Physicochemical Characterization and Effect of Additives of Membrane Vesicles from Brassica oleracea L. to Be Used in Nanofertilization †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Membrane Vesicles Isolation
2.3. Particle Size, Zeta Potential, and Polydispersity Index Analysis of Membrane Vesicles
2.4. Samples Preparation
2.5. Stopped-Flow Light Scattering
2.6. Statistical Analysis
3. Results
3.1. Physico-Chemical Characterization
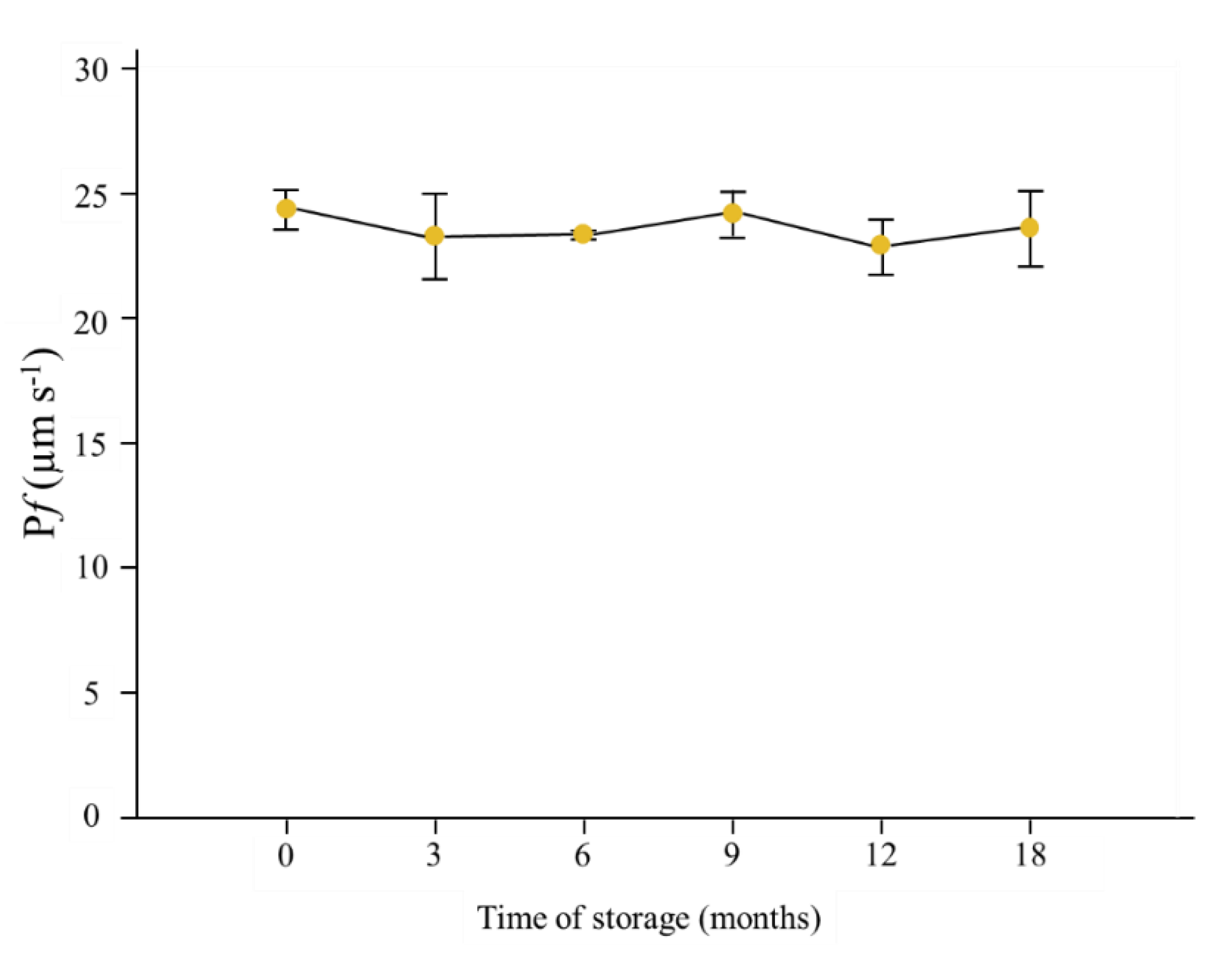
3.2. Stability of Vesicles Functionality over Time
3.3. Effect of Surfactant in Membrane Vesicle Functionallity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, X.; Davidson, E.A.; Mauzerall, D.L.; Searchinger, T.D.; Dumas, P.; Shen, Y. Managing nitrogen for sustainable development. Nature 2015, 528, 51–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, R.P.; Handa, R.; Manchanda, G. Nanoparticles in sustainable agriculture: An emerging opportunity. J. Control. Release 2021, 329, 1234–1248. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Liu, C.; Huang, M.; Liu, K.; Yan, D. Effects of Foliar Fertilization: A Review of Current Status and Future Perspectives. J. Soil Sci. Plant Nutr. 2021, 21, 104–118. [Google Scholar] [CrossRef]
- Karny, A.; Zinger, A.; Kajal, A.; Shainsky-Roitman, J.; Schroeder, A. Therapeutic nanoparticles penetrate leaves and deliver nutrients to agricultural crops. Sci. Rep. 2018, 8, 7589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramsey, R.J.L.; Stephenson, G.R.; Hall, J.C. A review of the effects of humidity, humectants, and surfactant composition on the absorption and efficacy of highly water-soluble herbicides. Pestic. Biochem. Physiol. 2005, 82, 162–175. [Google Scholar] [CrossRef]
- Rios, J.J.; Yepes-Molina, L.; Martinez-Alonso, A.; Carvajal, M. Nanobiofertilization as a novel technology for highly efficient foliar application of Fe and B in almond trees. R. Soc. Open Sci. 2020, 7, 200905. [Google Scholar] [CrossRef] [PubMed]
- Fanourakis, D.; Giday, H.; Milla, R.; Pieruschka, R.; Kjaer, K.H.; Bolger, M.; Vasilevski, A.; Nunes-Nesi, A.; Fiorani, F.; Ottosen, C.O. Pore size regulates operating stomatal conductance, while stomatal densities drive the partitioning of conductance between leaf sides. Ann. Bot. 2015, 115, 555–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rios, J.J.; Garcia-Ibañez, P.; Carvajal, M. The use of biovesicles to improve the efficiency of Zn foliar fertilization. Colloids Surf. B Biointerfaces 2019, 173, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Allahou, L.W.; Madani, S.Y.; Seifalian, A. Investigating the Application of Liposomes as Drug Delivery Systems for the Diagnosis and Treatment of Cancer. Int. J. Biomater. 2021, 2021, 3041969. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Karsch-Bluman, A.; Avraham, S.; Assayag, M.; Schwob, O.; Benny, O. Encapsulated carbenoxolone reduces lung metastases. Cancers 2019, 11, 1383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maurel, C.; Tacnet, F.; Güclü, J.; Guern, J.; Ripoche, P. Purified vesicles of tobacco cell vacuolar and plasma membranes exhibit dramatically different water permeability and water channel activity. Proc. Natl. Acad. Sci. USA 1997, 94, 7103–7108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eichert, T.; Kurtz, A.; Steiner, U.; Goldbach, H.E. Size exclusion limits and lateral heterogeneity of the stomatal foliar uptake pathway for aqueous solutes and water-suspended nanoparticles. Physiol. Plant. 2008, 134, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Yepes-Molina, L.; Martínez-Ballesta, M.C.; Carvajal, M. Plant plasma membrane vesicles interaction with keratinocytes reveals their potential as carriers. J. Adv. Res. 2020, 23, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Yepes-Molina, L.; Hernández, J.A.; Carvajal, M. Nanoencapsulation of Pomegranate Extract to Increase Stability and Potential Dermatological Protection. Pharmaceutics 2021, 13, 271. [Google Scholar] [CrossRef] [PubMed]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milani, D.; Athiyah, U.; Hariyadi, D.M.; Pathak, Y. V Surface Modifications of Liposomes for Drug Targeting. In Surface Modification of Nanoparticles for Targeted Drug Delivery; Springer International Publishing: Cham, Switzerland, 2019; pp. 207–220. [Google Scholar]
- Martínez-Ballesta, M.d.C.; García-Gomez, P.; Yepes-Molina, L.; Guarnizo, A.L.; Teruel, J.A.; Carvajal, M. Plasma membrane aquaporins mediates vesicle stability in broccoli. PLoS ONE 2018, 13, e0192422. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Applied Surfactant in Membrane Vesicles | Pf (µm s−1) |
|---|---|
| Control | 22.5 ± 2.8 |
| 1% Tween-20 | 21.8 ± 1.3 |
| 2% Tween-20 | 23.6 ± 2.4 |
| 1% PEG | 25.4 ± 5.0 |
| 2% PEG | 22.8 ± 3.1 |
| 0.1% PMP | 15.0 ± 1.2 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yepes-Molina, L.; Ríos, J.J.; Carvajal, M. Physicochemical Characterization and Effect of Additives of Membrane Vesicles from Brassica oleracea L. to Be Used in Nanofertilization. Biol. Life Sci. Forum 2022, 11, 21. https://doi.org/10.3390/IECPS2021-11954
Yepes-Molina L, Ríos JJ, Carvajal M. Physicochemical Characterization and Effect of Additives of Membrane Vesicles from Brassica oleracea L. to Be Used in Nanofertilization. Biology and Life Sciences Forum. 2022; 11(1):21. https://doi.org/10.3390/IECPS2021-11954
Chicago/Turabian StyleYepes-Molina, Lucía, Juan José Ríos, and Micaela Carvajal. 2022. "Physicochemical Characterization and Effect of Additives of Membrane Vesicles from Brassica oleracea L. to Be Used in Nanofertilization" Biology and Life Sciences Forum 11, no. 1: 21. https://doi.org/10.3390/IECPS2021-11954
APA StyleYepes-Molina, L., Ríos, J. J., & Carvajal, M. (2022). Physicochemical Characterization and Effect of Additives of Membrane Vesicles from Brassica oleracea L. to Be Used in Nanofertilization. Biology and Life Sciences Forum, 11(1), 21. https://doi.org/10.3390/IECPS2021-11954








