PPO enzyme activity assay was carried out using the previously described method [4]. A total of 200 μL of the enzyme extract sample was added with 1500 μL of phosphate buffer solution pH 6.5, then homogenized. The mixture was then incubated for 1 min at 25 °C. The absorbance was measured at a wavelength of 495 nm with a microplate reader Infinite 200 PRO Nanoquant Microplate Reader (Tecan) (Switzerland) for 3 min by recording the absorbance every 30 s (0, 30, 60, 90, 120, 150, and 180 s) and averaged at the end. The activity was expressed as μmol/min.
2.6. Phenylalanine Ammonia Lyase (PAL) Enzyme Activity Assay
PAL enzyme activity assay was carried out using the previously described method [5]. A mixture of sample solutions, blanks, and standard solutions was prepared. After that, the solution mixture was heated using a water bath at 37 °C for 60 min. Then, 0.4 mL of 5 M HCl was added to stop the enzymatic reaction of the solution. The absorbance was measured at a wavelength of 290 nm using the microplate reader Infinite 200 PRO Nanoquant Microplate Reader (Tecan) (Switzerland). The standard curve equation was determined using the absorbance value of the standard solution. The activity was expressed as μmol/min.
2.7. Measurement of Total Phenolic Content in Plant Tissue
The total phenolic content in the tissue was measured by the previously described method [6]. The 0.5 g samples were ground using liquid nitrogen. The obtained powder was mixed with 5 mL of 80% methanol (MeOH) and incubated for 2 h in a dark room at room temperature. Then, the extracted solution was centrifuged at 10,000 rpm for 10 min to form a supernatant which would then be used as a sample for the content of plant phenolic compounds. A sample of 200 μL of phenolic compounds was taken and put into a test tube, which was then added with 200 μL of Folin-Ciocalteu reagent and homogenized. The solution was incubated for 10 min at room temperature and then added with 2 mL of 20% Na2CO3 solution. The solution mixture was homogenized and incubated again for 90 min in the dark at room temperature. The absorbance was measured by UV Vis Shimadzu UV-1280 spectrophotometry at a wavelength of 750 nm. For the standard, gallic acid solutions with concentrations of 0, 20, 40, 60, 80, and 100 g/mL were used. The blank used was distilled water with the same treatment as the sample. The total phenolic content in the tissue was determined based on the calculation of the standard gallic acid curve, which was further described as micrograms of gallic acid equivalent per gram (mg GAE/g) of the wet weight of the sample based on the Equation (1):
2.8. Statistical Analysis
Statistical analysis was performed using IBM SPSS Statistics version 25. Statistical tests were considered significant if p < 0.05. All data were tested for normal distribution according to the Kolmogorov–Smirnov test and for homogeneity of variance among groups using Levene’s test. After passing the normality test, the analysis of variance between groups was conducted using one-way ANOVA with Duncan posthoc test. When data failed the normality test and Levene’s test, they were analyzed by the non-parametric Kruskal–Wallis test, followed by Kruskal–Wallis stepwise step-down multiple comparison posthoc test.
3. Results
Biochemical assay of different enzyme and phenolic content showed different results among root and shoot samples. Detailed results of each assay are described below.
3.1. PO Enzyme Activity Assay
It was observed that PO activity increased and reached its highest activity 72 h after inoculation in shoots (0.1640 ± 0.0335 μmol/min) and 24 h in the root (0.0129 ± 0.0024 μmol/min) (Figure 1). PO enzyme activity showed a significant difference between plants treated with endophyte treatment and control, across organ and sampling time points (P: 0.00 Kruskal–Wallis test, followed by a stepwise step down multiple comparison posthoc test). PO enzyme activity in roots treated with endophytic bacteria showed a decrease in PO enzyme activity at 6 h, then an increase at 24 h and a decrease at 72 h. The increase in PO enzyme activity at 24 h was thought to be due to the plant starting to produce systemic resistance. PO enzyme activity in shoots treated with endophytic bacteria showed a significant increase in enzyme activity. The highest PO enzyme activity was obtained at 72 h; this could have happened because the systemic resistance occurs in the roots and then spreads to the shoots [7], but, overall, the PPO enzyme activity was higher in shoots than in roots. This is not in accordance with the literature, which states that the activity of the PPO enzyme should fluctuate in the roots of banana plants, while the activity of the peroxidase enzyme tends to decrease in the leaves. This difference might have happened because the root tissue is a point of entrance and colonization of microorganisms, meaning that the focus of the physiological response of banana plants is prioritized in the roots [8].
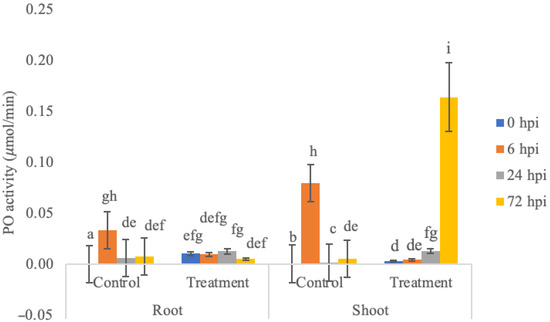
Figure 1.
PO enzyme activity at 0, 6, 24, and 72 hpi in control and treatment of roots and shoots of banana plants. Bars represent ± SE of the mean. Different letters indicate significant differences among treatments within a time point (p < 0.05).
3.2. PPO Enzyme Activity Assay
PPO activity in banana plants treated with K. rhizophila was significantly increased when challenged with the pathogen and reached 24 hpi in roots (0.0131 ± 0.0026 μmol/min) and 6 hpi in shoots (0.0201 ± 0.0065 μmol/min) (Figure 2). PPO enzyme activity showed a significant difference between treatment and control across organ and sampling time points (P: 0.00 Kruskal-Wallis test, followed by a stepwise step down multiple comparison posthoc test). PPO enzyme activity in roots and shoots treated with endophytic bacteria and control had relatively the same trend, but overall, PPO enzyme activity was higher in shoots than in roots. This is not in accordance with the literature, which states that the activity of the PPO enzyme should fluctuate in the roots of banana plants, while the activity of the peroxidase enzyme tends to decrease in the leaves.
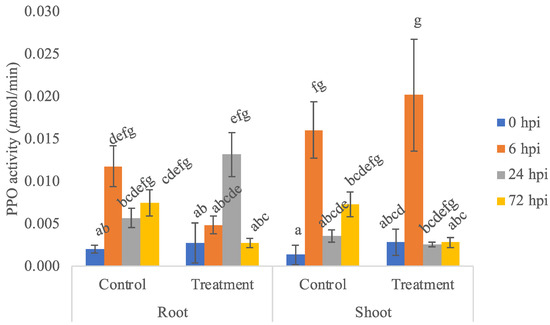
Figure 2.
PPO enzyme activity at 0, 6, 24, and 72 hpi in control and treatment of roots and shoots of banana plants. Bars represent ± SE of the mean. Different letters indicate significant differences among treatments within a time point (p < 0.05).
3.3. PAL Enzyme Activity Assay
PAL enzyme activity was higher in roots treated with endophytic bacteria. In general, roots and shoots treated with endophytic bacteria at 0, 6, 24, and 72 h were generally slightly higher than the control treatment (Figure 3), which showed the systemic induction of plant-defense enzymes. PAL enzyme activity on roots (1.776 μmol/min) and shoots (1.2170 μmol/min) inoculated with endophytic bacteria showed the highest value at 24 h. The activity of PAL enzymes in roots and shoots treated with endophytic bacteria and control had relatively the same trend. PAL activity in roots and shoots treated with endophytic bacteria at 6 h decreased enzyme activity, increased at 24 h, and decreased at 72 h. This is presumably due to the fact that banana plants treated with endophytic bacteria had synthesized PAL enzymes since 0 hpi, so they were able to induce other defense systems [9].
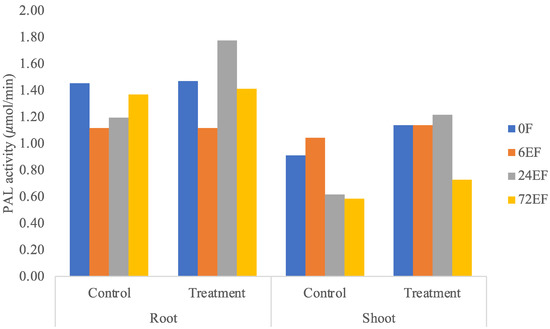
Figure 3.
PAL enzyme activity in the control treatment and samples of roots and shoots of banana plants.
3.4. Total Phenolic Content
Roots and shoots treated with endophytic bacteria showed the total phenolic content tended to be stable in both roots and shoots, which could be seen from a similar pattern (Figure 4). The total phenolic content in roots and shoots treated with endophytic bacteria decreased at 24 h and increased at 72 h. Total phenolic content in roots (41.15384 mg GAE/g samples) and shoots treated with endophytic bacteria at 72 h (39.6102 mg GAE/g samples) was greatly increased, which indicated that the plant was under stress, resulting in necrosis [10].
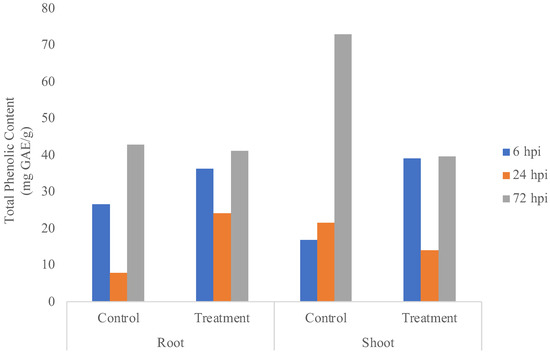
Figure 4.
Total phenolic content in the control treatment and samples of roots and shoots of banana plants.
4. Discussion
In general, roots and shoots treated with endophytic bacteria showed PO and PPO enzyme activities higher than the control treatment. This happened because the systemic resistance occurs in the roots and then spreads to the shoots. Therefore, the presence of high peroxidase enzyme activity in all treatment groups indicated that the plant response system in recognizing the presence of pathogens was running well; the induction of peroxidase activity indicated that there was stimulation of plant defenses to prevent the entry of pathogens [7].
PO is a component of an early response in plants to pathogen infection and plays a key role in the biosynthesis of lignin, which limits the extent of pathogen spread [7]. When the PO level increases due to the induced systemic resistance, a quick synthesis of reactive oxygen derivatives by oxidative burst leads to cell death and inhibits pathogenic activities that were observed [11]. Obtained results were supported by earlier studies that demarcated the induction of PO in plants infected by pathogens, resulting in faster and stronger resistance against them [12]. They experimentally supported the idea that peroxidase plays a defensive role against attacking pathogens [13].
In plant–pathogen interactions, PPO enzymes play a role in the oxidation process of phenol compounds into quinine compounds, which are quinone toxic compounds and will spread to injured plant tissues. In addition, these compounds will also cause environmental conditions that are not suitable for the development of pathogens, so the activity of this enzyme becomes important in plant resistance [14]. PO and PPO can catalyze the formation of lignin and other oxidative phenols and contribute to the formation of defense barriers by changing the cell structure defense system that gets actuated against pathogens [15]. In the present experiment, PPO activity was significantly enhanced by K. rhizophila treated banana plants. Moreover, PPO activity helps in disease resistance as it oxidizes the phenolic level increase during this stage to toxic molecules such as quinones, leading to the invasion of the pathogen [14].
PAL activity is an extremely sensitive indicator of stress conditions and fungal challenges, and elicitor treatment elevates the levels of the flux through the general phenylpropanoid pathway, thereby supplying the carbon skeletons for secondary products such as phenolics, which are the precursor molecules for lignin. Increased PAL enzyme activity can be induced in various ways, including elicitor, plant-pathogen interactions, and plant-biocontrol agent interactions. The observed increase in PAL activity in elicited roots is presumably related to the lignification process [9]. Figure 3 showed that the suspected banana plants treated with endophytic bacteria had synthesized PAL enzymes since 0 hpi, so they were able to induce other defense systems than the control.
PAL enzyme activity (Figure 3) and total phenolic content (Figure 4) showed that, between control and treatment, most of the endophytic effects were less visible because it was shown from previous research that K. rhizophila maintains primary metabolism instead of secondary metabolism under Foc TR4 infection [2]. K. rhizophila inoculation on soybeans also increased plant biomass [16]. Meanwhile, K. rhizophila inoculation on maize enhanced salt stress tolerances by regulating phytohormone levels, nutrient acquisition, redox potential, ion homeostasis, photosynthetic capacity, and stress-responsive gene expression [17].
Author Contributions
Conceptualization and methodology, L.U.K.; validation, L.U.K. and V.D.P.; formal analysis and investigation, R.T.C.; writing—original draft preparation, R.T.C.; writing—review and editing, L.U.K. and V.D.P.; supervision, L.U.K. and V.D.P.; project administration, R.T.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Atma Jaya Research and Community Service Center 2020, proposal number 1634.
Acknowledgments
We would like to thank Indonesian Tropical Fruit Research Institute for providing Foc TR4 culture.
Conflicts of Interest
The authors declare no conflict of interest.
References
- FAOSTAT Top 10 Country Production of Bananas. Available online: http://www.fao.org/faostat/en/#rankings/countries_by_commodity (accessed on 23 August 2018).
- Karmawan, L.U.; Dwivany, F.M.; Esyanti, R.R.; Aryantha, I.N.P. Improved in vitro bioassay for Musa acuminata cv. Pisang ambon kuning (AAA group) based on quantitative analysis of necrosis area and biomass changes during Foc4 infection. Arch. Phytopathol. Plant Prot. 2018, 51, 408–422. [Google Scholar] [CrossRef]
- Subramaniam, S.; Maziah, M.; Sariah, M.; Puad, M.P.; Xavier, R. Bioassay method for testing Fusarium wilt disease tolerance in transgenic banana. Sci. Hortic. 2006, 108, 378–389. [Google Scholar] [CrossRef]
- Siguemoto, É.S.; Gut, J.A.W. Validation of spectrophotometric microplate methods for polyphenol oxidase and peroxidase activities analysis in fruits and vegetables. Food Sci. Technol. 2017, 37, 148–153. [Google Scholar] [CrossRef] [Green Version]
- Dickerson, D.P.; Pascholati, S.F.; Hagerman, A.E.; Butler, L.G.; Nicholson, R.L. Phenylalanine ammonia-lyase and hydroxycinnamate: CoA ligase in maize mesocotyls inoculated with Helminthosporium maydis or Helminthosporium carbonum. Physiol. Plant Pathol. 1984, 25, 111–123. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Vidhyasekaran, P. Fungal Pathogenesis in Plants and Crops; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar] [CrossRef]
- Suswati, S.; Habazar, T.; Husin, E.F.; Nasir, N.; Putra, D.P.; Taylor, P. Senyawa Phenolik Akar Pisang CV. Kepok (Musa acuminata) yang Diinduksi dengan Fungi Mikoriza Arbuskular Indigenus PU10-Glomus sp 1 terhadap Penyakit Darah Bakteri. J. Nat. Indones. 2012, 13, 207–213. [Google Scholar] [CrossRef]
- De Ascensao, A.R.D.C.F.; Dubery, I.A. Panama disease: Cell wall reinforcement in banana roots in response to elicitors from Fusarium oxysporum f. sp. cubense Race four. Phytopathology 2000, 90, 1173–1180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Wang, Y.; Li, K.; Song, X.; Chen, J. Characterization and comparative expression profiling of browning response in Medinilla formosana after cutting. Front. Plant Sci. 2016, 7, 1897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasannath, K.; Dharmadasa, K.N.P.; De Costa, D.M.; Hemachandra, K.S. Variations of Incidence, Types of Virus Diseases and Insect Vector Populations of Tomato (Solanum lycopersicum L.), Grown in Different Agroecological Regions of Sri Lanka under Two Crop Management Systems. Trop. Agric. Res. 2015, 25, 376–395. [Google Scholar] [CrossRef] [Green Version]
- Surekha, C.; Neelapu, N.; Prasad, B.S.; Ganesh, P.S. Induction of Defense Enzymes and Phenolic Content by Trichoderma viride in Vigna mungo Infested with Fusarium oxysporum and Alternaria alternata. Int. J. Agric. Sci. Res. 2014, 4, 31–40. [Google Scholar]
- Caruso, C.; Chilosi, G.; Leonardi, L.; Bertini, L.; Magro, P.; Buonocore, V.; Caporale, C. A basic peroxidase from wheat kernel with antifungal activity. Phytochemistry 2001, 58, 743–750. [Google Scholar] [CrossRef]
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.L.; Marra, R.; Woo, S.L.; Lorito, M. Trichoderma-plant-pathogen interactions. Soil Biol. Biochem. 2008, 40, 1–10. [Google Scholar] [CrossRef]
- Li, L.; Steffens, J.C. Overexpression of polyphenol oxidase in transgenic tomato plants results in enhanced bacterial disease resistance. Planta 2002, 215, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Amna; Kamran, M.A.; Javed, M.T.; Hayat, K.; Farooq, M.A.; Ali, N.; Ali, M.; Manghwar, H.; Jan, F.; et al. Individual and combinatorial application of Kocuria rhizophila and citric acid on phytoextraction of multi-metal contaminated soils by Glycine max L. Environ. Exp. Bot. 2019, 159, 23–33. [Google Scholar] [CrossRef]
- Li, X.; Sun, P.; Zhang, Y.; Jin, C.; Guan, C. A novel PGPR strain Kocuria rhizophila Y1 enhances salt stress tolerance in maize by regulating phytohormone levels, nutrient acquisition, redox potential, ion homeostasis, photosynthetic capacity and stress-responsive genes expression. Environ. Exp. Bot. 2020, 174, 104023. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).