Gradual Replacement of Soybean Meal with Brewer’s Yeast in Fingerling Nile Tilapia (Oreochromis niloticus) Diet, Resulting in a Polynomial Growth Pattern, Independent of Whether Reared in a Biofloc or Clear-Water System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Conditions
2.2. Experimental Diets
2.3. Measurements and Calculations
2.4. Water Quality and Biofloc Monitoring
2.5. Chemical Analysis
2.6. Statistical Analyses
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bohlke, R.A.; Thaler, R.C.; Stein, H.H. Calcium, phosphorus, and amino acid digestibility in low-phytate corn, normal corn, and soybean meal by growing pigs. J. Anim. Sci. 2005, 83, 2396–2403. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.L.; Lu, L.; Li, S.F.; Luo, X.G.; Liu, B. Relative bioavailabilities of organic zinc sources with different chelation strengths for broilers fed a conventional corn-soybean meal diet. J. Anim. Sci. 2009, 87, 2038–2046. [Google Scholar] [CrossRef] [PubMed]
- Hammond, B.G.; Vicini, J.L.; Hartnell, G.F.; Naylor, M.W.; Knight, C.D.; Robinson, E.H.; Fuchs, R.L.; Padgette, S.R. The feeding value of soybeans fed to rats, chickens, catfish and dairy cattle is not altered by genetic incorporation of glyphosate tolerance. J. Nutr. 1996, 126, 717–727. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, A.-F.M. Alternative dietary protein sources for farmed tilapia, Oreochromis spp. Aquaculture 1999, 179, 149–168. [Google Scholar] [CrossRef]
- Naylor, R.L.; Liska, A.J.; Burke, M.B.; Falcon, W.P.; Gaskell, J.C.; Rozelle, S.D.; Cassman, K.G. The ripple effect: Biofuels, food security, and the environment. Environ. Sci. Policy Sustain. Dev. 2007, 49, 30–43. [Google Scholar] [CrossRef]
- Fearnside, P.M. Soybean cultivation as a threat to the environment in Brazil. Environ. Conserv. 2001, 28, 23–38. [Google Scholar] [CrossRef]
- Day, L. Proteins from land plants—Potential resources for human nutrition and food security. Trends Food Sci. Technol. 2013, 32, 25–42. [Google Scholar] [CrossRef]
- Barros, M.M.; Lim, C.; Klesius, P.H. Effect of soybean meal replacement by cottonseed meal and iron supplementation on growth, immune response and resistance of Channel Catfish (Ictalurus puctatus) to Edwardsiella ictaluri challenge. Aquaculture 2002, 207, 263–279. [Google Scholar] [CrossRef]
- Yue, Y.-R.; Zhou, Q.-C. Effect of replacing soybean meal with cottonseed meal on growth, feed utilization, and hematological indexes for juvenile hybrid tilapia, Oreochromis niloticus × O. aureus. Aquaculture 2008, 284, 185–189. [Google Scholar] [CrossRef]
- National Research Council, Bord on Agriculture. Subcommittee on Fish Nutrition. In Nutrient Requirements of Fish; National Academies Press: Washington, DC, USA, 1993; p. 114. [Google Scholar]
- White, L.A.; Newman, M.C.; Cromwell, G.L.; Lindemann, M.D. Brewers dried yeast as a source of mannan oligosaccharides for weanling pigs. J. Anim. Sci. 2002, 80, 2619–2628. [Google Scholar] [CrossRef]
- Jin, M.; Xiong, J.; Zhou, Q.-C.; Yuan, Y.; Wang, X.-X.; Sun, P. Dietary yeast hydrolysate and brewer’s yeast supplementation could enhance growth performance, innate immunity capacity and ammonia nitrogen stress resistance ability of Pacific white shrimp (Litopenaeus vannamei). Fish Shellfish Immunol. 2018, 82, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.-Y.; Jiang, G.-Z.; Wang, C.-C.; Abasubong, K.P.; Zou, Q.; Zhou, Y.-Y.; Liu, W.-B. Effects of partial replacement of fish meal by yeast hydrolysate on antioxidant capability, intestinal morphology, and inflammation-related gene expression of juvenile Jian carp (Cyprinus carpio var. Jian). Fish Physiol. Biochem. 2018, 45, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Burr, G.; Hume, M.; Ricke, S.; Nisbet, D.; Gatlin, D. A preliminary in vitro assessment of GroBiotic®-A, brewer’s yeast and fructooligosaccharide as prebiotics for the red drum Sciaenops ocellatus. J. Environ. Sci. Health Part B 2008, 43, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Ortuño, J.; Cuesta, A.; Rodríguez, A.; Esteban, M.A.; Meseguer, J. Oral administration of yeast, Saccharomyces cerevisiae, enhances the cellular innate immune response of gilthead seabream (Sparus aurata L.). Vet. Immunol. Immunopathol. 2002, 85, 41–50. [Google Scholar] [CrossRef]
- Siwicki, A.K.; Anderson, D.P.; Rumsey, G.L. Dietary intake of immunostimulants by rainbow trout affects non-specific immunity and protection against furunculosis. Vet. Immunol. Immunopathol. 1994, 41, 125–139. [Google Scholar] [CrossRef]
- Zhou, M.; Liang, R.; Mo, J.; Yang, S.; Gu, N.; Wu, Z.; Sarath Babu, V.; Li, J.; Huang, Y.; Lin, L. Effects of brewer’s yeast hydrolysate on the growth performance and the intestinal bacterial diversity of largemouth bass (Micropterus salmoides). Aquaculture 2018, 484, 139–144. [Google Scholar] [CrossRef]
- Abass, D.A.; Obirikorang, K.A.; Campion, B.B.; Edziyie, R.E.; Skov, P.V. Dietary supplementation of yeast (Saccharomyces cerevisiae) improves growth, stress tolerance, and disease resistance in juvenile Nile tilapia (Oreochromis niloticus). Aquac. Int. 2018, 26, 843–855. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M.; Abdel-Rahman, A.M.; Ismael, N.E. Evaluation of commercial live bakers’ yeast, Saccharomyces cerevisiae as a growth and immunity promoter for Fry Nile tilapia, Oreochromis niloticus (L.) challenged in situ with Aeromonas hydrophila. Aquaculture 2008, 280, 185–189. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M.; Mousa, M.A.; Mohammed, M.A. Use of live baker’s yeast, Saccharomyces cerevisiae, in practical diet to enhance the growth performance of Galilee tilapia, Sarotherodon galilaeus (L.), and its resistance to environmental copper toxicity. J. World Aquac. Soc. 2010, 41, 214–223. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Mirvaghefi, A.; Merrifield, D.L. The effects of dietary inactive brewer’s yeast Saccharomyces cerevisiae var. ellipsoideus on the growth, physiological responses and gut microbiota of juvenile beluga (Huso huso). Aquaculture 2011, 318, 90–94. [Google Scholar] [CrossRef]
- Li, P.; Gatlin Iii, D.M. Evaluation of brewers yeast (Saccharomyces cerevisiae) as a feed supplement for hybrid striped bass (Morone chrysops×M. saxatilis). Aquaculture 2003, 219, 681–692. [Google Scholar] [CrossRef]
- Li, P.; Gatlin Iii, D.M. Dietary brewers yeast and the prebiotic Grobiotic™ AE influence growth performance, immune responses and resistance of hybrid striped bass (Morone chrysops × M. saxatilis) to Streptococcus iniae infection. Aquaculture 2004, 231, 445–456. [Google Scholar] [CrossRef]
- Jarmołowicz, S.; Rożyński, M.; Kowalska, A.; Zakęś, Z. Growth in juvenile pikeperch (Sander lucioperca L.) stimulated with yeast, Saccharomyces cerevisiae, extract. Aquac. Res. 2018, 49, 614–620. [Google Scholar] [CrossRef]
- Lara-Flores, M.; Olvera-Novoa, M.A.; Guzmán-Méndez, B.E.; López-Madrid, W. Use of the bacteria Streptococcus faecium and Lactobacillus acidophilus, and the yeast Saccharomyces cerevisiae as growth promoters in Nile tilapia (Oreochromis niloticus). Aquaculture 2003, 216, 193–201. [Google Scholar] [CrossRef]
- Nhi, N.H.Y.; Da, C.T.; Lundh, T.; Lan, T.T.; Kiessling, A. Comparative evaluation of Brewer’s yeast as a replacement for fishmeal in diets for tilapia (Oreochromis niloticus), reared in clear water or biofloc environments. Aquaculture 2018, 495, 654–660. [Google Scholar] [CrossRef]
- Avnimelech, Y. Carbon/nitrogen ratio as a control element in aquaculture systems. Aquaculture 1999, 176, 227–235. [Google Scholar] [CrossRef]
- Avnimelech, Y. Feeding with microbial flocs by tilapia in minimal discharge bio-flocs technology ponds. Aquaculture 2007, 264, 140–147. [Google Scholar] [CrossRef]
- Azim, M.E.; Little, D.C. The biofloc technology (BFT) in indoor tanks: Water quality, biofloc composition, and growth and welfare of Nile tilapia (Oreochromis niloticus). Aquaculture 2008, 283, 29–35. [Google Scholar] [CrossRef]
- Azim, M.E.; Little, D.C.; Bron, J.E. Microbial protein production in activated suspension tanks manipulating C:N ratio in feed and the implications for fish culture. Bioresour. Technol. 2008, 99, 3590–3599. [Google Scholar] [CrossRef]
- El-Hawarry, W.N.; Shourbela, R.M.; Haraz, Y.G.; Khatab, S.A.; Dawood, M.A. The influence of carbon source on growth, feed efficiency, and growth-related genes in Nile tilapia (Oreochromis niloticus) reared under biofloc conditions and high stocking density. Aquaculture 2021, 542, 736919. [Google Scholar] [CrossRef]
- Shourbela, R.M.; Khatab, S.A.; Hassan, M.M.; Van Doan, H.; Dawood, M.A. The effect of stocking density and carbon sources on the oxidative status, and nonspecific immunity of Nile tilapia (Oreochromis niloticus) reared under biofloc conditions. Animals 2021, 11, 184. [Google Scholar] [CrossRef] [PubMed]
- Tinh, T.H.; Koppenol, T.; Hai, T.N.; Verreth, J.A.; Verdegem, M.C. Effects of carbohydrate sources on a biofloc nursery system for whiteleg shrimp (Litopenaeus vannamei). Aquaculture 2021, 531, 735795. [Google Scholar] [CrossRef]
- Xu, W.-J.; Pan, L.-Q. Enhancement of immune response and antioxidant status of Litopenaeus vannamei juvenile in biofloc-based culture tanks manipulating high C/N ratio of feed input. Aquaculture 2013, 412–413, 117–124. [Google Scholar] [CrossRef]
- Xu, W.-J.; Pan, L.-Q. Evaluation of dietary protein level on selected parameters of immune and antioxidant systems, and growth performance of juvenile Litopenaeus vannamei reared in zero-water exchange biofloc-based culture tanks. Aquaculture 2014, 426, 181–188. [Google Scholar] [CrossRef]
- Kim, S.K.; Pang, Z.; Seo, H.C.; Cho, Y.R.; Samocha, T.; Jang, I.K. Effect of bioflocs on growth and immune activity of Pacific white shrimp, Litopenaeus vannamei postlarvae. Aquac. Res. 2014, 45, 362–371. [Google Scholar] [CrossRef]
- Long, L.; Yang, J.; Li, Y.; Guan, C.; Wu, F. Effect of biofloc technology on growth, digestive enzyme activity, hematology, and immune response of genetically improved farmed tilapia (Oreochromis niloticus). Aquaculture 2015, 448, 135–141. [Google Scholar] [CrossRef]
- Mansour, A.T.; Esteban, M.Á. Effects of carbon sources and plant protein levels in a biofloc system on growth performance, and the immune and antioxidant status of Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2017, 64, 202–209. [Google Scholar] [CrossRef]
- Da Silva, M.A.; de Alvarenga, É.R.; Alves, G.F.d.O.; Manduca, L.G.; Turra, E.M.; de Brito, T.S.; de Sales, S.C.M.; da Silva Junior, A.F.; Borges, W.J.; Teixeira, E.D.A. Crude protein levels in diets for two growth stages of Nile tilapia (Oreochromis niloticus) in a biofloc system. Aquac. Res. 2018, 49, 2693–2703. [Google Scholar] [CrossRef]
- Ekasari, J.; Rivandi, D.R.; Firdausi, A.P.; Surawidjaja, E.H.; Zairin, M., Jr.; Bossier, P.; De Schryver, P. Biofloc technology positively affects Nile tilapia (Oreochromis niloticus) larvae performance. Aquaculture 2015, 441, 72–77. [Google Scholar] [CrossRef]
- Luo, G.; Gao, Q.; Wang, C.; Liu, W.; Sun, D.; Li, L.; Tan, H. Growth, digestive activity, welfare, and partial cost-effectiveness of genetically improved farmed tilapia (Oreochromis niloticus) cultured in a recirculating aquaculture system and an indoor biofloc system. Aquaculture 2014, 422–423, 1–7. [Google Scholar] [CrossRef]
- Nguyen, H.Y.N.; Trinh, T.L.; Baruah, K.; Lundh, T.; Kiessling, A. Growth and feed utilisation of Nile tilapia (Oreochromis niloticus) fed different protein levels in a clear-water or biofloc-RAS system. Aquaculture 2021, 536, 736404. [Google Scholar] [CrossRef]
- Gao, L.; Shan, H.-W.; Zhang, T.-W.; Bao, W.-Y.; Ma, S. Effects of carbohydrate addition on Litopenaeus vannamei intensive culture in a zero-water exchange system. Aquaculture 2012, 342–343, 89–96. [Google Scholar] [CrossRef]
- Wang, G.; Yu, E.; Xie, J.; Yu, D.; Li, Z.; Luo, W.; Qiu, L.; Zheng, Z. Effect of C/N ratio on water quality in zero-water exchange tanks and the biofloc supplementation in feed on the growth performance of crucian carp, Carassius auratus. Aquaculture 2015, 443, 98–104. [Google Scholar] [CrossRef]
- Xu, W.-J.; Pan, L.-Q. Effects of bioflocs on growth performance, digestive enzyme activity and body composition of juvenile Litopenaeus vannamei in zero-water exchange tanks manipulating C/N ratio in feed. Aquaculture 2012, 356–357, 147–152. [Google Scholar] [CrossRef]
- Xu, W.-J.; Pan, L.-Q.; Zhao, D.-H.; Huang, J. Preliminary investigation into the contribution of bioflocs on protein nutrition of Litopenaeus vannamei fed with different dietary protein levels in zero-water exchange culture tanks. Aquaculture 2012, 350–353, 147–153. [Google Scholar] [CrossRef]
- Zimmermann, S.; Kiessling, A.; Zhang, J. The future of intensive tilapia production and the circular bioeconomy without effluents: Biofloc technology, recirculation aquaculture systems, bio-RAS, partitioned aquaculture systems and integrated multitrophic aquaculture. Rev. Aquac. 2023, 15, 22–31. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Trinh, L.T.; Chau, D.T.; Baruah, K.; Lundh, T.; Kiessling, A. Spent brewer’s yeast as a replacement for fishmeal in diets for giant freshwater prawn (Macrobrachium rosenbergii), reared in either clear water or a biofloc environment. Aquac. Nutr. 2019, 25, 970–979. [Google Scholar] [CrossRef]
- Ekasari, J.; Napitupulu, A.D.; Djurstedt, M.; Wiyoto, W.; Baruah, K.; Kiessling, A. Production performance, fillet quality and cost effectiveness of red Tilapia (Oreochromis sp.) culture in different biofloc systems. Aquaculture 2023, 563, 738956. [Google Scholar] [CrossRef]
- Dey, M.M.; Eknath, A.E.; Sifa, L.; Hussain, M.G.; Thien, T.M.; Van Hao, N.; Aypa, S.; Pongthana, N. Performance and nature of genetically improved farmed tilapia: A bioeconomic analysis. Aquac. Econ. Manag. 2000, 4, 83–106. [Google Scholar] [CrossRef]
- Pérez-Fuentes, J.A.; Hernández-Vergara, M.P.; Pérez-Rostro, C.I.; Fogel, I. C:N ratios affect nitrogen removal and production of Nile tilapia Oreochromis niloticus raised in a biofloc system under high density cultivation. Aquaculture 2016, 452, 247–251. [Google Scholar] [CrossRef]
- Nhi, N.H.Y. Brewer’s Yeast as a Protein Source in the Diet of Tilapia (Oreochromis niloticus) and Freshwater Prawns (Macrobrachium rosenbergii) Reared in a Clear Water or Biofloc Environment. Ph.D. Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2019. [Google Scholar]
- Zhang, N.; Luo, G.Z.; Tan, H.X.; Liu, W.C.; Hou, Z.W. Growth, digestive enzyme activity and welfare of tilapia (Oreochromis niloticus) reared in a biofloc-based system with poly-beta-hydroxybutyric as a carbon source. Aquaculture 2016, 464, 710–717. [Google Scholar] [CrossRef]
- Da, C.T.; Lundh, T.; Lindberg, J.E. Evaluation of local feed resources as alternatives to fish meal in terms of growth performance, feed utilisation and biological indices of striped catfish (Pangasianodon hypophthalmus) fingerlings. Aquaculture 2012, 364–365, 150–156. [Google Scholar] [CrossRef]
- American Public Health Association; American Water Works Association; Water Environment Federation. Standard Methods for the Examination of the Water and Waste Water; American Public Health Association: Washington, DC, USA, 1998; p. 541. [Google Scholar]
- AOAC. Official Methods of Analysis, 17th ed.; The Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000; p. 741. [Google Scholar]
- Vázquez-Ortiz, F.; Caire, G.; Higuera-Ciapara, I.; Hernández, G. High performance liquid chromatographic determination of free amino acids in shrimp. J. Liq. Chromatogr. Relat. Technol. 1995, 18, 2059–2068. [Google Scholar] [CrossRef]
- Jauncey, K. Nutritional requirements. In Tilapias: Biology and Exploitation; Beveridge, M.C.M., McAndrew, B., Eds.; Springer: Dordrecht, The Netherlands, 2000; pp. 327–375. [Google Scholar]
- Langeland, M.; Vidakovic, A.; Vielma, J.; Lindberg, J.; Kiessling, A.; Lundh, T. Digestibility of microbial and mussel meal for Arctic charr (Salvelinus alpinus) and Eurasian perch (Perca fluviatilis). Aquac. Nutr. 2016, 22, 485–495. [Google Scholar] [CrossRef]
- Li, Z.; Ye, W.; He, X. The nutritional value of commercial feed ingredients for Nile tilapia (Oreochromis niloticus) in China. In Proceedings of the Fish Nutrition Research in Asia: Proceedings of the Fourth Asian Fish Nutrition Workshop, September 1990, Vijayawada, Andhra Pradesh, India; Asian Fish Society Special Publication; Asian Fisheries Society: Manila, Philippines, 1991; pp. 101–106. [Google Scholar]
- Chou, B.-S.; Shiau, S.-Y. Optimal dietary lipid level for growth of juvenile hybrid tilapia, Oreochromis niloticus × Oreochromis aureus. Aquaculture 1996, 143, 185–195. [Google Scholar] [CrossRef]
- Abdel-Ghany, H.M.; Mohamed El-S, S.; Ezzat, A.A.; Essa, M.A.; Helal, A.M.; Ismail, R.F.; El-Sayed, A.-F.M. Effects of different levels of dietary lipids on growth performance, liver histology and cold tolerance of Nile tilapia (Oreochromis niloticus). J. Therm. Biol. 2021, 96, 102833. [Google Scholar] [CrossRef]
- Paul, M.; Sardar, P.; Sahu, N.P.; Jana, P.; Deo, A.D.; Harikrishna, V.; Varghese, T.; Shamna, N.; Kumar, P.; Krishna, G. Effect of dietary lipid level on growth performance, body composition, and physiometabolic responses of genetically improved farmed tilapia (GIFT) juveniles reared in inland ground saline water. Aquac. Nutr. 2022, 2022, 5345479. [Google Scholar] [CrossRef]
- Furuya, W.M.; Cruz, T.P.D.; Gatlin, D.M., III. Amino acid requirements for nile tilapia: An update. Animals 2023, 13, 900. [Google Scholar] [CrossRef]
- Oliva-Teles, A.; Gonçalves, P. Partial replacement of fishmeal by brewers yeast (Saccaromyces cerevisae) in diets for sea bass (Dicentrarchus labrax) juveniles. Aquaculture 2001, 202, 269–278. [Google Scholar] [CrossRef]
- OzóRio, R.O.A.; Turini, B.G.S.; MôRo, G.V.; Oliveira, L.S.T.; Portz, L.; Cyrino, J.E.P. Growth, nitrogen gain and indispensable amino acid retention of pacu (Piaractus mesopotamicus, Holmberg 1887) fed different brewers yeast (Saccharomyces cerevisiae) levels. Aquac. Nutr. 2010, 16, 276–283. [Google Scholar] [CrossRef]
- Øverland, M.; Karlsson, A.; Mydland, L.T.; Romarheim, O.H.; Skrede, A. Evaluation of Candida utilis, Kluyveromyces marxianus and Saccharomyces cerevisiae yeasts as protein sources in diets for Atlantic salmon (Salmo salar). Aquaculture 2013, 402–403, 1–7. [Google Scholar] [CrossRef]
- Hassaan, M.S.; Mahmoud, S.A.; Jarmolowicz, S.; El-Haroun, E.R.; Mohammady, E.Y.; Davies, S.J. Effects of dietary baker’s yeast extract on the growth, blood indices and histology of Nile tilapia (Oreochromis niloticus L.) fingerlings. Aquac. Nutr. 2018, 24, 1709–1717. [Google Scholar] [CrossRef]
- Huyben, D. Effects of Feeding Yeasts on Blood Physiology and Gut Microbiota of Rainbow Trout. Doctoral Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2017. [Google Scholar]
- Huyben, D.; Sun, L.; Moccia, R.; Kiessling, A.; Dicksved, J.; Lundh, T. Dietary live yeast and increased water temperature influence the gut microbiota of rainbow trout. J. Appl. Microbiol. 2018, 124, 1377–1392. [Google Scholar] [CrossRef] [PubMed]
| Diet | ||||
|---|---|---|---|---|
| Ingredients | SBY0 (Control) a | SBY30 b | SBY60 c | SBY100 d |
| Blood meal | 1.3 | 1.7 | 2.1 | 2.8 |
| Fish meal | 1.0 | 1.0 | 1.0 | 1.0 |
| Soybean meal | 40 | 28 | 16 | 0 |
| Spent brewer’s yeast | 0 | 15.2 | 30.4 | 50.5 |
| Rice bran | 25.0 | 23.8 | 22.7 | 21.0 |
| Wheat flour | 23.0 | 21.0 | 18.6 | 16.0 |
| Fish oil | 5.7 | 5.3 | 4.8 | 4.3 |
| Methionine | 0.36 | 0.36 | 0.36 | 0.36 |
| Premix (vitamin-mineral) e | 2.0 | 2.0 | 2.0 | 2.0 |
| CMC f | 2.0 | 2.0 | 2.0 | 2.0 |
| Total | 100 | 100 | 100 | 100 |
| Diet | ||||
|---|---|---|---|---|
| SBY0 (Control) a | SBY30 b | SBY60 c | SBY100 d | |
| Dry matter | 92.6 | 93.9 | 91.7 | 92.2 |
| Ash | 7.5 | 7.6 | 7.2 | 6.9 |
| Crude protein | 28.6 | 28.8 | 28.9 | 28.8 |
| Crude lipid | 11.3 | 9.9 | 9.0 | 7.5 |
| Crude fibre | 3.2 | 3.3 | 3.2 | 3.2 |
| NFE | 49.5 | 50.4 | 49.6 | 50.9 |
| Gross energy (MJ.kg−1) | 19.6 | 19.1 | 19.8 | 19.1 |
| Essential amino acids | ||||
| Arginine | 1.54 | 1.66 | 1.63 | 1.52 |
| Histidine | 0.55 | 0.69 | 0.76 | 0.39 |
| Isoleucine | 0.73 | 0.72 | 0.70 | 0.65 |
| Leucine | 2.00 | 2.08 | 2.11 | 2.06 |
| Lysine | 1.60 | 1.75 | 2.08 | 1.99 |
| Methionine | 0.59 | 0.54 | 0.65 | 0.67 |
| Phenylalanine | 1.62 | 1.85 | 1.73 | 1.51 |
| Threonine | 0.75 | 0.85 | 0.95 | 0.97 |
| Valine | 0.89 | 0.93 | 0.96 | 1.05 |
| Total | 10.27 | 11.07 | 11.57 | 10.81 |
| Dietary Treatment | SEM | ptotal *** Value | pY Value | pw Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C0 | C30 | C60 | C100 | B0 | B30 | B60 | B100 | |||||
| Initial length, cm | 14.5 | 14.5 | 14.4 | 14.4 | 14.5 | 14.4 | 14.4 | 14.5 | 0.04 | 0.69 | 0.38 | 0.23 |
| Final length, cm | 18.0 a | 18.8 ab | 18.8 ab | 18.5 a | 19.4 b | 19.6 b | 19.0 b | 18.7 ab | 0.30 | 0.002 | 0.1 | 0.002 |
| Initial body weight (g) | 50.4 | 50.4 | 49.8 | 50.6 | 50.3 | 50.4 | 50.4 | 50.0 | 0.48 | 0.94 | 0.93 | 0.93 |
| Body weight (g) day 30 | 101 ab | 102 ab | 102 ab | 94 b | 108 ac | 114 c | 106 ac | 106 ac | 2.3 | <0.0001 | 0.01 | <0.0001 |
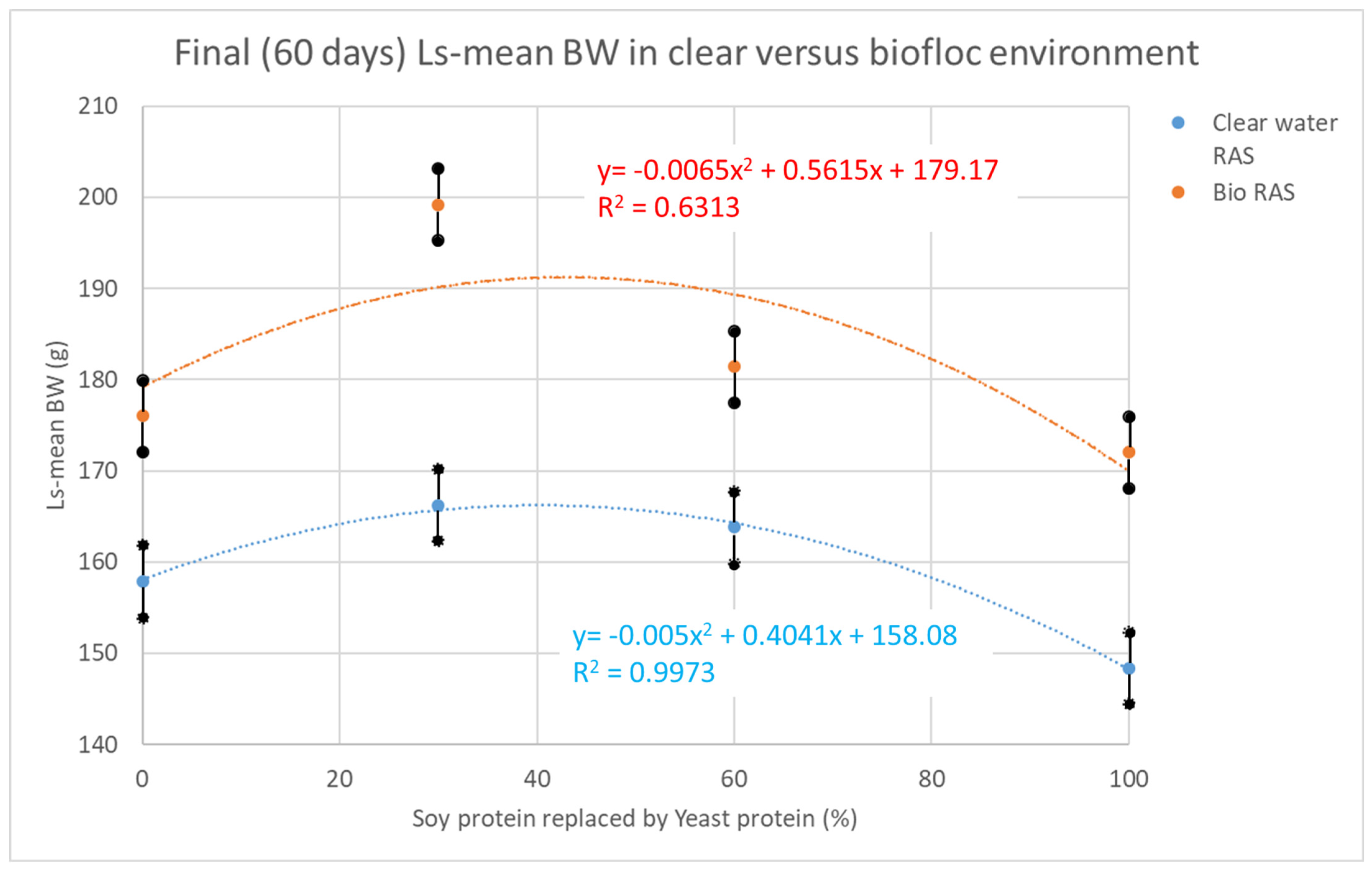
| Body weight (g) day 60 (final) ** | 158 a | 166 a | 164 a | 148 c | 176 a | 199 b | 181 a | 172 a | 3.9 | 0.02 | <0.0001 | <0.0001 |
| Specific growth rate (SGR), %/d | 1.9 ab | 2.0 ab | 2.0 ab | 1.8 b | 2.1 ac | 2.3 c | 2.2 cd | 2.0 abd | 0.1 | 0.002 | 0.05 | 0.0006 |
| Feed conversion rate (FCR) | 1.65 ab | 1.64 ab | 1.59 ad | 1.86 b | 1.46 ac | 1.28 c | 1.37 cd | 1.60 a | 0.09 | 0.0005 | 0.015 | 0.0003 |
| Protein conversion ratio (PCR), % | 39 ab | 40 ab | 40 ab | 35 a | 45 bd | 51 c | 47 cd | 41 ab | 2.3 | 0.004 | 0.02 | 0.0004 |
| PCR + Biofloc *, % | -‖- | -‖- | -‖- | -‖- | 65 ce | 71 d | 66 c | 60 e | 2.3 | 0.0001 | 0.02 | 0.0001 |
| Survival rate, % | 97 | 98 | 100 | 100 | 98 | 100 | 100 | 100 | 1.4 | 0.30 | 0.24 | 0.39 |
| Period | SEM | p-Value | |||
|---|---|---|---|---|---|
| Start 1 | Middle 2 | End 3 | |||
| Dry matter (g m−3) | 8.20 c | 25.78 b | 71.41 a | 3.92 | <0.0001 |
| Protein | 39.58 b | 43.80 ab | 44.62 a | 0.98 | 0.02 |
| Lipid | 2.79 a | 1.50 ab | 0.61 b | 0.41 | 0.03 |
| Essential amino acids | |||||
| Arginine | 1.44 | 1.60 | 1.52 | 0.05 | 0.19 |
| Histidine | 0.60 b | 0.71 ab | 0.77 a | 0.03 | 0.02 |
| Isoleucine | 0.77 | 0.82 | 0.82 | 0.04 | 0.52 |
| Leucine | 2.06 | 2.00 | 2.10 | 0.04 | 0.20 |
| Lysine | 1.34 a | 1.20 b | 1.21 b | 0.02 | <0.0001 |
| Methionine | 0.56 a | 0.44 b | 0.49 ab | 0.02 | <0.0001 |
| Phenylalanine | 1.53 b | 1.59 ab | 1.68 a | 0.03 | 0.05 |
| Threonine | 1.21 | 1.23 | 1.23 | 0.04 | 0.89 |
| Valine | 1.24 | 1.28 | 1.35 | 0.04 | 0.15 |
| Total | 10.72 | 10.86 | 11.18 | 0.22 | 0.37 |
| Temperature, °C | DO, mg L−1 | pH | TAN, mg L−1 | NO2-N, mg L−1 | |||
|---|---|---|---|---|---|---|---|
| Morning | Afternoon | Morning | Afternoon | ||||
| Bio-RAS | 28.6 ± 0.36 | 29.2 ± 0.44 | 4.81 ± 0.47 | 7.06 ± 0.22 | 7.95 ± 0.30 | 0.753 ± 0.203 | 0.014 ± 0.002 |
| Cw-RAS | 28.3 ± 0.57 | 29.2 ± 0.60 | 4.65 ± 0.36 | 6.68 ± 0.23 | 7.24 ± 0.31 | 0.409 ± 0.299 | 0.015 ± 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nhi, N.H.Y.; Lan, T.T.; Baruah, K.; Lundh, T.; Kiessling, A. Gradual Replacement of Soybean Meal with Brewer’s Yeast in Fingerling Nile Tilapia (Oreochromis niloticus) Diet, Resulting in a Polynomial Growth Pattern, Independent of Whether Reared in a Biofloc or Clear-Water System. Aquac. J. 2024, 4, 255-269. https://doi.org/10.3390/aquacj4040019
Nhi NHY, Lan TT, Baruah K, Lundh T, Kiessling A. Gradual Replacement of Soybean Meal with Brewer’s Yeast in Fingerling Nile Tilapia (Oreochromis niloticus) Diet, Resulting in a Polynomial Growth Pattern, Independent of Whether Reared in a Biofloc or Clear-Water System. Aquaculture Journal. 2024; 4(4):255-269. https://doi.org/10.3390/aquacj4040019
Chicago/Turabian StyleNhi, Nguyen Huu Yen, Trinh Thi Lan, Kartik Baruah, Torbjörn Lundh, and Anders Kiessling. 2024. "Gradual Replacement of Soybean Meal with Brewer’s Yeast in Fingerling Nile Tilapia (Oreochromis niloticus) Diet, Resulting in a Polynomial Growth Pattern, Independent of Whether Reared in a Biofloc or Clear-Water System" Aquaculture Journal 4, no. 4: 255-269. https://doi.org/10.3390/aquacj4040019
APA StyleNhi, N. H. Y., Lan, T. T., Baruah, K., Lundh, T., & Kiessling, A. (2024). Gradual Replacement of Soybean Meal with Brewer’s Yeast in Fingerling Nile Tilapia (Oreochromis niloticus) Diet, Resulting in a Polynomial Growth Pattern, Independent of Whether Reared in a Biofloc or Clear-Water System. Aquaculture Journal, 4(4), 255-269. https://doi.org/10.3390/aquacj4040019






