Abstract
In bipartite ecological networks involving higher and lower trophic levels, these components are a reflection of community interaction. The present investigation of butterflies and exotic nectar plant communities across the Darjeeling district of West Bengal, India, is a significant event in generating awareness for the protection of such plant communities. Analysis of these bipartite networks characterizing butterfly-plant community interaction could help in elaborating different aspects of species assemblage. Different indices (based on unweighed links and weighed links) were used for the exploration of such networks. A total of 28 exotic plant species served as nectaring resources for 44 butterfly species. Some ecologically significant descriptors of this network include the network dimension (no. of species in higher trophic level: 44; no. of species in lower trophic level: 28), links per species (1.042), connectance (0.061) and network asymmetry (−0.222), generality (3.608), vulnerability (3.166), linkage density (3.387) and Shannon’s evenness of network interaction (0.441). Thus, the above predictions provide a probable clue to the involvement of exotic plant species in the maintenance of community structure. Significantly, exotic plants serve as key service providers to a community’s pollinator assemblages, thereby attempting to fill up an otherwise “empty coevolutionary niche”.
1. Introduction
The feeding relationship between different individuals belonging to a particular species usually involves a complex network of food web series. Although food webs are occasionally compartmentalised by habitats, they, however, retain connections to interact with each other [1,2]. Significantly, compartmentalisation within food webs helps in creating mutually beneficial, heterogeneous, nestedness within such webs. Mutualistic network theory is centred on animal-mediated pollination with an integrative approach highlighting trophic level interaction and reproductive preferences [3,4]. Mutualistic networks are found to be compartmentalised or modular with a group of species, well connected amongst themselves but weakly connected with others within the same network [5]. Interesting observations have revealed the structure and co-evolutionary dynamics within ecological networks [6,7,8,9,10,11]. Interaction networks are considered to be a direct indicator of habitat conditions altering trophic interactions [12,13,14]. Among pollination networks, specialised species associate with few species in contrast to generalist species interacting with many [15]. Significantly the occurrence of exotic plants may influence the availability and utilisation of nectar and pollen resources of pollinators over the entire season, which in turn directly influence the network properties [16]. Thus, such interactions in the network could be considered to be a suitable tool for monitoring the community-wide influence of exotic plant species on pollinators [17,18,19,20].
Therefore, the major objectives of the present study are to address the contribution of exotic nectar plant species of butterflies in determining the plant-pollinator network structure. In this regard, the present study will also investigate the different exotic nectaring plant species of the adult butterfly community across the Darjeeling district of West Bengal, India. The construction and analysis of the bipartite network characterising butterfly-nectaring plant resources and its relevant role in species assemblages were investigated. Several ecologically relevant descriptors of such network structure (links per species, connectance, network asymmetry, generality, vulnerability, linkage density and Shannon’s evenness of network interaction) were also analysed.
2. Materials and Methods
Study Area: The entire study was conducted across the montane broad-leaved temperate forests in the Darjeeling district, West Bengal, India. Sampling was undertaken at forest patches during the study period (July 2020–June 2021).
2.1. Sampling Design
The sampling for butterfly visitation of nectar plant species at different quadrates was undertaken weekly for a month with the help of two trained field assistants. The sampling procedure was repeated at an interval of seven days. Plants were identified from published literature [21,22,23,24] along with assistance from the plant taxonomist as and when required. Specific observations were made on each plant species visited by butterflies.
The observation of the nectar plants was carried out twice a day (i.e., between 1000 and 1300 h in the morning and 1400–1600 h in the afternoon). The flower visitors were observed for 5–8 min per flowering, standing at a distance of 1.5 m. Nectar probing by species was ascertained from the moment of inserting the proboscis in the corolla till the end of its withdrawal.
The butterflies were observed (using Bushnell binoculars) and photographed (using Nikon COOLPIX-P90) occasionally for identification from published literature [24,25].
2.2. Data Analysis
A bipartite graph depicting the association between lower trophic level species and higher trophic level species was produced. Such a mutualistic relationship was represented by connecting links between two trophic nodes. Several different indices based on weighed links and unweighed links were investigated.
(a) Indices based on weighted links (Quantitative webs):
- (i)
- Generality: It represents the mean number of prey species per predator. In the case of weighted links, Hj, the Shannon diversity of interactions for predator species j has been calculated as follows [26]:Hj = −Σ[(aij/Aj) × ln(aij/Aj)]
- (ii)
- Vulnerability: It represents the mean number of predator species per prey. In the case of weighted links, Hi, the Shannon diversity of interactions for prey species i is calculated as follows [26]:Hi = −Σ[(aji/Ai) × ln(aji/Ai)]
- (iii)
- Weighted Linkage density: Since the generality and vulnerability are known. The weighted linkage density (Lq) is obtained as their arithmetic mean. In this case, Lq is calculated as follows [27]:Lq = 0.5(Hj + Hi)
- (iv)
- Interaction Evenness: Shannon’s evenness of network interactions has been calculated as follows [28]:
(b) Indices based on unweighted links (Quanlitative webs):
- (i)
- Links per species: The mean number of links per species has been calculated as follows [28]:Lx (mean) = l/(I + J)
- (ii)
- Connectance: Is represented by the realised proportion of possible links. Connectance (C) has been expressed as [29]:C = L/(IJ)
- (iii)
- Web asymmetry: It denotes a balance between the number of species in two trophic levels. Web asymmetry has been calculated as follows [30]:W = (I − J)/(I + J)
3. Results
A total of 28 exotic plant species served as nectaring resources for 44 butterfly species across the entire study site. Butterfly species representing five subfamilies i.e., Nymphalidae: 50%, Lycaenidae:18.18%, Papilionidae: 15.91%, Pieridae:11.36%, Hesperiidae:4.54% were reported. Further investigations into this bipartite network revealed the existence of 75 connecting links between 28 lower trophic level species and 44 higher trophic level species (Figure 1).
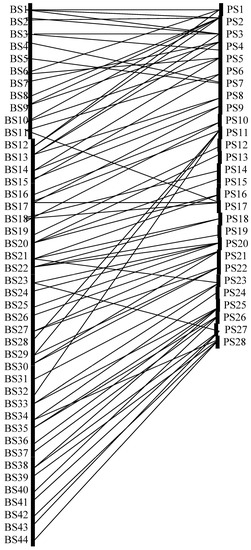
Figure 1.
Bipartite graph representing two trophic levels (i.e., butterfly species expressed as BS and nectaring plant species expressed as PS). where: BS1: Tirumala limniace, BS2: T. septentrionis, BS3: Danaus genutia, BS4: D. melanippus, BS5: D. chrysippus, BS6: Parantica aglea. BS7: Euploea sylvestor, BS8: E. mulciber, BS9: E. core, BS10: Ypthima baldus, BS11: Y. sacra, BS12: Arceea violae, BS13: Argyreus hyperbius, BS14: Cethosia cyane BS15:Athyma perius, BS16: Ariadne merione, BS17: Vanessa indica, BS18: V. cardui, BS19: Aglaia cashmirensis, BS20: Junonia orithiya, BS21: J. atlites, BS22: Hypolimnas misippus, BS 23: Deudorix epijarbas, BS24: Rapala manae, BS25: Spindasis lohita, BS26: Lycaena phlaeas, BS27: Heliophorus brahma, BS28: Prosatas nora, BS29: Zizeeria karsandra, BS30: Pseudozizeeria maha, BS31: Sarangosa dasahara, BS32: Pseudoborbo bevani, BS33: Eurema hecabe, BS34: Catopsilia pyranthe, BS35: Appias lyncida, BS36: Pieris brassicae, BS37: Delias eucharis, BS38: Graphium sarpedon, BS39: G. agamemnon, BS40: Papilio helenus, BS41: P. polytes, BS42: P. polymnestor, BS43: P. paris, BS44: Aristolochia hector. PS1: Ageratum haustomiianum, PS2: Anemone japonica, PS3: Argemone mexicana, PS4: Asclepias curassavica, PS5: Cassia siamea, PS6: Catharanthus roseus, PS7: Chenopodium ambrosoides, PS8: Cleome rutidosperma, PS9: Coronopus didymus, PS10: Crassocephalum crepidiodes, PS11: Croton bonplandianus, PS12: Drymaria villosa, PS13: Erigeron karwinskianus, PS14: Eupatorium adenophorum, PS15: E. ligustrinum, PS16: Galinsoga parviflora, PS17: G. quadrifolia, PS18: Ipomoea purpurea, PS19: Kerria japonica, PS20: Lepidium sativum, PS21: Linoria cymbalaria, PS22: Mikania micrantha, PS23: Passiflora foetida, PS24: Peperomia pellucida, PS25: Ranunuculus repens, PS26: Setaria geniculata, PS27: Tridax procumbens, PS28: Veronica persica.
Tirumala limniace, Argyreus hyperbius, Vanessa indica, V. cardui, Junonia orithiya, J. atlites, Hypolimnas misippus, displayed a maximum of three connecting links with their respective nectar plants. In contrast, Anemone japonica was associated with a maximum of six butterfly species. Additionally, Argemone mexicana, Asclepias curassavica, Croton bonplandianus, Lepidium sativum, Ranunuculus repens, Tridax procumbens and Veronica persica showed linkage with four butterfly species (Figure 1).
The mean number of links per species in the above bipartite mutualistic interaction network was 1.042. The network connectance of the above plant-pollinator network was found to be 0.061 (6.10%). Additionally, the web asymmetry of the studied network was −0.222, with negative values being an indicator of the assemblages of higher trophic level species. Values of indices based on weighted links (quantitative webs) were also obtained by analysing the above mutualistic plant-pollinator network (Table 1). Generality (Shannon diversity of interaction for predator/butterfly species) was 3.608. Additionally, vulnerability (Shannon’s diversity of interaction for prey species/exotic nectar plant species) was 3.166. Therefore, the weighted linkage density was deduced as 3.387. Finally, Shannon’s evenness of this network interaction was found to be 0.441 (Table 1).

Table 1.
Table indicating different values of network indices.
4. Discussion
The construction of the above mutualistic bipartite interactive network helps to highlight the significance of nectaring exotic plant resources of butterflies in the Himalayan landscape of Darjeeling, West Bengal, India. Analysis of this bipartite network generated new network indices [26,31], which in turn explored different patterns among them [15,32,33,34]. This, in turn, also contributed to the ongoing evolutionary processes affecting different communities [35,36,37].
Dual trophic level species interaction in bipartite networks appears to be highly unstable both on temporal and spatial scales [38,39]. The present study emphasizes that for areas where the utilization of exotic plants as nectaring resources occurs, this could probably be driven by a need to explore such resources, as the native plant species have been replaced by exotic invasive species. One possible reason behind the switching over of preference towards exotic plant resources could be the greater abundance of predator/butterfly species as displayed by higher values of “generality” as compared to “vulnerability”. Such dynamism in switching over species preference could probably explain the asymmetrical food web (as denoted by negative values) due to a greater number of higher trophic level members. Such an asymmetry in the plant-pollinator network could probably be attributed to the presence of specialist species, a common phenomenon as observed by previous studies [3,34].
Several studies have hypothesized that species abundance and morphological traits are the key factors of a mutualistic network [40,41]. It could also be assumed that the utilization of exotic or introduced plants could lead to the homogenization of butterfly fauna. Such exotic plants (ornamental or cultivated plants) have also facilitated the entry of butterflies into previously unexplored regions [42]. Such supplementation of the flowering native plants by the exotic resources (pollen and nectar) could help enrich the plant-pollinator integration network further [43,44].
Author Contributions
Conceptualization and Methodology by N.G. Entire work and preparation of manuscript by P.S. Interpretation of results by N.G. & P.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not Applicable.
Informed Consent Statement
Not Applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are highly grateful to the faculty members of the Department of Zoology, West Bengal State University, Barasat, for their help and support. The individuals mentioned here have also consented to the acknowledgement.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dicks, L.V.; Corbet, S.A.; Pywell, R.F. Compartmentalization in plant-insect-flower visitor webs. J. Anim. Ecol. 2002, 71, 32–43. [Google Scholar] [CrossRef]
- Eichhorn, M.P. Natural Systems: The Organisation of Life; John Wiley, Blackwell & Sons: Hoboken, NJ, USA, 2016; p. 368. [Google Scholar]
- Bascompte, J.; Jordano, P.; Melian, C.J.; Olesen, J.M. The nested assembly of plant-animal mutualistic networks. Proc. Natl. Acad. Sci. USA 2003, 100, 9383–9387. [Google Scholar] [CrossRef] [PubMed]
- Valdovinos, F.S. Mutualistic networks: Moving closer to a predictive theory. Ecol. Lett. 2019, 22, 1517–1534. [Google Scholar] [CrossRef] [PubMed]
- Olesen, J.M.; Bascompte, J.; Dupont, Y.; Jordano, P. The modularity of pollination networks. Proc. Natl. Acad. Sci. USA 2007, 104, 19891–19896. [Google Scholar] [CrossRef]
- Bascompte, J.; Jordano, P. Plant-animal mutualistic networks: The architecture of biodiversity. Annu. Rev. Ecol. Syst. 2007, 38, 567–593. [Google Scholar] [CrossRef]
- Ebeling, A.; Klein, A.-M.; Tscharntke, T. Plant-flower visitor interaction webs: Temporal stability and pollinator specialization increases along an experimental plant diversity gradient. Basic Appl. Ecol. 2011, 12, 300–309. [Google Scholar] [CrossRef]
- Allesina, S.; Tang, S. Stability criteria for complex ecosystems. Nature 2012, 483, 205–208. [Google Scholar] [CrossRef]
- Nuismer, S.L.; Jordano, P.; Bascompte, J. Coevolution and the architecture of mutualistic networks. Evolution 2013, 67, 338–354. [Google Scholar] [CrossRef]
- Suweis, S.; Simini, F.; Banavar, J.; Maritan, A. Emergence of structural and dynamical properties of ecological mutualistic networks. Nature 2013, 500, 449–452. [Google Scholar] [CrossRef]
- James, A.; Plank, M.; Rossberg, A.; Beecham, J.; Emmerson, M.; Pitchford, J. Constructing random matrices to represent real ecosystems. Am. Nat. 2015, 185, 680–692. [Google Scholar] [CrossRef]
- Ballantyne, G.; Baldock, K.C.R.; Willmer, P.G. Constructing more informative plant-pollinator networks: Visitation and pollen deposition networks in a heathland plant community. Proc. R. Soc. Lond. 2015, 282, 20151130. [Google Scholar] [CrossRef]
- Staab, M.; Blüthgen, N.; Klein, A.M. Tree diversity alters the structure of a tri-trophic network in a biodiversity experiment. Oikos 2015, 124, 827–834. [Google Scholar] [CrossRef]
- Pereira-Peixoto, M.H.; Pufal, G.; Staab, M.; Martins, C.; Klein, A.M. Diversity and specificity of host-natural enemy interactions in an urban-rural interface. Ecol. Entomol. 2016, 41, 241–252. [Google Scholar] [CrossRef]
- Jordano, P.; Bascompte, J.; Olesen, J.M. Invariant properties in coevolutionary networks of plant-animal interactions. Ecol. Lett. 2003, 6, 69–81. [Google Scholar] [CrossRef]
- Staab, M.; Pereira-Peixoto, M.H.; Klein, A.-M. Exotic garden plants partly substitute for native plants as resources for pollinators when native plants become seasonally scarce. Oecologia 2020, 194, 465–480. [Google Scholar] [CrossRef]
- Memmott, J.; Waser, N.M. Integration of alien plants into a native flower pollinator visitation web. Proc. R. Soc. Lond. 2002, 269, 2395–2399. [Google Scholar] [CrossRef]
- Bartomeus, I.; Vilà, M.; Santamaría, L. Contrasting effects of invasive plants in plant-pollinator networks. Oecologia 2008, 155, 761–770. [Google Scholar] [CrossRef]
- Valdovinos, F.S.; Ramos-Jiliberto, R.; Flores, J.D.; Espinoza, C.; Lopez, G. Structure and dynamics of pollination networks: The role of alien plants. Oikos 2009, 118, 1190–1200. [Google Scholar] [CrossRef]
- Lowenstein, D.M.; Matteson, K.C.; Minor, E.S. Evaluating the dependence of urban pollinators on ornamental, non-native, and weedy floral resources. Urban Ecosyst. 2019, 22, 293–302. [Google Scholar] [CrossRef]
- Cowan, A.; Cowan, J.M. The Trees of Northern Bengal Including Shrubs, Woody Climbers, Bamboos, Palms and Tree Ferns; Naaz Offset Press: Delhi, India, 1979; p. 178. [Google Scholar]
- Polunin, O.; Stainton, A. Flowers of the Himalaya; Seventh Impression; Oxford University Press: New Delhi, India, 2005; p. xxx+580. [Google Scholar]
- Maity, D.; Maiti, G.G. The Wild Flowers of Kanchenjunga Biosphere Reserve, Sikkim; Naya Udyog: Kolkata, India, 2007; p. 174. [Google Scholar]
- Kehimkar, I. The Book of Indian Butterflies Bombay Natural History Society; Oxford University Press: New Delhi, India, 2008; p. xvi+497. [Google Scholar]
- Haribal, M. The Butterflies of Sikkim Himalaya and Their Natural History; Sikkim Natural Conservation Foundation: Gangtok, India, 1992; p. 217. [Google Scholar]
- Bersier, L.F.; Banasek-Richter, C.; Cattin, M.F. Quantitative descriptors of food-web matrices. Ecology 2002, 83, 2394–2407. [Google Scholar] [CrossRef]
- Tylianakis, J.M.; Tscharntke, T.; Lewis, O.T. Habitat modification alters the structure of tropical host-parasitoid food webs. Nature 2007, 445, 202–205. [Google Scholar] [CrossRef]
- Dormann, C.F.; Fründ, J.; Blüthgen, N.; Gruber, B. Indices, Graphs and Null Models: Analyzing Bipartite Ecological Networks. Open Ecol. J. 2009, 2, 7–24. [Google Scholar] [CrossRef]
- Gotelli, N.J.; Graves, G.R. Null Models in Ecology; Smithsonian Institution Press: Washington, DC, USA, 1996. [Google Scholar]
- Blüthgen, N.; Menzel, F.; Hovestadt, T.; Fiala, B.; Blüthgen, N. Specialization, constraints and conflicting interests in mutualistic networks. Curr. Biol. 2007, 17, 1–6. [Google Scholar] [CrossRef]
- Blüthgen, N.; Menzel, F.; Blüthgen, N. Measuring specialization in species interaction networks. BMC Ecol. 2006, 6, 12. [Google Scholar] [CrossRef][Green Version]
- Dunne, J.A.; Williams, R.J.; Martinez, N.D. Food-web structure and network theory: The role of connectance and size. Proc. Natl. Acad. Sci. USA 2002, 99, 12917–12922. [Google Scholar] [CrossRef]
- Olesen, J.M.; Jordano, P. Geographic patterns in plant-pollinator mutualistic networks. Ecology 2002, 83, 2416–2424. [Google Scholar]
- Vázquez, D.P.; Aizen, M.A. Asymmetric specialization: A pervasive feature of plant-pollinator interactions. Ecology 2004, 85, 1251–1257. [Google Scholar] [CrossRef]
- Lewinsohn, T.M.; Prado, P.I.; Jordano, P.; Bascompte, J.; Olesen, J.M. Structure in plant-animal interaction assemblages. Oikos 2006, 113, 174–184. [Google Scholar] [CrossRef]
- Rezende, E.L.; Lavebre, J.E.; Guimarães, P.R.; Jordano, P.; Bascompte, J. Non-random coextinctions in phylogenetically structured mutualistic networks. Nature 2007, 448, 925–928. [Google Scholar] [CrossRef] [PubMed]
- Mouillot, D.; Krasnov, B.R.; Shenbrot, G.I.; Poulin, R. Connectance and parasite diet breadth in flea-mammal webs. Oikos 2008, 31, 16–20. [Google Scholar] [CrossRef]
- Whittall, J.B.; Hodges, S.A. Pollinator shifts drive increasingly long nectar spurs in columbine flowers. Nature 2007, 447, 706–709. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.N.; Laine, A.L.; Thompson, J.F. Retention of mutualism in a geographically diverging interaction. Ecol. Lett. 2010, 13, 1368–1377. [Google Scholar] [CrossRef] [PubMed]
- Ollerton, J.; Johnson, S.D.; Cranmer, L.; Kellie, S. The pollination ecology of an assemblage of grassland asclepiads in South Africa. Ann. Bot. 2003, 92, 807–834. [Google Scholar] [CrossRef] [PubMed]
- Olesen, J.M.; Bascompte, J.; Elberling, H.; Jordano, P. Temporal dynamics in a pollination network. Ecology 2008, 89, 1573–1582. [Google Scholar] [CrossRef]
- Graves, S.D.; Shapiro, A.M. Exotics as host plants of the California butterfly fauna. Biol. Conserv. 2003, 110, 413–433. [Google Scholar] [CrossRef]
- Martins, K.T.; Gonzalez, A.; Lechowicz, M.J. Patterns of pollinator turnover and increasing diversity associated with urban habitats. Urban Ecosyst. 2017, 20, 1359–1371. [Google Scholar] [CrossRef]
- Buchholz, S.; Kowarik, I. Urbanisation modulates plant-pollinator interactions in invasive vs. native plant species. Sci. Rep. 2019, 9, 6375. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).