Biofloc Formation Strategy Effects on Halophyte Integration in IMTA with Marine Shrimp and Tilapia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Location and Organism Origin
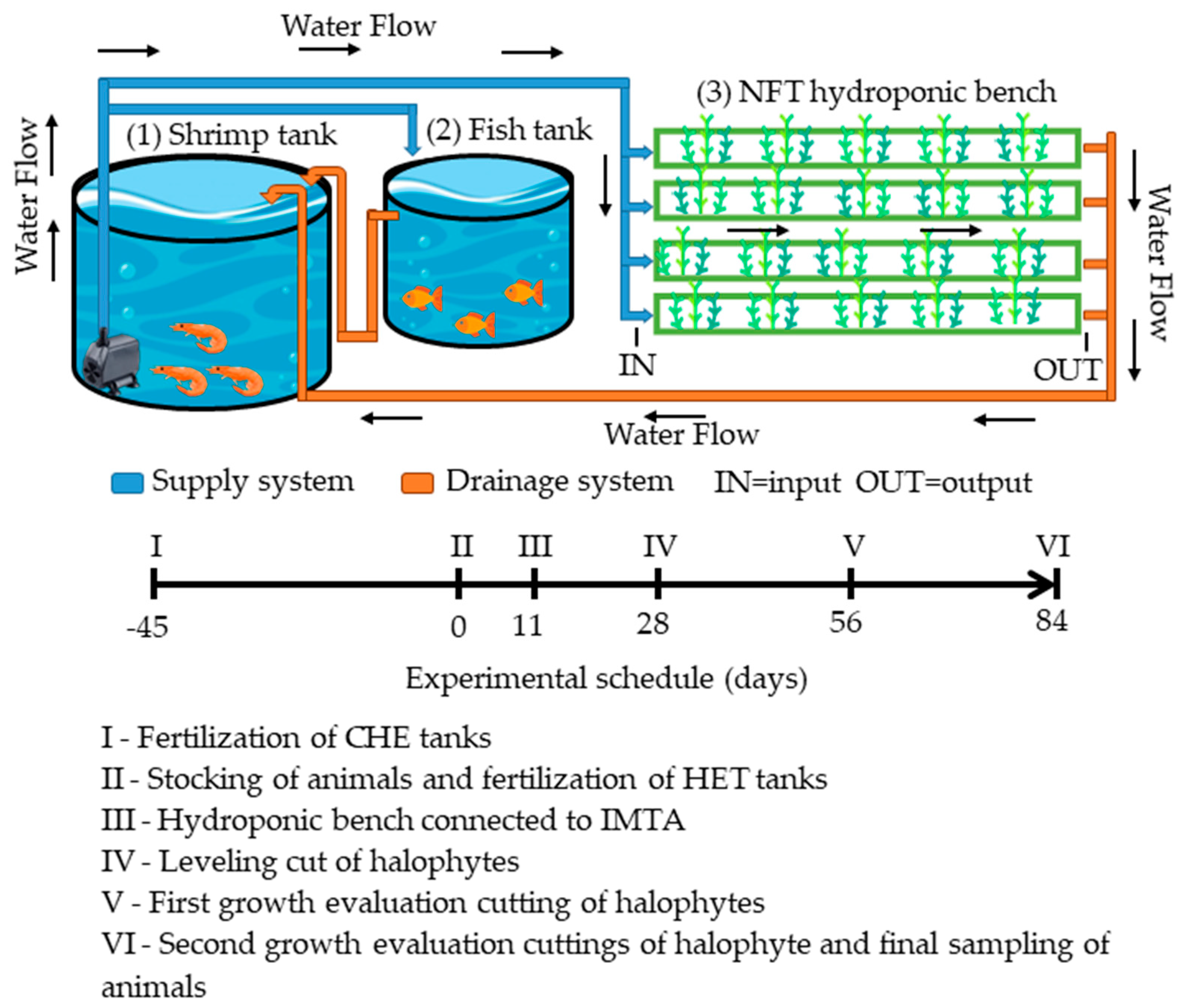
2.2. Experimental Design and Systems
2.3. Assessment of Meteorological and Physical–Chemical Parameters of Water
2.4. Assessment of Zootechnical Parameters and Growth and Productivity of S. neei
2.5. Statistical Analysis
3. Results
3.1. Environmental Conditions and Water Quality
3.2. Growth of S. neei in the Waters of the Two IMTA Biofloc Formation Strategies
3.3. Zootechnical Development of Shrimp and Tilapia in Waters of the Two IMTA Biofloc Formation Strategies
4. Discussion
4.1. Water Quality Parameters in CHE and HET Treatments
4.2. Effect of Inserting Aquaponic Benches with Halophytes on IMTA Water Quality
4.3. Zootechnical Development of Shrimp and Fish
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Knowler, D.; Chopin, T.; Martínez-Espiñeira, R.; Neori, A.; Nobre, A.; Noce, A.; Reid, G. The economics of Integrated Multi-Trophic Aquaculture: Where are we now and where do we need to go? Rev. Aquac. 2020, 12, 1579–1594. [Google Scholar] [CrossRef]
- Biswas, G.; Kumar, P.; Ghoshal, T.K.; Kailasam, M.; De, D.; Bera, A.; Mandal, B.; Sukumaran, K.; Vijayan, K.K. Integrated multi-trophic aquaculture (IMTA) outperforms conventional polyculture with respect to environmental remediation, productivity and economic return in brackishwater ponds. Aquaculture 2020, 516, 734626. [Google Scholar] [CrossRef]
- Rosa, J.; Lemos, M.F.; Crespo, D.; Nunes, M.; Freitas, A.; Ramos, F.; Leston, S. Integrated multitrophic aquaculture systems—Potential risks for food safety. Trends Food Sci. Technol. 2020, 96, 79–90. [Google Scholar] [CrossRef]
- Pinheiro, I.; Arantes, R.; Espírito Santo, C.M.; Nascimento Vieira, F.; Lapa, K.R.; Gonzaga, L.V.; Seiffert, W.Q. Production of the halophyte Sarcocornia ambigua and Pacific white shrimp in an aquaponic system with biofloc technology. Ecol. Eng. 2017, 100, 261–267. [Google Scholar] [CrossRef]
- Fierro-Sañudo, J.F.; Rodríguez-Montes de Oca, G.A.; Páez-Osuna, F. Co-culture of shrimp with commercially important plants: A review. Rev. Aquac. 2020, 12, 2411–2428. [Google Scholar] [CrossRef]
- Pinho, S.M.; Lima, J.P.; David, L.H.; Oliveira, M.S.; Goddek, S.; Carneiro, D.J.; Keesman, K.J.; Portella, M.C. Decoupled FLOCponics systems as an alternative approach to reduce the protein level of tilapia juveniles’ diet in integrated agri-aquaculture production. Aquaculture 2021, 543, 736932. [Google Scholar] [CrossRef]
- Samocha, T.M.; Patnaik, S.; Speed, M.; Ali, A.M.; Burger, J.M.; Almeida, R.V.; Brock, D.L. Use of molasses as carbon source in limited discharge nursery and grow-out systems for Litopenaeus vannamei. Aquac. Eng. 2007, 36, 184–191. [Google Scholar] [CrossRef]
- Emerenciano, M.; Gaxiola, G.; Cuzon, G. Biofloc technology (BFT): A review for aquaculture application and animal food industry. In Biomass Now-Cultivation and Utilization; Matovic, M.D., Ed.; InTech: Rijeka, Croatia, 2013; pp. 301–328. [Google Scholar] [CrossRef]
- Furtado, P.S.; Campos, B.R.; Serra, F.P.; Klosterhoff, M.; Romano, L.A.; Wasielesky, W. Effects of nitrate toxicity in the Pacific white shrimp, Litopenaeus vannamei, reared with biofloc technology (BFT). Aquacult. Int. 2015, 23, 315–327. [Google Scholar] [CrossRef]
- Binalshikh-Abubkr, T.; Mohd Hanafiah, M. Effect of Supplementation of Dried Bioflocs Produced by Freeze-Drying and Oven-Drying Methods on Water Quality, Growth Performance and Proximate Composition of Red Hybrid Tilapia. J. Mar. Sci. Eng. 2022, 10, 61. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2020; Sustainability in Action; FAO: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- Silva, K.R.; Wasielesky, W.; Abreu, P.C. Nitrogen and phosphorus dynamics in the biofloc production of the pacific white shrimp, Litopenaeus vannamei. J. World Aquac. Soc. 2013, 44, 30–41. [Google Scholar] [CrossRef]
- Ebeling, J.M.; Timmons, M.B.; Bisogni, J.J. Engineering analysis of the stoichiometry of photoautotrophic, autotrophic, and heterotrophic removal of ammonia–nitrogen in aquaculture systems. Aquaculture 2006, 257, 346–358. [Google Scholar] [CrossRef]
- Azim, M.E.; Little, D.C. The biofloc technology (BFT) in indoor tanks: Water quality, biofloc composition, and growth and welfare of Nile tilapia (Oreochromis niloticus). Aquaculture 2008, 283, 29–35. [Google Scholar] [CrossRef]
- Krummenauer, D.; Samocha, T.; Poersch, L.; Lara, G.; Wasielesky, W., Jr. The reuse of water on the culture of Pacific white shrimp, Litopenaeus vannamei, in BFT system. J. World Aquac. Soc. 2014, 45, 3–14. [Google Scholar] [CrossRef]
- Gaona, C.A.P.; Serra, F.D.P.; Furtado, P.S.; Poersch, L.H.; Wasielesky, W., Jr. Biofloc management with different flow rates for solids removal in the Litopenaeus vannamei BFT culture system. Aquacult. Int. 2016, 24, 1263–1275. [Google Scholar] [CrossRef]
- Poli, M.A.; Legarda, E.C.; Lorenzo, M.A.; Pinheiro, I.; Martins, M.A.; Seiffert, W.Q.; Nascimento Vieira, F. Integrated multitrophic aquaculture applied to shrimp rearing in a biofloc system. Aquaculture 2019, 511, e734274. [Google Scholar] [CrossRef]
- Holanda, M.; Ravagnan, E.; Lara, G.; Santana, G.; Furtado, P.; Cardozo, A.; Wasielesky, W., Jr.; Poersch, L.H. Integrated multitrophic culture of shrimp Litopenaeus vannamei and tilapia Oreochromis niloticus in biofloc system: A pilot scale study. Front. Mar. Sci. 2023, 10, e1060846. [Google Scholar] [CrossRef]
- Ferreira, G.S.; Santos, D.; Schmachtl, F.; Machado, C.; Fernandes, V.; Boegner, M.; Vieira, F.N. Heterotrophic, chemoautotrophic and mature approaches in biofloc system for Pacific white shrimp. Aquaculture 2021, 533, 736099. [Google Scholar] [CrossRef]
- Ray, A.J.; Lotz, J.M. Comparing a chemoautotrophic-based biofloc system and three heterotrophic-based systems receiving different carbohydrate sources. Aquac. Eng. 2014, 63, 54–61. [Google Scholar] [CrossRef]
- Costa, C.S.B.; Herrera, O.B. Halófitas brasileiras: Formas de cultivo e usos. In Manejo da Salinidade na Agricultura: Estudos Básicos e Aplicados, 2nd ed.; Gheyi, H.R., Dias, N.S., Lacerda, C.F., Filho, E.G., Eds.; INCTSal/CNPq: Fortaleza, Brazil, 2016; pp. 243–258. [Google Scholar]
- Doncato, K.B.; Costa, C.S.B. Micronutrient supplementation needs for halophytes in saline aquaponics with BFT system water. Aquaculture 2021, 531, e735815. [Google Scholar] [CrossRef]
- Kotzen, B.; Emerenciano, M.G.C.; Moheimani, N.; Burnell, G.M. Aquaponics: Alternative types and approaches. In Aquaponics Food Production Systems; Chapter 12; Goddek, S., Joyce, A., Kotzen, B., Burnell, G.M., Eds.; Springer Nature: Cham, Switzerland, 2019; pp. 301–330. [Google Scholar] [CrossRef]
- Beyer, C.P.; Gómez, S.; Lara, G.; Monsalve, J.P.; Orellana, J.; Hurtado, C.F. Sarcocornia neei: A novel halophyte species for bioremediation of marine aquaculture wastewater and production diversification in integrated systems. Aquaculture 2021, 543, e736971. [Google Scholar] [CrossRef]
- Doncato, K.B.; Costa, C.S.B. Evaluation of nitrogen and phosphorus nutritional needs of halophytes for saline aquaponics. Hortic. Environ. Biotechnol. 2023, 64, 355–370. [Google Scholar] [CrossRef]
- Pinheiro, I.; Carneiro, R.F.S.; Nascimento Vieira, F.; Gonzaga, L.V.; Fett, R.; Oliveira Costa, A.C.; Magallón-Barajas, F.J.; Seiffert, W.Q. Aquaponic production of Sarcocornia ambigua and Pacific white shrimp in biofloc system at different salinities. Aquaculture 2020, 519, e734918. [Google Scholar] [CrossRef]
- Silva, H.V.; Martins, M.A.; Espírito Santo, C.M.; Nascimento Vieira, F.; Rezende, P.C.; Gonzaga, L.V.; Fett, R.; Seiffert, W.Q. Aquaponic production of sea asparagus and Pacific white shrimp using biofloc technology: Different irrigation regimes affect plant production of bioactive compounds and antioxidant capacity. Aquac. Res. 2022, 53, 1001–1010. [Google Scholar] [CrossRef]
- Jory, D.E.; Cabrera, T.R.; Dugger, D.M.; Fegan, D.; Lee, P.G.; Lawrence, A.L.; Jackson, C.J.; Mcintosh, R.P.; Castañeda, J. A global review of shrimp feed management: Status and perspectives. In The New Wave: Proceedings of the Special Session on Sustainable Shrimp Culture; Browdy, C.L., Jory, D.E., Eds.; The World Aquaculture Society: Baton Rouge, LA, USA, 2001; pp. 104–152. [Google Scholar]
- Doncato, K.B.; Costa, C.S.B. Effects of cutting on vegetative development and biomass quality of perennial halophytes grown in saline aquaponics. Hortic. Bras. 2022, 40, 432–440. [Google Scholar] [CrossRef]
- APHA American Public Health Association; AWWA American Water Works Association; WEF Water Enviroment Federation. Standard Methods for the Examination of Water and Wastewater, 16th ed.; American Public Health Association: New York, NY, USA, 1998. [Google Scholar]
- Strickland, J.D.H.; Parsons, T.R. A Practical Handbook of Seawater Analysis, 2nd ed.; Fisheries Research Board of Canada: Ottawa, Canada, 1972; p. 310. [Google Scholar]
- Aminot, A.; Chaussepied, M. Manuel des Analyses Chimiques en Milieu Marin; Éditions Jouve: Paris, France, 1983; p. 395. [Google Scholar]
- Baumgarten, M.G.Z.; Wallner-kersanach, M.; Niencheski, L.F.H. Manual de Análises em Oceanografia Química, 2a ed.; Rio Grande Ed. da Universidade Federal do Rio Grande: Rio Grande, Brazil, 2010; p. 172. [Google Scholar]
- Zar, J.H. Biostatistical Analysis; Prentice-Hall: New York, NY, USA, 2010; p. 944. [Google Scholar]
- Lin, Y.C.; Chen, J.C. Acute toxicity of nitrite on Litopenaeus vannamei (Boone) juveniles at different salinity levels. Aquaculture 2003, 224, 193–201. [Google Scholar] [CrossRef]
- Valencia-Castañeda, G.; Vanegas-Pérez, R.C.; Frías-Espericueta, M.G.; Chávez-Sánchez, M.C.; Ramírez-Rochín, J.; Páez-Osuna, F. Comparison of four treatments to evaluate acute toxicity of nitrite in shrimp Litopenaeus vannamei postlarvae: Influence of feeding and the renewal water. Aquaculture 2018, 491, 375–380. [Google Scholar] [CrossRef]
- Souza, D.M.; Martins, Á.C.; Jensen, L.; Wasielesky, W., Jr.; Monserrat, J.M.; Garcia, L.D.O. Effect of temperature on antioxidant enzymatic activity in the Pacific white shrimp Litopenaeus vannamei in a BFT (Biofloc technology) system. Mar. Freshw. Behav. Physiol. 2014, 47, 1–10. [Google Scholar] [CrossRef]
- Santos, D.; Pinheiro, I.C.; Seiffert, W.Q. Cultivo aquapônico de Salicornia ambigua e camarão marinho em duas fases do sistema de bioflocos: Heterotrófica e quimioautotrófica. In Aquicultura no Sébculo XXI: Desenvolvimento Econômico, Meio Ambiente e Pesquisas; Valença, A.R., Santos, P.R.D., Guzella, L., Eds.; Universidade Federal de Santa Caterina: Florianópolis, Brazil, 2020; pp. 25–39. Available online: https://semaqui.paginas.ufsc.br/files/2018/09/aquicultura-no-seculo-xxi.pdf (accessed on 16 July 2024). [CrossRef]
- Soares, J.; Martins, M.A.; Castilho-Barros, L.; Espírito Santo, C.M.; Nascimento Vieira, F.; Seiffert, W.Q. Reducing the feed input per unit of plant area as a means to improve the efficiency of sea asparagus and Pacific white shrimp biofloc technology-based aquaponics. Aquac. Res. 2022, 53, 6536–6544. [Google Scholar] [CrossRef]
- Ventura, Y.; Wuddineh, W.A.; Myrzabayeva, M.; Alikulov, Z.; Khozin-Goldberg, I.; Shpigel, M.; Sagi, M. Effect of seawater concentration on the productivity and nutritional value of annual Salicornia and perennial Sarcocornia halophytes as leafy vegetable crops. Sci. Hortic. 2011, 128, 189–196. [Google Scholar] [CrossRef]
- Souza, M.M.; Mendes, C.R.; Doncato, K.B.; Badiale-Furlong, E.; Costa, C.S.B. Growth, Phenolics, Photosynthetic Pigments, and Antioxidant Response of Two New Genotypes of Sea Asparagus (Salicornia neei Lag.) to Salinity under Greenhouse and Field Conditions. Agriculture 2018, 8, 115. [Google Scholar] [CrossRef]
- Vinatea, L.; Galvez, A.O.; Browdy, C.L.; Stokes, A.; Venero, J.; Haveman, J.; Lewis, B.L.; Lawson, A.; Shuler, A.; Leffler, J.W. Photosynthesis, water respiration and growth performance of Litopenaeus vannamei in a super-intensive raceway culture with zero water exchange: Interaction of water quality variables. Aquac. Eng. 2010, 42, 17–24. [Google Scholar] [CrossRef]



| Parameter | Treatment | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CHE | HET | |||||||||||
| IN | OUT | IN | OUT | |||||||||
| DO | 5.78 | ± | 0.05 a | 5.69 | ± | 0.05 a | 6.21 | ± | 0.06 c | 6.10 | ± | 0.05 b |
| Temperature | 25.37 | ± | 0.24 c | 25.03 | ± | 0.25 ab | 25.09 | ± | 0.26 bc | 24.78 | ± | 0.27 a |
| pH | 7.78 | ± | 0.02 a | 7.82 | ± | 0.02 ab | 7.83 | ± | 0.03 ab | 7.88 | ± | 0.02 b |
| Alkalinity | 158.13 | ± | 3.86 | 157.71 | ± | 4.75 | 165.83 | ± | 4.12 | 163.75 | ± | 3.96 |
| NAT | 0.14 | ± | 0.01 b | 0.16 | ± | 0.01 c | 0.11 | ± | 0.01 a | 0.14 | ± | 0.01 b |
| Nitrite | 1.06 | ± | 0.14 a | 1.04 | ± | 0.12 a | 5.42 | ± | 1.18 b | 5.16 | ± | 1.14 b |
| Nitrate | 95.09 | ± | 5.70 b | 101.78 | ± | 6.52 b | 59.80 | ± | 5.40 a | 64.61 | ± | 5.87 a |
| Phosphate | 4.49 | ± | 0.36 b | 4.74 | ± | 0.35 b | 3.32 | ± | 0.30 a | 3.27 | ± | 0.26 a |
| TSS | 350.83 | ± | 16.64 b | 343.54 | ± | 17.98 b | 305.00 | ± | 19.60 a | 316.26 | ± | 22.32 a |
| Salinity | 20.74 | ± | 0.35 a | 20.76 | ± | 0.34 a | 24.44 | ± | 0.31 b | 24.37 | ± | 0.31 b |
| Parameter | Treatment | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CHE | HET | |||||||||||
| 1st Cut | 2nd Cut | 1st Cut | 2nd Cut | |||||||||
| Survival rate (%) | − | − | − | 91.52 | ± | 0.29 | − | − | − | 85.67 | ± | 2.88 |
| Shoot height (cm) | 15.60 | ± | 0.28 b | 17.53 | ± | 0.23 c | 12.60 | ± | 0.27 a | 16.97 | ± | 0.25 c |
| Branch ≥ 10 cm (per shoot) | 6.93 | ± | 0.37 b | 10.43 | ± | 0.36 c | 3.73 | ± | 0.26 a | 7.97 | ± | 0.29 b |
| Shoot biomass (g) | 15.80 | ± | 0.77 b | 30.99 | ± | 0.81 d | 9.63 | ± | 0.53 a | 27.01 | ± | 0.79 c |
| Shoot productivity (kg m−2) | 0.34 | ± | 0.08 ab | 0.67 | ± | 0.03 c | 0.19 | ± | 0.04 a | 0.53 | ± | 0.03 bc |
| Shoot succulence (%) | 89.20 | ± | 0.34 ab | 88.84 | ± | 0.64 ab | 88.10 | ± | 0.25 a | 89.95 | ± | 0.16 b |
| Root biomass (g) | − | − | − | 2.21 | ± | 0.08 | − | − | − | 2.38 | ± | 0.09 |
| Shoot allocation (%) | − | − | − | 95.42 | ± | 0.14 a | − | − | − | 93.87 | ± | 0.16 b |
| Parameter | t−test | ANOVA | ||||||
|---|---|---|---|---|---|---|---|---|
| t | p | Ft | p | Fc | P | FtXc | p | |
| Survival rate (%) | 2.02 | 0.11 | ||||||
| Shoot height (cm) | − | − | 37.92 | <0.001 | 196.08 | <0.001 | 29.91 | <0.001 |
| Branch ≥ 10 cm (per shoot) | − | − | 56.50 | <0.001 | 204.05 | <0.001 | 1.83 | 0.18 |
| Shoot biomass (g) | − | − | 31.73 | <0.001 | 913.88 | <0.01 | 3.99 | 0.05 |
| Shoot productivity (kg m−2) | − | − | 4.97 | 0.09 | 94.79 | <0.001 | 0.04 | 0.85 |
| Shoot succulence (%) | − | − | <0.001 | 1.00 | 5.89 | 0.02 | 14.04 | <0.001 |
| Root biomass (g) | −1.38 | 0.17 | ||||||
| Biomass allocation–Shoot (%) | 7.34 | <0.001 | ||||||
| Parameter | Treatment | |||||||
|---|---|---|---|---|---|---|---|---|
| CHE | HET | t | p | |||||
| L. vannamei | ||||||||
| Survival (%) | 90.50 | ± | 3.75 | 85.09 | ± | 4.21 | 1.17 | 0.31 |
| Final average weight (g) | 11.24 | ± | 1.39 a | 8.25 | ± | 1.06 b | 10.42 | <0.01 |
| Yield (kg m−3) | 3.86 | ± | 0.08 a | 2.83 | ± | 0.08 b | 10.42 | <0.01 |
| SGR (% day−1) | 2.88 | ± | 0.02 a | 2.51 | ± | 0.03 b | 10.20 | <0.01 |
| O. niloticus | ||||||||
| Survival (%) | 75.05 | ± | 17.27 | 83.81 | ± | 6.31 | −0.58 | 0.59 |
| Final average weight (g) | 171.60 | ± | 12.59 | 180.28 | ± | 6.53 | −0.79 | 0.47 |
| Yield (kg m−3) | 1.11 | ± | 0.26 | 1.29 | ± | 0.13 | −0.77 | 0.48 |
| SGR (% day−1) | 2.29 | ± | 0.07 | 2.35 | ± | 0.04 | −0.82 | 0.46 |
| Reference | Individual Shoot (g) | Production (kg m−2) | Time (Week) | Productivity (kg m−2 mês−1) | D (m−2) | Experimental Evaluation |
|---|---|---|---|---|---|---|
| [4] | 85.1 | 8.10 | 10.4 | 3.31 | 100 | Aquaponics with shrimp. |
| [26] | 12.2−21.4 | 0.38−0.61 | 8.1 | 0.19−0.30 | 40 | Aquaponics with shrimp; different salinities. |
| [38] | 13.0−41.2 | 0.49−1.69 | 5.7 | 0.34−1.19 | 40 | Aquaponics with shrimp; BFT strategies. |
| [27] | 10.8−19.0 | 1.10−1.90 | 10.0 | 0.44−0.76 | 100 | Aquaponics with shrimp; irrigation time. |
| [39] * | 9.4–11.5 | 0.67−0.77 | 12.0 | 0.24–0.28 | 100 | Aquaponics with shrimp; animal feeding rate. |
| [17] | 23.0 | 2.27 | 8.1 | 1.12 | 97 | IMTA with BFT. |
| Present study * | 37.0−46.8 | 0.72−1.01 # | 8.0 | 0.19–0.67 | 24 | IMTA; BFT strategies. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonçalves, M.d.S.; Carvalho, A.; Santos, J.; Holanda, M.; Poersch, L.H.; Costa, C.S.B. Biofloc Formation Strategy Effects on Halophyte Integration in IMTA with Marine Shrimp and Tilapia. Aquac. J. 2024, 4, 217-231. https://doi.org/10.3390/aquacj4040016
Gonçalves MdS, Carvalho A, Santos J, Holanda M, Poersch LH, Costa CSB. Biofloc Formation Strategy Effects on Halophyte Integration in IMTA with Marine Shrimp and Tilapia. Aquaculture Journal. 2024; 4(4):217-231. https://doi.org/10.3390/aquacj4040016
Chicago/Turabian StyleGonçalves, Mayra da Silva, Andrezza Carvalho, Jorge Santos, Mariana Holanda, Luís Henrique Poersch, and César Serra Bonifácio Costa. 2024. "Biofloc Formation Strategy Effects on Halophyte Integration in IMTA with Marine Shrimp and Tilapia" Aquaculture Journal 4, no. 4: 217-231. https://doi.org/10.3390/aquacj4040016
APA StyleGonçalves, M. d. S., Carvalho, A., Santos, J., Holanda, M., Poersch, L. H., & Costa, C. S. B. (2024). Biofloc Formation Strategy Effects on Halophyte Integration in IMTA with Marine Shrimp and Tilapia. Aquaculture Journal, 4(4), 217-231. https://doi.org/10.3390/aquacj4040016






