Establishment and Optimization of Micrografting Assays with Almond (Prunus dulcis) Portuguese Varieties †
Abstract
1. Introduction
2. Materials and Methods
2.1. Establishment of In Vitro Cultures for Scion and Rootstock
2.2. Rooting Assays
2.3. Micrografting Assays
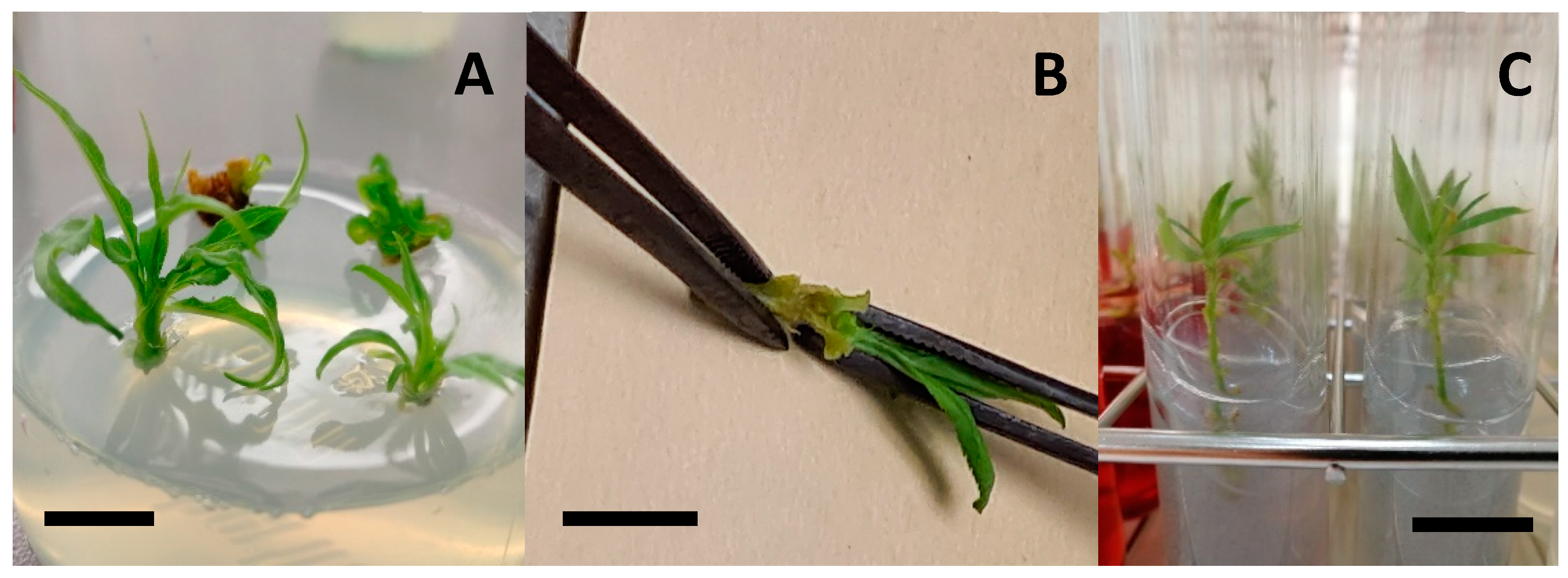
2.3.1. Effect of the Final Medium in Micrografting
2.3.2. Rooting during Micrografting
2.4. Statistical Analysis
3. Results and Discussion
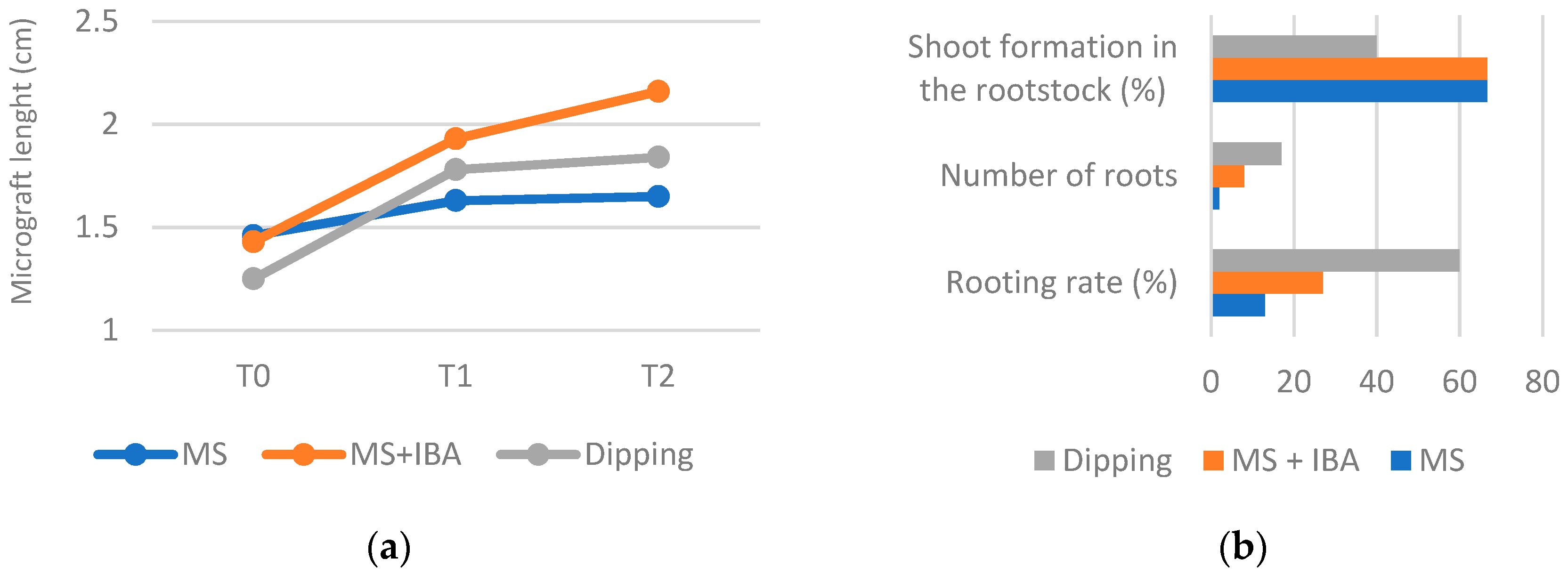
3.1. Rooting Assay
3.2. Micrografts Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barreca, D.; Nabavi, S.M.; Sureda, A.; Raskhian, M.; Raciti, R.; Silva, A.S.; Annunziata, G.; Arnone, A.; Tenore, G.C.; Süntar, I.; et al. Almonds (Prunus Dulcis Mill. D. A. Webb): A Source of Nutrients and Health- Promoting Compounds. Nutrients 2020, 12, 672. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, I.; Meyer, A.S.; Afonso, S.; Aires, A.; Goufo, P.; Trindade, H.; Gonçalves, B. Phenolic and fatty acid profiles, α-tocopherol and sucrose contents, and antioxidant capacities of understudied Portuguese almond cultivars. J. Food Biochem. 2019, 43, e12887. [Google Scholar] [CrossRef] [PubMed]
- Yıldırım, H.; Onay, A.; Süzerer, V.; Tilkat, E.; Ozden-Tokatli, Y.; Akdemir, H. Micrografting of almond (Prunus dulcis Mill.) cultivars “Ferragnes” and “Ferraduel”. Sci. Hortic. 2010, 125, 361–367. [Google Scholar] [CrossRef]
- Navarro, L.; Llácer, G.; Cambra, M.; Arregui, J.M.; Juárez, J. Shoot-tip grafting “in vitro” for elimination of viruses in peach plants. Acta Hortic. 1982, 130, 185–192. [Google Scholar]
- Gebhardt, K.; Goldbach, H. Establishment, graft union characteristics and growth of Prunus micrografts. Physiol. Plant. 1988, 72, 153–159. [Google Scholar] [CrossRef]
- Jonard, R.; Lukman, D.; Schall, F.; Villemur, P. Early testing of graft incompatibilities in apricot and lemon trees using in vitro techniques. Sci. Hortic. 1990, 43, 117–128. [Google Scholar] [CrossRef]
- Ewald, D.; Kretzschmar, U. The influence of micrografting in vitro culture behavior and vegetative propagation of old European larch trees. Plant Cell Tissue Organ Cult. 1996, 44, 249–252. [Google Scholar]
- Martinez-Gomez, P.; Gradziel, T.M. In vivo micrografts in almond and their application in breeding programs. HortTechnology 2001, 11, 313–315. [Google Scholar] [CrossRef]
- Hussain, G.; Wani, M.S.; Mir, M.A.; Rather, Z.A.; Bhat, K.M. Micrografting for fruit crop improvement. Afr. J. Biotechnol. 2014, 13, 2474–2483. [Google Scholar] [CrossRef][Green Version]
- Pahnekolayi, M.D.; Tehranifar, A.; Samiei, L.; Shoor, M. Optimizing culture medium ingredients and micrografting devices can promote in vitro micrografting of cut roses on different rootstocks. Plant Cell Tissue Organ Cult. 2019, 137, 265–274. [Google Scholar] [CrossRef]
- Yıldırım, H.; Akdemir, H.; Süzerer, V.; Ozden, Y.; Onay, A. In vitro micrografting of the almond cultivars “Texas”, “Ferrastar” and “Nonpareil”. Biotechnol. Biotechnol. Equip. 2013, 27, 3493–3501. [Google Scholar] [CrossRef]
- Isıkalan, Ç.; Namli, S.; Akbas, F.; Ak, B.E. Micrografting of almond (Amygdalus communis) cultivar ‘Nonpareil’. AJCS Austral. J. Crop Sci. 2011, 5, 61–65. [Google Scholar]
- Ainsley, P.J.; Collins, G.G.; Sedgley, M. In vitro rooting of almond (Prunus dulcis Mill.). In Vitro Cell Dev. Biol. Plant 2001, 37, 778–785. [Google Scholar] [CrossRef]
- Namli, S.; Isıkalan, Ç.; Akbas, F.; Basaran, D. Improved in vitro rooting of almonds (Amygdalus communis) cultivar ‘Nonpareil’. Plant OMICS 2011, 4, 14–18. [Google Scholar]
- Köse, C.; Güleryüz, M. Effects of auxins and cytokinins on graft union of grapevine (Vitis vinifera) New Zealand. J. Crop Hortic. Sci. 2006, 34, 145–150. [Google Scholar] [CrossRef]
- Bourrain, L.; Charlot, G. In vitro micrografting of cherry (Prunus avium L. ‘Regina’) onto ‘Piku® 1’ rootstock [P. avium (P. canescens P. tomentosa)]. J. Hortic. Sci. 2014, 89, 47–52. [Google Scholar]
- Thomas, T.D. The role of activated charcoal in plant tissue culture. Biotechnol. Adv. 2008, 26, 618–631. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.; Tereso, S.; Nolasco, G.; Oliveira, M.M. Cellular location of Prune dwarf virus in Almond Sections by In Situ Reverse Transcription-Polymerase Chain Reaction. Phytopathology 2003, 93, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Yegül, M.; Baloğlu, S. Determination and characterization of several virus diseases on almond trees in the Eastern Mediterranean Region. Plant Prot. Bull. 2020, 60, 71–84. [Google Scholar]
| Rooting Rate (%) | Total Number of Roots | |
|---|---|---|
| Bitter almond | 60 | 13 |
| Canhota | 6.6 | 1 |
| MS + BAP | |||||
| N | Micrograft Length (cm) | Healing (%) | Micrograft Success (%) | Shoot Formation in the Rootstock (%) | |
| Bitter almond × Canhota | 4 | 2.75 | 100 | 100 | 100 |
| R. Zorra × R. Zorra | 5 | 1.42 | 80 | 40 | 40 |
| 2.01 ± 1.2 a | 88.9 | 62.5 | 66.67 | ||
| MS + AC | |||||
| N | Micrograft Length (cm) | Healing (%) | Micrograft Success (%) | Shoot Formation in the Rootstock (%) | |
| Bitter Almond × Canhota | 8 | 3.3 | 75 | 75 | 75 |
| R. Zorra × R. Zorra | 6 | 2.08 | 100 | 33.33 | 83.3 |
| Canhota × Canhota | 7 | 1.98 | 100 | 85.7 | 57.14 |
| G. Dura × G. Dura | 10 | 1.75 | 100 | 30 | 30 |
| 2.26 ± 1.5 a | 87.1 | 45.16 | 58.06 | ||
| MS | |||||
| N | Micrograft Length (cm) | Healing (%) | Micrograft Success (%) | Shoot Formation in the Rootstock (%) | |
| Bitter Almond × Canhota | 10 | 2.18 | 80 | 70 | 20 |
| R. Zorra × R. Zorra | 9 | 1.75 | 88.89 | 88.89 | 77.78 |
| Canhota × Canhota | 6 | 1.8 | 100 | 16.67 | 66.67 |
| G. Dura × G. Dura | 8 | 1.84 | 100 | 100 | 62.5 |
| 1.91 ± 0.4 a | 90.9 | 72.72 | 54.54 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faustino, A.; Pires, R.C.; Caeiro, S.; Rosa, A.; Marreiros, A.; Canhoto, J.; Correia, S.; Marum, L. Establishment and Optimization of Micrografting Assays with Almond (Prunus dulcis) Portuguese Varieties. Biol. Life Sci. Forum 2022, 11, 47. https://doi.org/10.3390/IECPS2021-11918
Faustino A, Pires RC, Caeiro S, Rosa A, Marreiros A, Canhoto J, Correia S, Marum L. Establishment and Optimization of Micrografting Assays with Almond (Prunus dulcis) Portuguese Varieties. Biology and Life Sciences Forum. 2022; 11(1):47. https://doi.org/10.3390/IECPS2021-11918
Chicago/Turabian StyleFaustino, Ana, Rita Costa Pires, Sandra Caeiro, Armindo Rosa, António Marreiros, Jorge Canhoto, Sandra Correia, and Liliana Marum. 2022. "Establishment and Optimization of Micrografting Assays with Almond (Prunus dulcis) Portuguese Varieties" Biology and Life Sciences Forum 11, no. 1: 47. https://doi.org/10.3390/IECPS2021-11918
APA StyleFaustino, A., Pires, R. C., Caeiro, S., Rosa, A., Marreiros, A., Canhoto, J., Correia, S., & Marum, L. (2022). Establishment and Optimization of Micrografting Assays with Almond (Prunus dulcis) Portuguese Varieties. Biology and Life Sciences Forum, 11(1), 47. https://doi.org/10.3390/IECPS2021-11918








