Abstract
This study aimed to characterize the behavior of different cultivars of Vigna unguiculata L. Walp. to osmotic stress, from germination to vigor parameters. The experimental design used was completely randomized in a 14 × 4 factorial arrangement, with fourteen cultivars and four levels of osmotic potential (0, −0.1, −0.2, and −0.4 MPa) of the germination solution. BRS-Novaera and BRS-Pajeú cultivars were characterized with possible tolerance at both the −0.1 and −0.2 MPa levels. The study pointed to the BRS-Pujante cultivar as the most sensitive to the −0.4 MPa level. The multivariate technique used allowed for a satisfactory characterization of the treatments adopted.
1. Introduction
Cowpea (Vigna unguiculata L. Walp.), also known as Macassar and white bean, is one of the most cultivated and consumed legumes in the world, and one of the main sources of vegetable protein for populations, especially for those with lower purchasing power [1]. In Brazil, above all, it is cultivated in the North and Northeast, presenting important socio-economic functions for family farming in these regions [2], considering it is configured as a source of employment and income for numerous families.
Although cowpea is well adapted to different edaphoclimatic conditions and is considered tolerant to situations of low soil water availability, some studies have shown that osmotic stress is one of the abiotic stresses that most affect this crop, which can cause changes in seed germination and early development processes, among other things, which are the phases considered most vulnerable to water scarcity [3,4].
The osmotic stress influences practically all aspects related to plant development, which ends up culminating in the reduction of crop productivity [3]. This type of stress tends to compromise the initial establishment of the seedling stand, as water limitation ends up reducing the speed of germination and may even impede it [5]. This is because water is the starting factor for germination and is, directly and indirectly, involved in all other stages of germination metabolism [6].
Studies with seeds of different species have been conducted to assess germination and vigor under low humidity conditions [7]. However, studies related to tolerance of different cowpea cultivars to abiotic stresses at these stages of development are still scarce in the literature, especially when referring to data interpreted from multivariate analysis, which consists of a set of statistical methods that allows the simultaneous evaluation of several variables of a sample or population, whose purpose is to simplify and facilitate the interpretation of certain studied phenomena [8,9]. In this sense, the objective of this work was to characterize, through multivariate data analysis, the behavior of different cowpea bean cultivars to osmotic stress, based on germination and vigor variables.
2. Material and Methods
The experiment was carried out at the Seed Analysis Laboratory of the Federal University of Ceará (UFC), in Fortaleza, from September to October 2018. Cowpea seeds of the cultivars were used: BRS-Pujante, BRS-Guariba, BRS-Potengi, BR 17-Gurgueia, BRS-Tumucumaque, BRS-Pajeú, BRS-Rouxinol, BRS-Novaera, BRS-Xiquexique, BRS-Milênio, BRS-Acauã, Patativa, BR 3-Tracuateua and BRS-Aracê. They were subjected to germination at different osmotic potentials, simulating situations of osmotic stress: 0 (distilled water), −0.1, −0.2 and −0.4 MPa, induced by polyethylene glycol 6000 (PEG) solutions and prepared according to [10].
Initially, we selected uniform seeds with an intact integument of each cultivar. Then, the seeds were disinfected in a 1% sodium hypochlorite solution for 1 min and then washed with distilled water. For each treatment, 200 seeds were used and divided into four replicates of 50 seeds, with each group being distributed among three sheets of blotting paper moistened with distilled water or PEG 6000 solution in the proportion of 2.5 times the weight of the dry paper [11], obeying the different osmotic potentials previously established. Then, the three sheets were rolled and the resulting rolls were placed inside polyethylene pots covered with transparent plastic, which were kept in a BOD-type germination chamber at a constant temperature of 25 °C [11] and under a 12 h light/12 h dark photoperiod.
The count of germinated seeds was performed daily for nine days, considering as germination criterion the emission of a radicle with at least 2.0 mm [12]. The germination variables evaluated were: final germination percentage [13], germination speed index [14] and mean germination time [13].
For vigor evaluation, we experimented with the same conditions and treatments mentioned for the germination test. However, the four repetitions of each treatment had twenty seeds each [15]. The evaluations were carried out nine days after sowing and the lengths of the aerial part (LAP) and root (LR) of the normal seedlings of each repetition were measured with a ruler.
Subsequently, the seedlings of each repetition had shoots and roots sectioned, which were conditioned separately in properly identified paper bags, and taken to an oven with forced air circulation at 65 °C for 48 h. After this period, with the aid of a precision scale, the aerial part dry mass (APDM) and root dry mass (RDM) data were obtained, and the RDM/APDM ratio was calculated.
The experimental design used was completely randomized in a 14 × 4 factorial arrangement, referring to the fourteen cowpea cultivars and four levels of osmotic potential, with four replications. Statistical analysis of data was performed using the multivariate method of Principal Component Analysis (PCA), an exploratory technique that aims to reduce the number of variables to a smaller number of indices (principal components), which are combinations of linear variables of the original variables [9]. This analysis was performed using the R statistical package v. 4.0.2 [16].
This methodology is widely used when one wants to analyze several characteristics simultaneously and its efficiency is based on a greater correlation between the original variables, whether positive or negative [8]. Therefore, the correlation matrix used in this study was calculated using Spearman’s correlation coefficient, due to the abnormality of the data found through the Shapiro-Wilk test, in which a p-value < 0.05 was obtained for all analyzed variables.
The data set consisting of the means of the variables for each treatment was standardized (μ = 0; σ2 = 1), removing the influence of the different measurement units of the variables on the final result. To determine the number of main components, the Kaiser criterion was used, which consists of selecting the components that presented eigenvalues greater than 1 (λi > 1) [9,17].
3. Results and Discussion
Table 1 shows the matrix of correlations between the variables and their respective p-values. We observed that the variables were significantly correlated (p < 0.05), both positively and negatively, except for the correlations between RDM/APDM and GSI (ρ = 0.25), RDM/APDM and LAP (ρ = 0.23) and RDM/APDM and APDM (ρ = 0.22), which were not significant. Thus, we can conclude that there is evidence of a correlation between the characteristics, indicating that the use of multivariate analysis techniques is adequate.

Table 1.
Correlation matrix and their respective significance values (p-value) between the studied variables.
The results obtained with PCA reveal that the first two components generated presented eigenvalues greater than 1 (λi > 1), meeting the criterion established by [17] and therefore, selected for the interpretation of the results (Table 2). The PCA showed that the two selected components explained 91.50% of the total variance of the data, with 74.77% explained by component 1 (CP1) and 16.73% by component 2 (CP2), as shown in Table 2. The first two main components effectively summarize a large part of the total sample variance and can be used to study the dataset.

Table 2.
Weight coefficients (eigenvectors), eigenvalues, explained variance (EV) and accumulated explained variance (AEV) for each principal component, based on the studied variables.
In the degree of importance of the variables in each of the main components, those with weight coefficients greater than 0.3 in the module were considered relevant (Table 2). Thus, the variables that most contributed to CP1 were G, GSI, MGT, LR, RDM and APDM, with the variable MGT showing low values; for CP2, the variables that most influenced it were LAP (characterized by low values) and RDM/APDM ratio (characterized by high values). Thus, CP1 indicates germination performance and root growth, while CP2 indicates differences in seedling size.
When analyzing Figure 1, in general, the treatments distributed in the positive region of CP1 and CP2 are characterized by presenting higher values of the variables G, RDM, LR and RDM/APDM. However, with increasing stress intensity (from −0.1 MPa to −0.2 MPa), there are reductions in the values of these variables according to each cultivar evaluated. However, even with the stress of −0.2 MPa applied, some cultivars managed to practically maintain the germination potential presented at the level of −0.1 MPa and also increase their root growth at the expense of the aerial part as a way of tolerance to water scarcity, which was the case of cultivars BRS-Novaera and BRS-Pajeú.
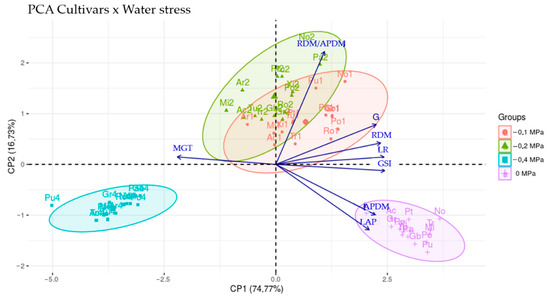
Figure 1.
Biplot showing the relationship between variables and treatments for the first two main components (CP1 e CP2). Pu = BRS-Pujante; Gb = BRS-Guariba; Po = BRS-Potengi; Gr = BR 17-Gurgueia; Tu = BRS-Tumucumaque; Pa = BRS-Pajeú; Ro = BRS-Rouxinol; No = BRS-Novaera; Xi = BRS-Xiquexique; Mi = BRS-Milênio; Ac = BRS-Acauã; Pt = Patativa; Tr = BRS-Tracuateua; Ar = BRS-Aracê.
Therefore, most of the reserves metabolized by the seeds of these two treatments were destined for root development (with an increase in lateral roots) and not for shoot growth, which was reflected in the increase in the RDM/APDM ratio. According to [18], the osmotic stress condition suggests a prioritization of root growth, an important feature in the escape from this type of stress, where it can favor water absorption precisely by increasing the surface of contact with the substrate. This continuity in root growth depends on maintaining a minimum turgor pressure in the cells, which is sufficient to allow elongation and cell growth [19,20].
When analyzing the treatments in the negative region of CP1 and positive region of CP2 (Figure 1), there are higher values of the MGT variable and lower values of GSI, LAP and APDM. That is to say, the levels of osmotic stress (−0.1 and −0.2 MPa) imposed on these cultivars led to a delay in the seed germination process and impaired shoot growth, possibly due to a lower translocation of reserves to the axis, whereas embryonic (growth region) was due to the low availability of water. It is important to emphasize the responses of the cultivars BRS-Milênio, BRS-Acauã and BRS-Aracê that, when submitted to the level of −0.1 MPa, already presented difficulties to germinate and develop, and intensified at the level of −0.2 MPa, mainly for BRS-Milênio.
Reductions in seedling LAP values can be explained by the decrease in seed metabolism, since there is less water availability for the digestion of reserves and translocation of metabolized products [20]. Similar results were obtained by [21], who, while working with the viability of sunflower seeds after water stress, found a reduction in seedling growth as the concentrations of PEG 6000 increased, with the lowest values found in the osmotic potential of −0.8 MPa. In Triticum aestivum L., a significant reduction was also found for all genotypes evaluated, with increasing levels of osmotic stress [22].
When evaluating the mean germination time in seeds of Poincianela pyramidalis and Anadenanthera colubrina under water stress induced by polyethylene glycol, ref. [23] found that at the levels of more negative osmotic potentials it took a greater number of days for the seeds to germinate. These data corroborate the results obtained in this research. Therefore, we suggest that as the osmotic potential increases, the seed needs more time to germinate [24].
These decreases in the GSI and increases in the MGT, which were due to the increase in the expressiveness of stress, are widely reported in the literature [25,26]. When exposed to unfavorable conditions of water availability, the time for seed germination to occur tends to increase both due to the reduction in the absorption of water necessary for the activation of the metabolism, which ends up making the digestion of reserves and the translocation of the metabolized products difficult, as well as the time, which can increase until the seed can activate tolerance mechanisms for this situation; or, if this does not happen, the germination process may even be completely inhibited [20,24,27], as has happened with most of the cultivar seeds which were subjected to the level of −0.4 MPa.
The results showed that, regardless of the studied cultivars, all evaluated variables (except MGT) were severely affected when the seeds were subjected to the highest stress level (−0.4 MPa), where seedlings could not develop to the point of accounting for the variables LR, LAP, RDM, APDM and RDM/APDM. In Figure 1, this behavior can be seen and the low values for the germinative performance variables (G and GSI) can also be seen, especially for the BRS-Pujante cultivar, which was very sensitive. According to [28], when seeds are subjected to water deficiency by osmotic solutions, vigor is more affected than germination.
As in the present experiment, the osmotic potential of −0.4 MPa promoted decreases in the germination percentage of Apuleia leiocarpa seeds [29,30]. Working with the simulation of water stress in cowpea genotypes, we also found that there was a reduction in the percentage of seed germination and a reduction in the osmotic potential. This same response was found in soybean seeds subjected to osmotic stress conditions [31].
By analyzing the responses of cultivars at the 0 MPa level, we observed that they presented high values of the LAP and APDM variables and low values of MGT. This answer evidences the adequate water availability, since the seed has the necessary amount of water for the entire germination process, activation of the metabolism and translocation of reserves to the embryo’s growth region, which culminates in greater development of the aerial part, without the need for greater investments of its reserves for the growth of the root system [20,27].
In general, the LAP demonstrated that the cowpea cultivars presented similar responses and performances when compared within each of the stress levels, which can be observed through the 95% confidence ellipses, except the cultivar BRS-Pujante in the level −0.4 MPa, which was characterized as a discrepant point. The superposition of the ellipses of the treatments with levels −0.1 and −0.2 MPa indicates that the groups share similar characteristics.
4. Conclusions
The Principal Component Analysis allowed the characterization of the treatments, pointing to the cultivar BRS-Pujante as the most sensitive at the −0.4 MPa level. The technique showed that the cultivars BRS-Milênio, BRS-Acauã and BRS-Aracê had difficulties in tolerating the stresses of −0.1 and −0.2 MPa imposed, with emphasis on the cultivar BRS-Mi-lênio at the level −0.2 MPa. The cultivars BRS-Novaera and BRS-Pajeú were characterized with possible tolerance at both the −0.1 and −0.2 MPa levels.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/IECPS2021-11994/s1.
Author Contributions
Conceptualization, L.K.B.d.O.; methodology, A.B.d.O.; software, C.T.d.S.D.; validation, R.O.M.; formal analysis, L.K.B.d.O. and C.T.d.S.D.; investigation, M.L.d.S.S.; resources, L.K.B.d.O.; data curation, R.O.M.; writing—original draft preparation, L.K.B.d.O.; writing—review and editing, A.V.A. and R.O.M.; visualization, L.K.B.d.O.; supervision, A.B.d.O., A.V.A. and R.O.M.; project administration, A.B.d.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), with grant number 140807/2021-7.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank CNPq for granting the scholarship and the Federal University of Ceara for laboratory support.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study, the collection, analyses, or interpretation of data, the writing of the manuscript, or in the decision to publish the results.
References
- Vijaykumar, A.; Saini, A.; Jawali, N. Phylogenetic analysis of subgenus vigna species using nuclear ribosomal RNA ITS: Evidence of hybridization among Vigna unguiculata subspecies. J. Hered. 2010, 101, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Vale, J.C.; Bertini, C.; Borém, A. Feijão-Caupi do Plantio à Colheita, 1st ed.; Editora UFV: Viçosa, Brazil, 2017. [Google Scholar]
- Nascimento, S.P.; Bastos, E.A.; Araújo, E.C.E.; Freire Filho, F.R.; Silva, E.M. Tolerância ao déficit hídrico em genótipos de feijão-caupi. Rev. Bras. Eng. Agrícola E Ambient. 2011, 15, 853–861. [Google Scholar] [CrossRef]
- Freitas, R.M.O.; Dombroski, J.L.D.; Freitas, F.C.L.; Nogueira, N.W.; Pinto, J.R.S. Physiological responses of cowpea under water stress and rewatering in no-tillage and conventional tillage systems. Rev. Caatinga 2017, 30, 559–567. [Google Scholar] [CrossRef]
- Możdżeń, K.; Bojarski, B.; Rut, G.; Migdałek, G.; Repka, P.; Rzepka, A. Effect of drought stress induced by mannitol on physiological parameters of maize (Zea mays L.) seedlings and plants. J. Microbiol. Biotechnol. Food Sci. 2015, 4, 86–91. [Google Scholar] [CrossRef]
- Oliveira, A.B.; Gomes Filho, E.; Enéas Filho, J. O problema da salinidade na agricultura e as adaptações das plantas ao estresse salino. Enciclopédia Biosf. 2010, 6, 1–16. [Google Scholar]
- Colman, B.A.; Nunes, C.M.; Masson, G.L.; Barbosa, R.H.; Nunes, A.S. Indução de tolerância ao estresse hídrico na germinação de sementes de feijão-caupi. Comun. Sci. 2014, 5, 449–455. [Google Scholar] [CrossRef]
- Vicini, L.; Souza, A.M.; Morales, F.E.C.; Souza, F.M. Técnicas Multivariadas Exploratórias: Teorias e Aplicações No Software Statistica; Editora UFSM: Santa Maria, Brazil, 2018; p. 240. [Google Scholar]
- Manly, B.F.J.; Alberto, J.A.N. Métodos Estatísticos Multivariados: Uma Introdução, 4th ed.; Bookman: Porto Alegre, Brazil, 2019; p. 254. [Google Scholar]
- Villela, F.A.; Filho, L.D.; Sequeira, E.L. Tabela de potencial osmótico em função da concentração de polietileno-glicol 6.000 e da temperatura. Pesqui. Agropecuária Bras. 1991, 26, 1957–1968. [Google Scholar]
- Brasil Ministério da Agricultura, Pecuária e Abastecimento. Regras Para Análise de Sementes; Secretaria de Defesa Agropecuária/Mapa/ACS: Brasília, Brazil, 2009.
- Rosa, L.S.; Felippi, M.; Nogueira, A.C.; Grossi, F. Avaliação da germinação sob diferentes potenciais osmóticos e caracterização morfológica da semente e plântula de Ateleia glazioviana Baill (timbó). Cerne 2005, 11, 306–314. [Google Scholar]
- Labouriau, L.G. A Germinação das Sementes, 24th ed.; OEA: Washington, DC, USA, 1983; p. 174. [Google Scholar]
- Maguire, J.D. Speed of germination-aid in selection and evaluation for seedling emergence and vigor. Crop Sci. 1962, 2, 176–177. [Google Scholar] [CrossRef]
- Nakagawa, J. Testes de vigor baseados no desempenho das plântulas. In Vigor de sementes: Conceitos e Testes; KrzyzanoskI, F.C., Vieira, R.D., França Neto, J.B., Eds.; ABRATES: Londrina, Brazil, 1999; pp. 20–31. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Kaiser, H.F. The varimax criterion for analytic rotation in fator analysis. Psychometrika 1958, 23, 187–200. [Google Scholar] [CrossRef]
- Scalon, S.P.Q.; Mussury, R.M.; Euzébio, V.L.D.M.; Kodama, F.M.; Kissmann, C. Estresse hídrico no metabolismo e crescimento inicial de mudas de mutambo (Guazuma ulmifolia Lam.). Ciência Florest. 2011, 21, 655–662. [Google Scholar] [CrossRef]
- Marcos Filho, J. Fisiologia de Sementes de Plantas Cultivadas, 5th ed.; Abrates: Londrina, Brazil, 2015; p. 659. [Google Scholar]
- Taiz, L.; Zeiger, E.; Moller, I.M.; Murphy, A. Fisiologia e Desenvolvimento Vegetal, 6th ed.; Artmed: Porto Alegre, Brazil, 2017. [Google Scholar]
- Carneiro, M.M.L.C.; Deuner, S.; Oliveira, P.V.; Teixeira, S.B.; Sousa, C.P.; Bacarin, M.A.; Moraes, D.M. Atividade antioxidante e viabilidade de sementes de girassol após estresse hídrico e salino. Rev. Bras. Sementes 2011, 33, 752–761. [Google Scholar] [CrossRef]
- Girotto, L.; Alves, J.D.; Deuner, S.; Albuquerque, A.C.S.; Tomazoni, A.P. Tolerância à seca de genótipos de trigo utilizando agentes indutores de estresse no processo de seleção. Rev. Ceres 2012, 59, 192–199. [Google Scholar] [CrossRef][Green Version]
- Santos, C.A.; Silva, N.V.; Walter, L.S.; Silva, E.C.A.; Nogueira, R.J.M.C. Germinação de duas espécies da caatinga sob déficit hídrico e salinidade. Pesqui. Florest. Bras. 2016, 36, 219–224. [Google Scholar] [CrossRef]
- Pelegrini, L.L.; Borcioni, E.; Nogueira, A.C.; Koehler, H.S.; Quoirin, M.G.G. Efeito do estresse hídrico simulado com NaCl, manitol e PEG (6000) na germinação de sementes de Erythrina falcata Benth. Ciência Florest. 2013, 23, 511–519. [Google Scholar] [CrossRef]
- Oliveira, A.B.; Gomes Filho, E. Efeito do condicionamento osmótico na germinação e vigor de sementes de sorgo com diferentes qualidades fisiológicas. Rev. Bras. Sementes 2010, 32, 25–34. [Google Scholar] [CrossRef]
- Agostini, E.A.T.; Machado-neto, N.B.; Custódio, C.C. Induction of water deficit tolerance by cold shock and salicylic acid during germination in the common bean. Acta Scientiarum. Agron. 2013, 35, 209–219. [Google Scholar] [CrossRef]
- Bewley, J.D.; Bradford, K.J.; Hilhorst, H.W.M.; Nonogaki, H. Seeds: Physiology of Development, Germination and Dormancy, 3rd ed.; Springer: New York, NY, USA, 2013; p. 392. [Google Scholar]
- Machado Neto, N.B.; Saturnino, S.M.; Bomfim, D.C.; Custódio, C.C. Water stress induced by mannitol and sodium chloride in soybean cultivars. Braz. Arch. Biol. Technol. 2004, 47, 521–529. [Google Scholar] [CrossRef]
- Spadeto, C.; Lopes, J.C.; Mengarda, L.H.G.; Matheus, M.T.; Bernardes, P.M. Estresse salino e hídrico na germinação de sementes de garapa (Apuleia leiocarpa (VOGEL.) JF Macbr.). Enciclopédia Biosf. 2012, 8, 539–551. [Google Scholar]
- Ferreira, D.F. Sisvar: A Guide for its Bootstrap procedures in multiple comparisons. Ciência E Agrotecnologia 2014, 38, 109–112. [Google Scholar] [CrossRef]
- Soares, M.M.; Santos Junior, H.C.; Simões, M.G.; Pazzin, D.; Silva, L.J. Estresse hídrico e salino em sementes de soja classificadas em diferentes tamanhos. Pesqui. Agropecuária Trop. 2015, 45, 370–378. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).