Different Strategies to Tolerate Salinity Involving Water Relations †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
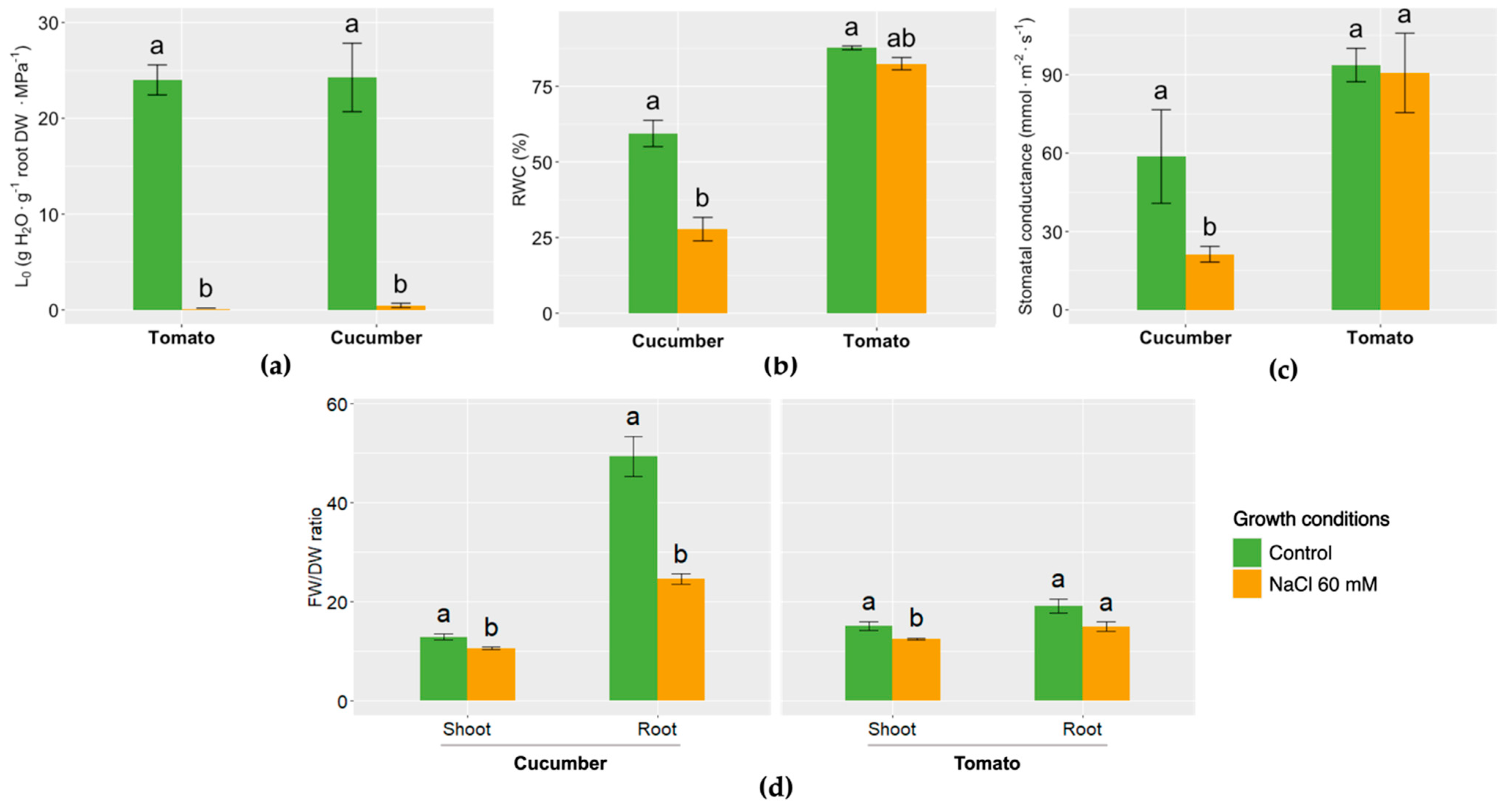
2.2. Root Hydraulic Conductance (L0)
2.3. Relative Water Content (RWC)
2.4. Stomatal Conductance
2.5. Fresh Weight and Dry Weight Ratio
2.6. Ions Concentration
2.7. Statistical Analysis
3. Results and Discussion
4. Conclusions
- The maintenance of the water balance in plants has a considerable influence on their adaptation to salinity stress.
- Tomatoes are able to resist salinity better than cucumbers, as most of the water relations in the plant are not altered.
- Membrane water transporters, like aquaporins, could have a key role in relieving the harmful effects of salinity in the plant, although more in-depth studies will be needed in order to confirm this fact.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grene, R.; Provart, N.J.; Pardo, J.M. Editorial: Resistance to Salinity and Water Scarcity in Higher Plants. Insights From Extremophiles and Stress-Adapted Plants: Tools, Discoveries and Future Prospects. Front. Plant Sci. 2019, 10, 3. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Farid, I.B.; Marghany, M.R.; Rowezek, M.M.; Sheded, M.G. Effect of Salinity Stress on Growth and MetabolomicProfiling of Cucumis sativus and Solanum lycopersicum. Plants 2020, 9, 1626. [Google Scholar] [CrossRef] [PubMed]
- Imran, Q.M.; Falak, N.; Hussain, A.; Mun, B.G.; Yun, B.W. Abiotic Stress in Plants; Stress Perception to Molecular Response and Role of Biotechnological Tools in Stress Resistance. Agronomy 2021, 11, 1579. [Google Scholar] [CrossRef]
- Chen, H.; Jiang, J.G. Osmotic adjustment and plant adaptation to environmental changes related to drought and salinity. Environ. Rev. 2010, 18, 309–319. [Google Scholar] [CrossRef]
- Fernandez-Garcia, N.; Martinez, V.; Carvajal, M. Effect of salinity on growth, mineral composition, and water relations of grafted tomato plants. J. Plant Nutr. Soil Sci. 2004, 167, 616–622. [Google Scholar] [CrossRef]
- Djanaguiraman, M.; Prasad, P.V.V. Effects of Salinity on Ion Transport, Water Relations and Oxidative Damage. In Ecophysiology and Responses of Plants under Salt Stress; Ahmad, P., Azooz, M.M., Prasad, M.N.V., Eds.; Springer: New York, NY, USA, 2013; pp. 89–114. [Google Scholar]
- Bidalia, A.; Vikram, K.; Yamal, G.; Rao, K.S. Effect of Salinity on Soil Nutrients and Plant Health; Springer-Verlag Singapore Pte Ltd.: Singapore, 2019; pp. 273–297. [Google Scholar]
- Munir, N.; Hasnain, M.; Roessner, U.; Abideen, Z. Strategies in improving plant salinity resistance and use of salinity resistant plants for economic sustainability. Crit. Rev. Environ. Sci. Technol. 2021, 47. [Google Scholar] [CrossRef]
- Alpaslan, M.; Gunes, A. Interactive effects of boron and salinity stress on the growth, membrane permeability and mineral composition of tomato and cucumber plants. Plant Soil 2001, 236, 123–128. [Google Scholar] [CrossRef]
- Chen, T.W.; Pineda, I.M.G.; Brand, A.M.; Stutzel, H. Determining Ion Toxicity in Cucumber under Salinity Stress. Agronomy 2020, 10, 677. [Google Scholar] [CrossRef]
- Aroca, R.; Amodeo, G.; Fernandez-Illescas, S.; Herman, E.M.; Chaumont, F.; Chrispeels, M.J. The role of Aquaporins and membrane damage in chilling and hydrogen peroxide induced changes in the hydraulic conductance of maize roots. Plant Physiol. 2005, 137, 341–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steudle, E.; Peterson, C. How does water get through roots? J. Exp. Bot. 1998, 49, 775–788. [Google Scholar] [CrossRef]
- González, L.; González-Vilar, M. Determination of Relative Water Content. In Handbook of Plant Ecophysiology Techniques; Reigosa Roger, M.J., Ed.; Springer: Dordrecht, The Netherlands, 2001; pp. 207–212. [Google Scholar]
- Chaumont, F.; Tyerman, S. Plant Aquaporins: From Transport to Signaling; Springer: Cham, Switzerland, 2017; pp. 1–353. [Google Scholar]
- Buchanan, B.; Gruissem, W.; Jones, R. Biochemistry & Molecular Biology of Plants, 2nd ed.; Wiley Blackwell: Chichester, UK, 2015; p. 1264. [Google Scholar]
- Chaumont, F.; Tyerman, S. Aquaporins: Highly Regulated Channels Controlling Plant Water Relations. Plant Physiol. 2014, 164, 1600–1618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afzal, Z.; Howton, T.; Sun, Y.; Mukhtar, M. The Roles of Aquaporins in Plant Stress Responses. J. Dev. Biol. 2016, 4, 9. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez-Alonso, A.; Carvajal, M.; Barzana, G. Different Strategies to Tolerate Salinity Involving Water Relations. Biol. Life Sci. Forum 2022, 11, 41. https://doi.org/10.3390/IECPS2021-12035
Martinez-Alonso A, Carvajal M, Barzana G. Different Strategies to Tolerate Salinity Involving Water Relations. Biology and Life Sciences Forum. 2022; 11(1):41. https://doi.org/10.3390/IECPS2021-12035
Chicago/Turabian StyleMartinez-Alonso, Alberto, Micaela Carvajal, and Gloria Barzana. 2022. "Different Strategies to Tolerate Salinity Involving Water Relations" Biology and Life Sciences Forum 11, no. 1: 41. https://doi.org/10.3390/IECPS2021-12035
APA StyleMartinez-Alonso, A., Carvajal, M., & Barzana, G. (2022). Different Strategies to Tolerate Salinity Involving Water Relations. Biology and Life Sciences Forum, 11(1), 41. https://doi.org/10.3390/IECPS2021-12035






