Novel Formula as Mosquito Larvicide †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Stage 1: Preparation, Characterization, Purification, and Identification of Prodigiosin
2.2. Stage 2: Preparation and Characterization of the Essential Oil Isolated from T. orientalis
2.3. Stage 3: The Fourth Stage: Maintaining the Mosquito by Rearing the Culture of Culex Pipiens
2.4. Stage 4: The Fifth Stage: Dose-Response Bioassay Separately PDG and E.O. as Mosquito Potential
2.5. Stage 5: Investigation of the Synergistic Effect of PDG with the E.O. as Mosquito Larvicidal Potential
2.6. Stage 6: The Seventh Stage: Investigating the Mode of Action of PDG and E.O. for Mosquito Larvicidal Potential
3. Results and Discussion
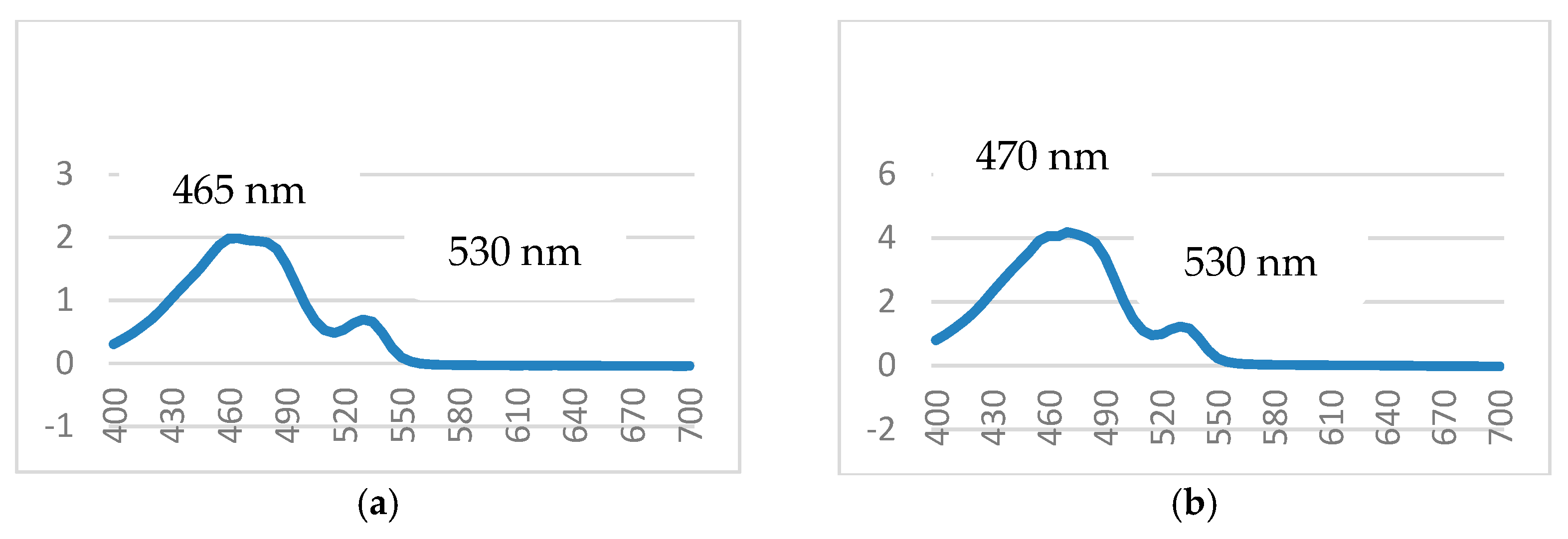
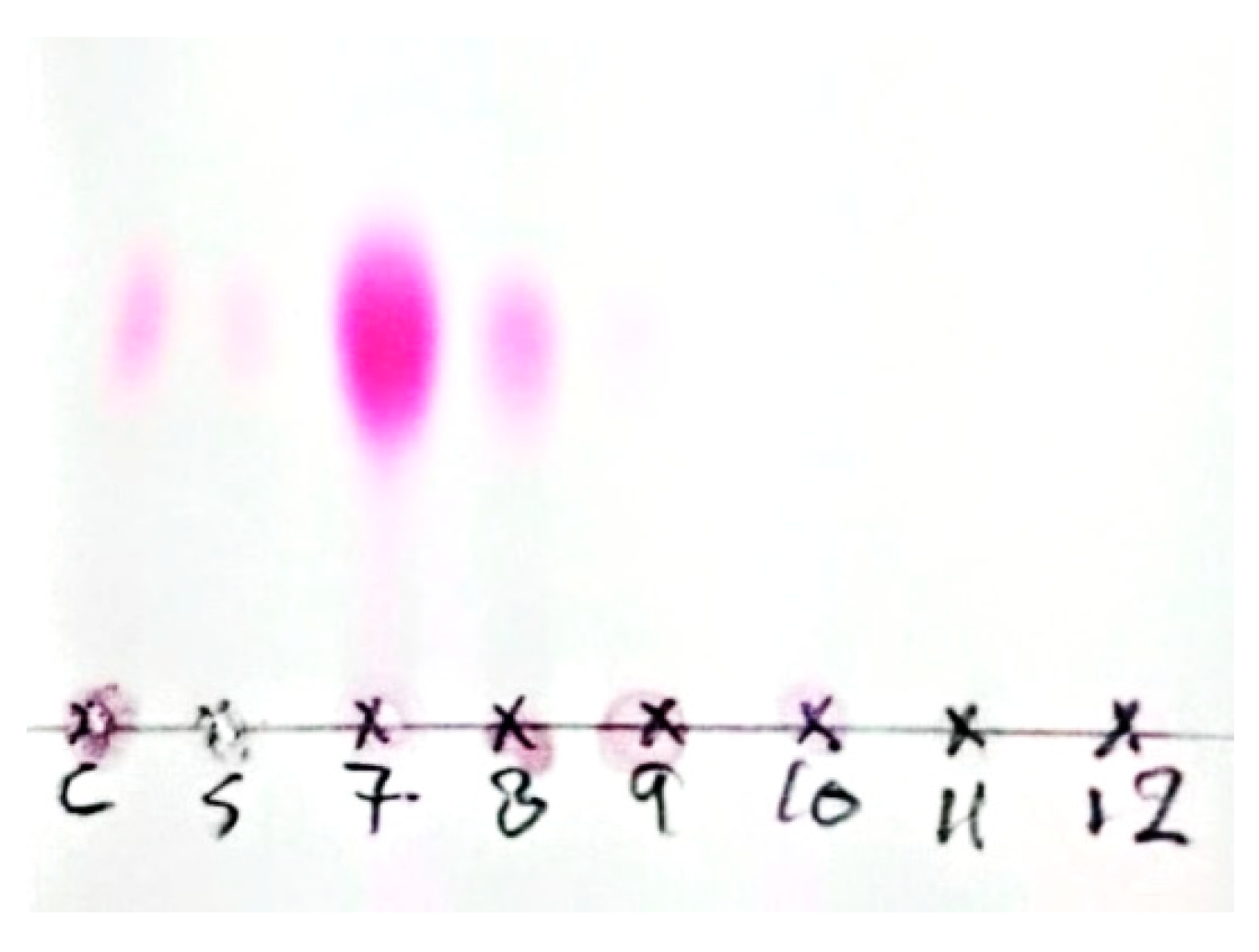
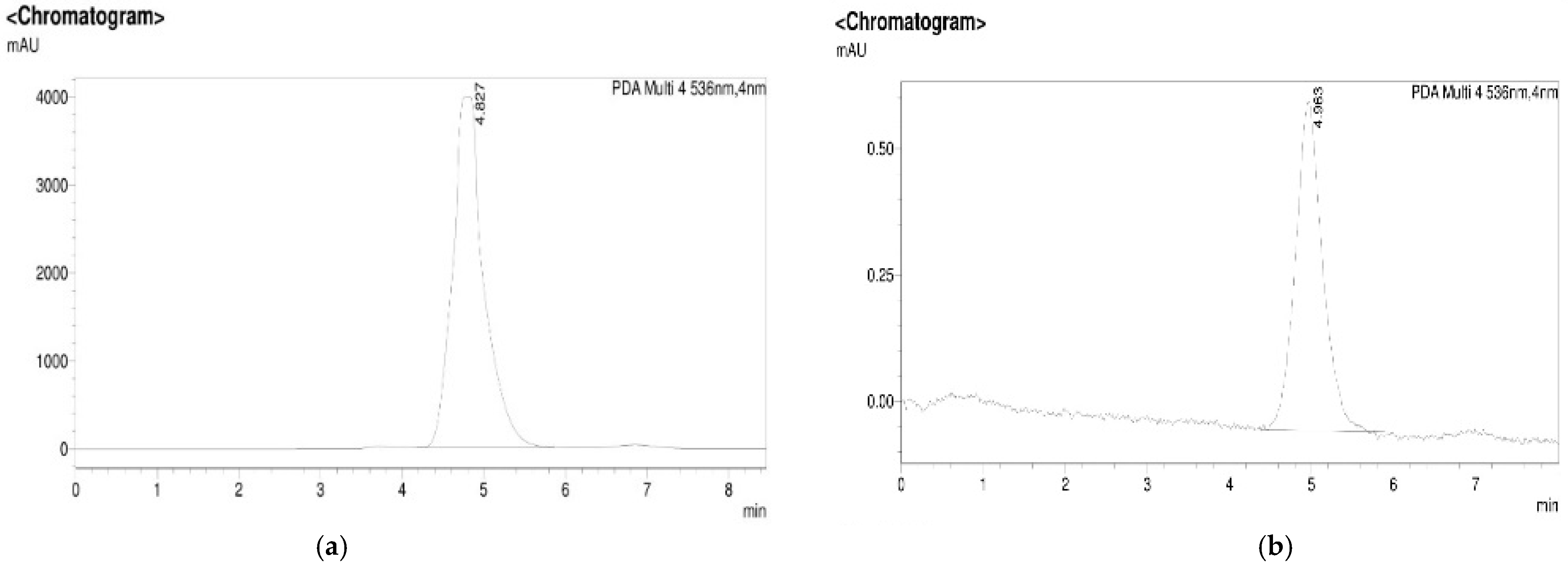
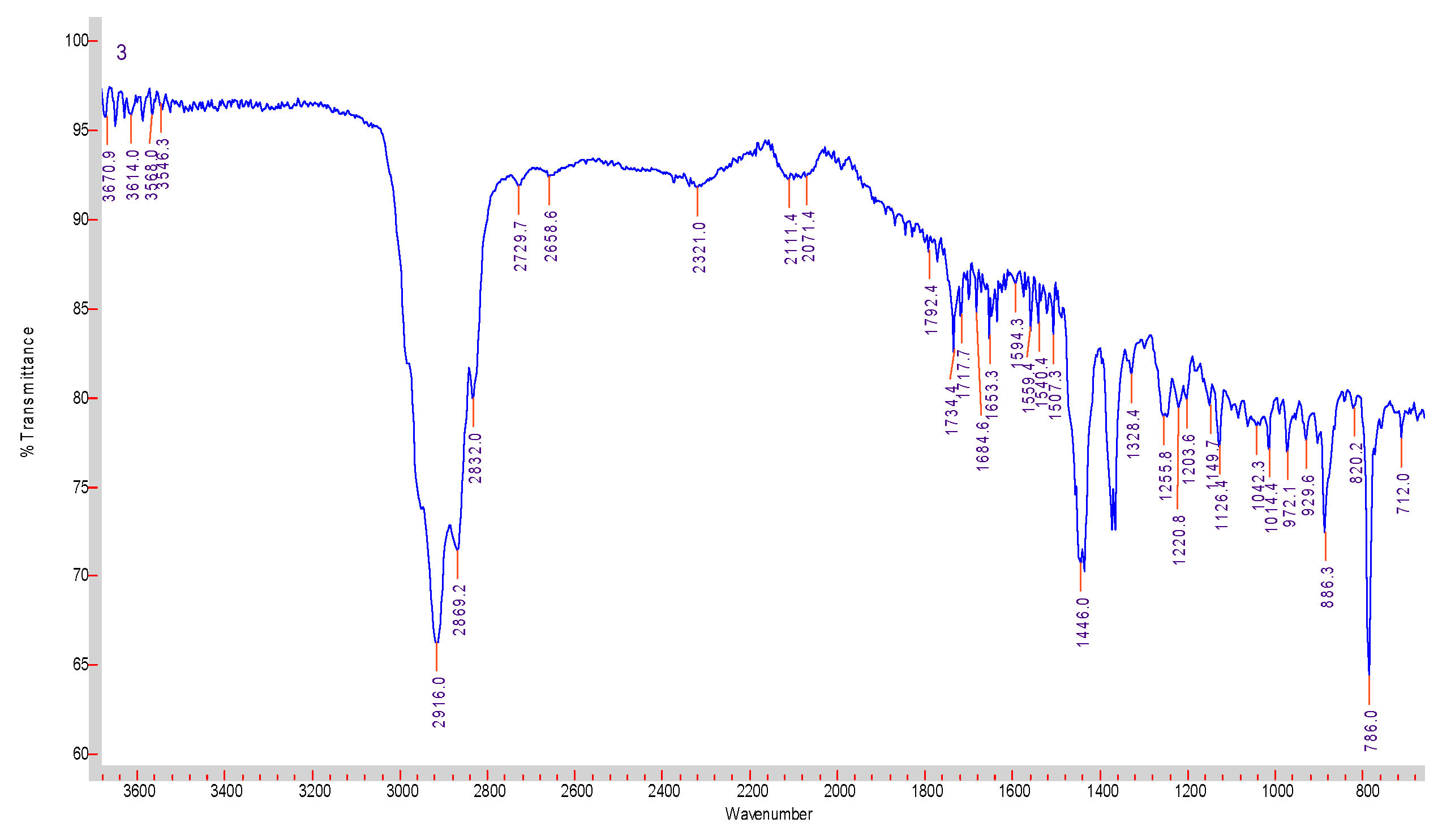
3.1. Isolation, Purification, and Characterization of PDG from SM
3.2. Preparation and Characterization of E.O. from Fresh Leaves of T. orientalis
3.3. Dose Response Bioassay Separately of the Preparations
3.4. Investigation of the Combination Effect of PDG with the E.O. as a Mosquito Larvicidal Potential after 24 h
3.5. Investigating the Mode of Action of PDG and E.O. for Mosquito Larvicidal Potential
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cerdeño, A.M.; Bibb, M.J.; Challis, G.L. Analysis of the prodiginine biosynthesis gene cluster of Streptomyces coelicolor A3(2): New mechanisms for chain initiation and termination in modular multienzymes. Chem. Biol. 2001, 8, 817–829. [Google Scholar] [CrossRef] [Green Version]
- Caraballo, H.; King, K. Emergency department management of mosquito-borne illness: Malaria, dengue, and West Nile virus. Emerg. Med. Pract. 2014, 16, 1–23. [Google Scholar] [PubMed]
- Centers for Disease Control and Prevention (CDC). Mosquito-Borne Diseases. Available online: https://www.cdc.gov/niosh/topics/outdoor/mosquito-borne/default.html (accessed on 21 March 2016).
- Becker, N.; Petric, D.; Zgomba, M.; Boase, C.; Madon, M.B.; Dahl, C.; Kaiser, A. Mosquitoes and Their Control, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2010; Volume 28, pp. 1–246. [Google Scholar]
- Nicolopoulou-Stamati, P.; Maipas, S.; Kotampasi, C.; Panagiotis Stamatis, P.; Hens, L. Chemical Pesticides and Human Health: The Urgent Need for a New Concept in Agriculture. Front. Public Health 2016, 4, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pimentel, D. Killer environment. Environ. Health Perspect. 1999, 107, A62–A63. [Google Scholar]
- Shirata, A.; Tsukamoto, T.; Yasui, H.; Hata, T.; Hayasaka, S.; Kojima, A.; Kato, H. Isolation of bacteria producing bluish-purple pigment and use for dyeing. Jpn. Agric. Res. Q. 2000, 34, 131–140. [Google Scholar]
- Li, H. A Simple HPLC Assay for Ginsenoside-Rh2 in Plasma and Its Application for Pharmacokinetic Study in Rats. Nat. Prod. Chem. Res. 2013, 1, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Raafat, K. Exploration of the Protective Effects of Some Natural Compounds against Neurodegeneration Exploiting Glycine Receptors in vivo Model. Nat. Prod. Chem. Res. 2013, 1, 1–6. [Google Scholar]
- Williams, R.P.; Green, J.A.; Rappo-Port, D.A. Studies on pigmentation of Serratia marcescens. I. Spectral and paper chromatographic properties of prodigiosin. J. Bacteriol. 1956, 71, 115–120. [Google Scholar] [CrossRef] [Green Version]
- Bhasin, M.T.A.; Harshey, R.M. Mutational analysis of flagellum-independent surface spreading of Serratia marcescens 274 on a low-agar medium. J. Bacteriol. 1995, 177, 987–991. [Google Scholar]
- Buchbauer, G.; Jirovetz, L.; Jäger, W.; Plank, C.; Dietrich, H. Fragrance compounds and essential oils with sedative effects upon inhalation. J. Pharm. Sci. 1993, 82, 660–664. [Google Scholar] [CrossRef]
- Jirovetz, L.; Buchbauerl, G.; Denkova, Z.; Al bena stoyanova, A.; Murgov, I.; Schrnidt, E.; Geissle, M. Antimicrobial testings and gas chromatographic analysis of pure oxygenated monoterpenes 1,8-cineole, α-terpineol, terpinen-4-ol and camphor as well as target compounds in essential oils of pine (Pinus pinaster), rosemary (Rosmarinus officinalis), tea tree (Melaleuca alternifolia). Sci. Pharm. 2005, 73, 27–38. [Google Scholar]
- Kim, T.H.; Li, H.; Wu, Q.; Lee, H.J.; Ryu, J.H. A new labdane diterpenoid with anti-inflammatory activity from Thuja orientalis. J. Ethnopharmacol. 2013, 146, 760–767. [Google Scholar] [CrossRef] [PubMed]
- Won, J.N.; Lee, S.Y.; Song, D.S.; Poo, H. Antiviral activity of the plant extracts from Thuja orientalis, Aster spathulifolius, and Pinus thunbergii against influenza virus A/PR/8/34. J. Microbiol. Biotechnol. 2013, 23, 125–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, N.N.; Park, D.K.; Park, H.J. Hair growth-promoting activity of hot water extract of Thuja orientalis. BMC Complement. Altern. Med. 2013, 13, 9. [Google Scholar] [CrossRef] [Green Version]
- Pradeep, B.V.; Akilandeswari, P.; Usha rani, V.; Palaniswamy, M. Application of multifactorial experimental design for optimization of Prodigiosin production using Serratia marcescens MBB01, MBB02 AND MBB05. Asian J. Pharm. Clin. Res. 2016, 9, 408–416. [Google Scholar]
- Vijayalakshmi, K.; Jagathy, K. Production of Prodigiosin from Serratia marcescens and its antioxidant and anticancer potential. Int. J. Adv. Res. Biol. Sci. 2016, 3, 75–88. [Google Scholar]
- Patil, C.D.; Patil, S.V.; Salunke, B.K.; Salunkhe, R.B. Prodigiosin produced by Serratia marcescens NMCC46 as a mosquito larvicidal agent against Aedes aegypti and Anopheles stephensi. Parasitol. Res. 2011, 109, 1179–1187. [Google Scholar] [CrossRef]
- Isman, M.B.; Machial, C.; Miresmailli, S.; Bainard, L. Essential oil based pesticides: New insights from old chemistry. In Pesticide Chemistry; Ohkawa, H., Miyagawa, H., Lee, P., Eds.; Wiley-VCH: Hoboken, NJ, USA, 2007; pp. 201–209. [Google Scholar]
- Tietz, N.W. Textbook of Clinical Chemistry; W.B. Saunders Co.: Philadelphia, PA, USA, 1999. [Google Scholar]
- Young, D.S. Effect of drugs on clinical lab Tests. Ann. Clin. Biochem. 1997, 34 Pt 6, 579–581. [Google Scholar] [CrossRef]
- Doumas, B.T.; Bayse, D.D.; Carter, R.J.; Peters, T., Jr.; Schaffer, R. A candidate reference method for determination of total protein in serum. I. Development and validation. Clin. Chem. 1981, 27, 1642–1650. [Google Scholar] [CrossRef]
- Song, M.; Bae, J.; Lee, D.; Kim, C.; Kim, J.; Kim, S.; Hong, S. Purification and characterization of prodigiosin produced by integrated bioreactor from Serratia sp. KH-95. J. Biosci. Bioeng. 2006, 101, 157–161. [Google Scholar] [CrossRef]
- Nakashima, T.; Kurachi, M.; Kato, Y.; Yamaguchi, K.; Oda, T. Characterization of bacterium isolated from the sediment at coastal area of Omura Bay in Japan and several biological activities of pigment produced by this isolate. Microbiol. Immunol. 2005, 49, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Mandal, R.; Adhikari, A.; Rana, G.; Mandal, T. Study of the useful characteristics of the red pigments of Serratia marcescens strains isolated from the soil. J. Appl. Pharm. Sci. 2017, 7, 142–148. [Google Scholar]
- Kumar, T.S.; Aparna, H. Anti-biofouling activity of Prodigiosin, a pigment extracted from Serratia marcescens. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 712–725. [Google Scholar]
- Al-Mammar, D. Decolorization of the aqueous Safranin O dye solution using Thuja orientalis as biosorbent. Iraqi. J. Sci. 2014, 55, 886–898. [Google Scholar]
- Ibrahim, M.T.; Abdel-Hady, N.M.; Hammad, L.N. 13-GC/MS Analysis and biochemical studies of the essential oil of Thuja orientalis L. Growing in Egypt. Bull. Fac. Pharm. Cairo Univ. 2004, 42, 151–156. [Google Scholar]
- Wang, K. 100 kg Cedar Leaf Essential Oil (Thuja Orientalis), Thuja Orientalis Leaf Oil, CAS 8000-27-9. Available online: https://www.tradesparq.com/products/2062017/100kg-Cedar-Leaf-Essential-Oil-Thuja-Orientalis-Thuja-Orientalis-Leaf-Oil-CAS-8000-27-9-manufacturers (accessed on 1 December 2021).
- Rehman, R.; Zubair, M.; Bano, A.; Hewitson, P.; Ignatova, S. Isolation of industrially valuable α-CedroAl from essential oil of Platycladus orientalis (Thuja orientalis) leaves using linear gradient counter current chromatography. Ind. Crops. Prod. 2022, 176, 114297. [Google Scholar] [CrossRef]
- Isaka, M.; Jaturapat, A.; Kramyu, J.; Tanticharoen, M.; Thebtaranonth, Y. Potent in vitro antimalarial activity of metacycloprodigiosin isolated from Streptomyces spectabilis BCC 4785. Antimicrob. Agents Chemother. 2002, 46, 1112–1113. [Google Scholar] [CrossRef] [Green Version]
- Jeon, J.-H.; Lee, S.-H.; Kim, M.-K.; Lee, H.-S. Larvicidal Activity of Chamaecyparis obtusa and Thuja orientalis Leaf Oils against Two Mosquito Species. J. Appl. Biol. Chem. 2005, 48, 26–28. [Google Scholar]
- Suryawanshi, R.K.; Patil, C.P.; Borase, H.P.; Narkhede, C.P.; Salunke, B.K.; Patil, S.V. Mosquito larvicidal and pupaecidal potential of prodigiosin from Serratia marcescens and understanding its mechanism of action. Pestic. Biochem. Physiol. 2015, 123, 49–55. [Google Scholar] [CrossRef]
- Govindarajan, M.; Rajeswary, M.; Hoti, S.L.; Bhattacharyya, A.; Benelli, G. Eugenol, alpha-pinene and beta-caryophyllene from Plectranthus barbatus essential oil as eco-friendly larvicides against malaria, dengue and Japanese encephalitis mosquito vectors. Parasitol. Res. 2016, 115, 807–815. [Google Scholar] [CrossRef]
- Sanei-Dehkordi, A.; Gholami, S.; Abai, M.R.; Sedaghat, M.M. Essential Oil Composition and Larvicidal Evaluation of Platycladus orientalis against Two Mosquito Vectors, Anopheles stephensi and Culex pipiens. J. Arthropod Borne Dis. 2018, 12, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Yankanchi, S.R.; Yadav, O.V.; Jadhav, G.S. Synergistic and individual efficacy of certain plant extracts against dengue vector mosquito. J Biopestic. 2014, 7, 22–28. [Google Scholar]
- Rukmini, V.; Reddy, C.Y.; Venkateswerlu, G. Bacillus thuringiensis crystal δ-endotoxin: Role of proteases in the conversion of protoxin to toxin. Biochimie 2000, 82, 109–116. [Google Scholar] [CrossRef]
- Hwang, I.; Mukhopadhyay, R.D.; Dhasaiyan, P.; Choi, S.; Kim, S.-Y.; Ko, Y.H.; Baek, K.; Kim, K. Audible sound-controlled spatiotemporal patterns in out-of-equilibrium systems. Nat. Chem. 2020, 12, 808–813. [Google Scholar] [CrossRef]
- Zhuang, Z.; Linser, P.J.; Harvey, W.R. Antibody to H(+) V-ATPase subunit E colocalizes with portasomes in alkaline larval midgut of a freshwater mosquito (Aedes aegypti). J. Exp. Biol. 1999, 202 Pt 12, 2449–2460. [Google Scholar] [CrossRef]
- Supuran, C.T. Drug interaction considerations in the therapeutic use of carbonic anhydrase inhibitors. Expert Opin. Drug Metab. Toxicol. 2016, 12, 423–431. [Google Scholar] [CrossRef]
- Bhaganna, P.; Volkers, R.J.M.; Bell, A.N.W.; Kluge, K.; Timson, D.J.; McGrath, J.W.; Ruijssenaars, H.J.; Hallsworth, J.E. Hydrophobic substances induce water stress in microbial cells. Microb. Biotechnol. 2010, 3, 701–716. [Google Scholar] [CrossRef] [Green Version]
- McCammick, E.M.; Gomase, V.S.; Timson, D.J.; McGenity, T.J.; Hallsworth, J.E. Water-hydrophobic compound interactions with the microbial cell. In Handbook of Hydrocarbon and Lipid Microbiology—Hydrocarbons, Oils and Lipids, Diversity, Properties and Formation, Vol. 2; Timmis, K.N., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 1451–1466. [Google Scholar] [CrossRef]

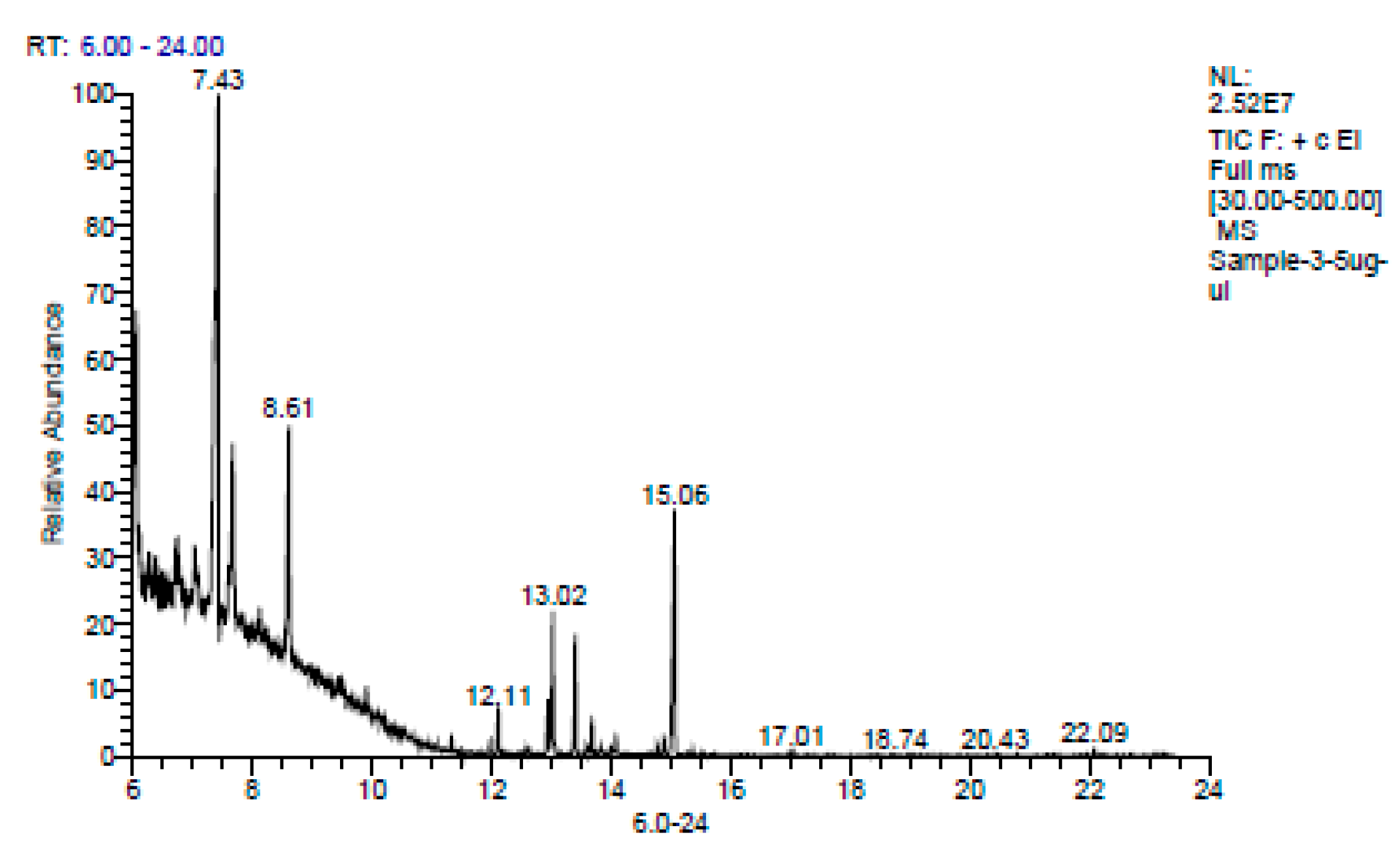
| Monoterpene Hydrocarbons | Oxygenated Monoterpene | ||||||
| Peak | RT | Constituents | % | Peak | RT | Constituents | % |
| 1 | 6.1 | α-Pinene | 17.19 | 8 | 8.61 | p-Menth-2-en-1-ol | 9.21 |
| 2 | 6.27 | α-Fenchene | 1.69 | 9 | 9.45 | Camphor | 0.49 |
| 3 | 6.81 | α- Phellandrene | 3.94 | 10 | 9.51 | Citronellal | 0.32 |
| 4 | 7.04 | β -Myrcene | 3.21 | 11 | 10.11 | α-Terpineol | 0.29 |
| 5 | 7.37 | 3-Carene | 30.26 | 12 | 10.56 | Citronellol | 0.26 |
| 6 | 7.65 | D-Limonene | 7.72 | 13 | 11.32 | iso- Bornyl acetate | 0.37 |
| 7 | 9.9 | α-Terpinene | 0.97 | 14 | 12.11 | α-Terpinyl acetate | 1.14 |
| Total | 64.98 | Total | 12.08 | ||||
| Sesquiterpene Hydrocarbons | Oxygenated Sesquiterpene | ||||||
| Peak | RT | Constituents | % | Peak | RT | Constituents | % |
| 15 | 12.44 | α-Copaene | 0.88 | 23 | 14.77 | Caryophyllene oxide | 0.24 |
| 16 | 12.92 | Cedrene | 0.26 | 24 | 14.88 | α-Acorenol | 0.48 |
| 17 | 12.94 | Di-epi- α-Cedrene | 1.4 | 25 | 15.02 | Cedrol | 8.87 |
| 18 | 12.98 | Caryophyllene | 3.67 | Total | 9.59 | ||
| 19 | 13.37 | α-Humulene | 2.87 | Total Monoterpene hydrocarbons% | 64.98 | ||
| 20 | 13.59 | α-Muurolene | 0.49 | Total Oxygenated Monoterpene% | 12.08 | ||
| 21 | 13.82 | Gurjunene | 0.29 | Total Sesquiterpene hydrocarbons% | 10.39 | ||
| 22 | 14.08 | β -Cubebene | 0.53 | Total Oxygenated Sesquiterpene% | 9.59 | ||
| Total | 10.39 | Total | 97.04% | ||||
| Larvicide | LC50/(ppm) 1 | 95% Confidence Limits | Slope 2 ± SE | Intercept 3 ± SE | (R2) 4 | (χ2) 5 | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| PDG | 39.5 | 29.7 | 52.5 | 2.9 | 0.321 | 0.946 | 0.924 |
| EO | 102.9 | 69.9 | 153.3 | 2.172 | 0.623 | 0.839 | 0.532 |
| Sample | LC10 of PDG with LC25 of Oil | LC10 of PDG with LC50 of Oil |
|---|---|---|
| % Death | 33.3 | 100 |
| Treatment | AChE Arbitrary Activity unit/gm Tissue 1 (%) 3 | Total Protein in mg/gm Tissue (%) 3 | AChE Arbitrary Specific Activity 2 (%) 3 |
|---|---|---|---|
| Untreated | 5.8 | 1.32 | 4.39 |
| PDG LC50 | 2.5 | 0.72 | 3.47 |
| EO LC50 | 3.5 | 1.12 | 3.13 |
| PDG LC10 + EO LC25 | 3.8 | 0.92 | 4.13 |
| PDG LC10 + EO LC50 | 3.2 | 0.52 | 0.91 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassanein, F.; Awad, O.; Harraz, F.M.; Saeed, H.; Hussein, A. Novel Formula as Mosquito Larvicide. Biol. Life Sci. Forum 2022, 11, 54. https://doi.org/10.3390/IECPS2021-12070
Hassanein F, Awad O, Harraz FM, Saeed H, Hussein A. Novel Formula as Mosquito Larvicide. Biology and Life Sciences Forum. 2022; 11(1):54. https://doi.org/10.3390/IECPS2021-12070
Chicago/Turabian StyleHassanein, Faika, Osama Awad, Fathallah M. Harraz, Hesham Saeed, and Ahmed Hussein. 2022. "Novel Formula as Mosquito Larvicide" Biology and Life Sciences Forum 11, no. 1: 54. https://doi.org/10.3390/IECPS2021-12070
APA StyleHassanein, F., Awad, O., Harraz, F. M., Saeed, H., & Hussein, A. (2022). Novel Formula as Mosquito Larvicide. Biology and Life Sciences Forum, 11(1), 54. https://doi.org/10.3390/IECPS2021-12070






