1. Introduction
Microalgae and protists are currently used for the production of new pharmaceuticals, cosmetics, nutraceuticals, functional foods, and in the energy industry. This motivates the search for new, suitable, high-yielding strains or genetically modified microorganisms [
1,
2].
The main problem of maintaining stable and efficient operation of biotechnological industries is minimizing the risk of contamination, maintaining the functional activity, and the phenotypic and genotypic stability of collection crops. The traditional methods of long-term storage of eukaryotic microalgae and protist, unfortunately, cannot fully meet the above requirements [
3,
4].
Cryopreservation is currently considered the best method for storing microorganisms. However, the existing methods of freezing–warming do not always ensure the preservation of the viability of different types of microalgae and protista [
5,
6,
7].
Cryopreservation protocols usually include the use of at least one cryoprotective substance; therefore, special attention should be paid to the stage of pre-preparation of any type of cells for freezing–thawing.
At the stage of adding cryoprotective substances, there is a violation in the osmolarity of the extracellular environment, a change in the volume of cells, and mechanical deformation of the membranes and cytoskeleton. This can cause of sublethal damage of cells.
The next critical moment for cells in the process of cryopreservation is the formation of extracellular ice. This creates hypertonic conditions for cells in the unfrozen fraction and leads to further dehydration of cells. During thawing, the reverse process takes place, where cells are subjected to hypotonic conditions.
Based on the above, the study of the osmotic parameters of the cell (permeability of the cell wall and membrane, surface-volumetric characteristics of cells) is an important stage in successful conservation.
The aim of this work is to study the osmotic reactions of cells of different taxonomic groups (euglenoid Astasia longa, microalga Dunaliella salina) in response to complementing with of promising cryoprotectants (Me2SO, glycerol, ethanol) under normothermic conditions.
2. Materials and Methods
The objects of the study were unicellular motile microorganisms—colorless euglenoid Astasia longa (Euglena longa) and halotolerant microalgae Dunaliella salina.
A. longa culture was obtained from the collection of the Department of Cryomicrobiology of the Institute for Problems of Cryobiology and Cryomedicine of the National Academy of Sciences of Ukraine. A. longa flagellates were grown without illumination on a modified synthetic Cramer–Myers medium containing ethanol—10 mL, as well as salts (g/L): NH4CL-1.0; K2HP04-1.0; KH2P04-1.0; MgSO4-0.8; NaCOOH-0.8; H2O-1000.0; microelements (mg/L): ZnSO4-20; CoCl2-75; NaMoO4-10; CuSO4-1; vitamins (mg/L): B1-0.02; B12-0.03; distilled water; initial value pH—6.8–7.2. The cultivation temperature was 25 °C.
The culture of
D. salina was obtained from the collection of microalgae cultures of the Department of Botany of V. N. Karazin Kharkiv National University. Microalgae were cultivated on Ramaraj nutrient medium [
8]. The accumulation of biomass was carried out in special vials with a volume of 40 mL (TPP, Switzerland) at a temperature of 25 ± 2 °C under round-the-clock illumination with white fluorescent light of 52.84 μmol photons m
−2 s
−1 (3 kLux).
Both cultures were grown under passive aeration until the onset of the stationary growth phase.
A number of cryoprotectants (CP) were used to study the osmotic reactions of A. longa and D. salina cells: glycerol, ethanol, dimethyl sulfoxide (Me2SO), and sucrose with final concentrations in the samples of 5%, 10%, and 20%. All CP solutions were prepared on Cramer–Myers and Ramaraj nutrient media, respectively. Then, aliquots of CP of a certain concentration were slowly added to cell suspensions of A. longa or D. salina cultures in test tubes (v/v) to the final test concentrations. The samples were exposed at room temperature for 1, 10, 20, and 30 min. Cell recovery after exposure to CP solutions was carried out in a “mirror” manner, namely, by gradually adding the appropriate culture medium to maximize the decrease in the CP concentration in the samples. The dynamics of the effect of CP and rehydration of cells in both cultures was recorded continuously (photographing).
Morphometric parameters of cells (integrity, size, relative area, shape and mobility of cells, presence or loss of flagella) were assessed using a microscope «LSM510-META» (Carl Zeiss, Germany) and the use of computer programs AimImageBrowser, AxioVision Rel.4.8. (Carl Zeiss MicroImaging).
Statistical processing was performed using PAST 3 and Mann–Whitney U test.
3. Results and Discussion
The reactions of various types of microorganisms to the action of cryoprotective substances or their mixtures are unpredictable [
9]. An example of this are toxic substances such as Me
2SO and methanol, which are most often used as the main cryoprotectants in the freezing of many flagellates [
10]; however, they were not very effective in cryopreservation of other microalgae and protists. Nevertheless, all cryoprotective compounds in contact with the intracellular space for a certain period can cause osmotic stress of cells even at the stage of adding them in the positive temperature range, which will lead to irreversible damage to cells.
Previously, we found out that 1–5% concentrations of carbohydrates are promising for use in cryoprotective mixtures for A. longa cells. When Me2SO solutions were added to the cell suspension at concentrations of 1%, 5%, 10%, and short-term exposure, the structural state of the cells changed. A change in the shape of cells was observed; a rounded or ellipsoidal shape dominated; flagella and mobility were lost; and the relative area of cells decreased by 10–27%, which was only partially restored. An increase in exposure time or concentration in samples with Me2SO leads to deformation of the cell shape and almost complete loss of flagella.
In this work, we carried out a further study of the effect of various cryoprotectants on the morphological state and osmotic reactions of A. longa cells. We used ethanol as an alternative to toxic methanol.
It was found that solutions of 1% ethanol and 1–5% glycerol did not affect the morphometric parameters of
A. longa cells up to 30 min. Increasing the concentration of ethanol up to 5–10% in samples with culture of
A. longa and exposure for more than 10 min led to a decrease in the relative area of cells (
Table 1). These changes quickly recovered to control values after the slow addition of Cramer–Myers medium. Further stay of flagellate cells in these solutions for up to 30 min did not significantly change their size (relative areas); however, the cells massively lost flagella and mobility, which indicates the damaging effect of these solutions upon prolonged exposure.
A. longa cells are osmotically resistant to glycerol solutions at concentrations of 1–5%, but an increase in concentration to 10% is already 10 min accompanied by the loss of flagella. Further exposure to this concentration of glycerol was accompanied by an increase in the proportion of cells that lost flagella and reduced the relative area of cells to 70.8 ± 9.61%.
Rehydration of euglenoid cells after short-term exposure to 10% solutions of both substances occurred instantly (2–3 s).
Changes in the morphological state of the cells of the A. longa population were also observed upon the addition of sucrose solutions, but the relative cell area did not change significantly during equilibration for 5 min in a 5% sucrose solution. In the case of a 10% solution, the cells decrease their relative area to 26%. An increase in the exposure period in these solutions significantly reduced the area of cells and their viability. Upon rehydration, the cells completely restored their original sizes.
Thus, the use of solutions of 1–5% alcohols and glycerol in cryoprotective mixtures can be promising in the development of effective methods for cryopreservation of A. longa.
Exposure of D. salina cells with different concentrations of Me2SO did not significantly affect the relative area, and the change in cell shape occurred in the interval of 20–30 min in all studied solutions. Starting with 5% Me2SO concentration at 20 min of exposure, the cells changed the nature of motility. However, even 30 min of exposure with a 10% concentration of Me2SO did not lead to irreversible plasmolysis and other visible abnormalities in the cells.
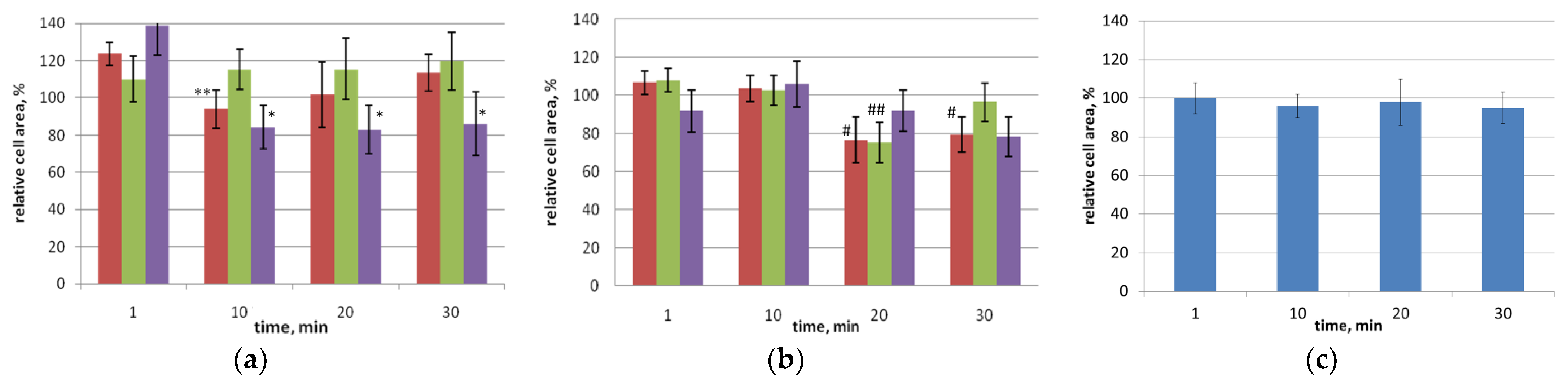
The addition of various concentrations of glycerol in the range of 5% to 30% to the suspension of
D. salina cells did not disrupt the integrity of the cells and did not affect their area (
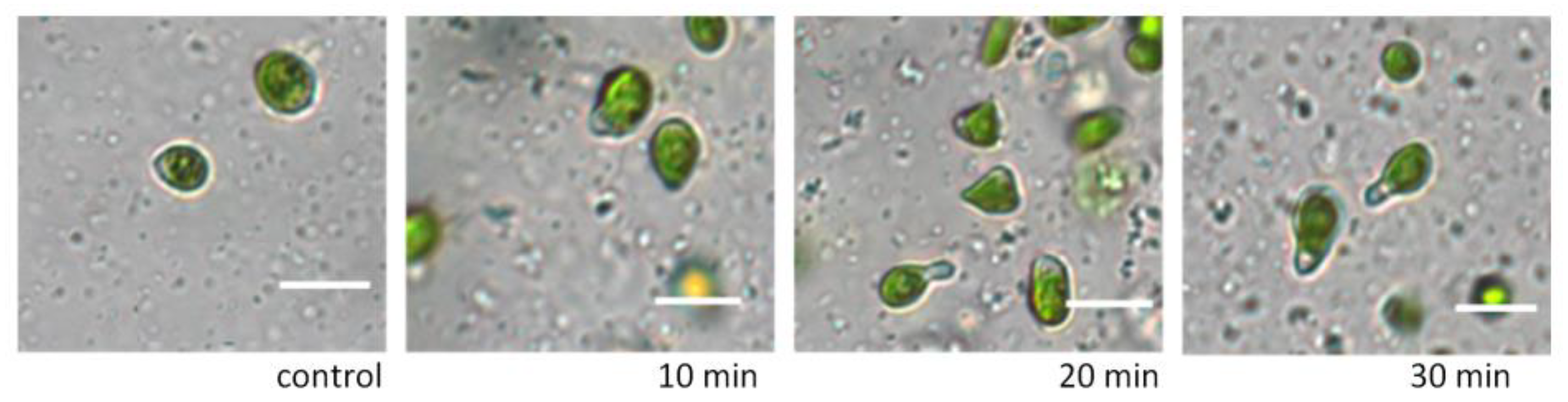
Figure 1) and motility. By 30 min of exposure, glycerol concentrations above 3% resulted in changes in cell shape only (
Figure 2). When appropriate nutrient media were added to the microalgae cells after exposure to glycerol solutions, the cells acquired their original shape.
Addition to the suspension of D. salina cells of solutions of ethanol of various concentrations (1–10%) did not lead to cell death even up to 30 min of exposure. There was a loss of activity, but the cells did not lose plaits. By 5 min of exposure, the shape of the cells changed; they became rounded; and by 30 min, they were stretched. Almost all concentrations of ethanol by 30 min reduced the relative cell area by 3–30%. Rehydration helped to restore the relative area and shape.
4. Conclusions
When choosing a cryoprotective substance, it is necessary to take into account that each taxon is characterized by individual resistance and permeability level. Typically, algal cells with rigid cell walls have a limited ability to change the volume and shape of the cells and are, thus, highly dependent on solutes for osmoregulation. In addition, many microalgae form palmeloids in response to stress. However,
A. longa euglenoid cells lack a cell wall, and
D. salina also lacks a rigid cell wall and lacks the ability to form palmeloids [
11]. Therefore, it was assumed that the cells of
A. longa and
D. salina would rapidly change size upon addition of cryoprotective substances, regulating the intracellular concentration of ions and CP, ultimately restoring the turgor pressure of the cells. The results obtained suggest that a high concentration of intracellular glycerol (a natural osmoregulator for culture) allows
D. salina cells exposed to osmotic stress to quickly restore their original cell sizes.
Thus, D. salina cells are osmotically tolerant to different concentrations of promising solutions of cryoprotectants, whereas A. longa is more vulnerable to osmotic stress and can withstand only short-term exposure with 1–5% solutions of ethanol and glycerol.
Therefore, the type, concentration of CP or a combination of CP, equilibration rate, and the exposure time of the cells must be determined empirically to select the optimal conditions before cryopreservation, taking into account the individual differences in the structure of cells of each taxon.