Larviculture of Brycon amazonicus under Different Food and Farming Systems
Abstract
1. Introduction
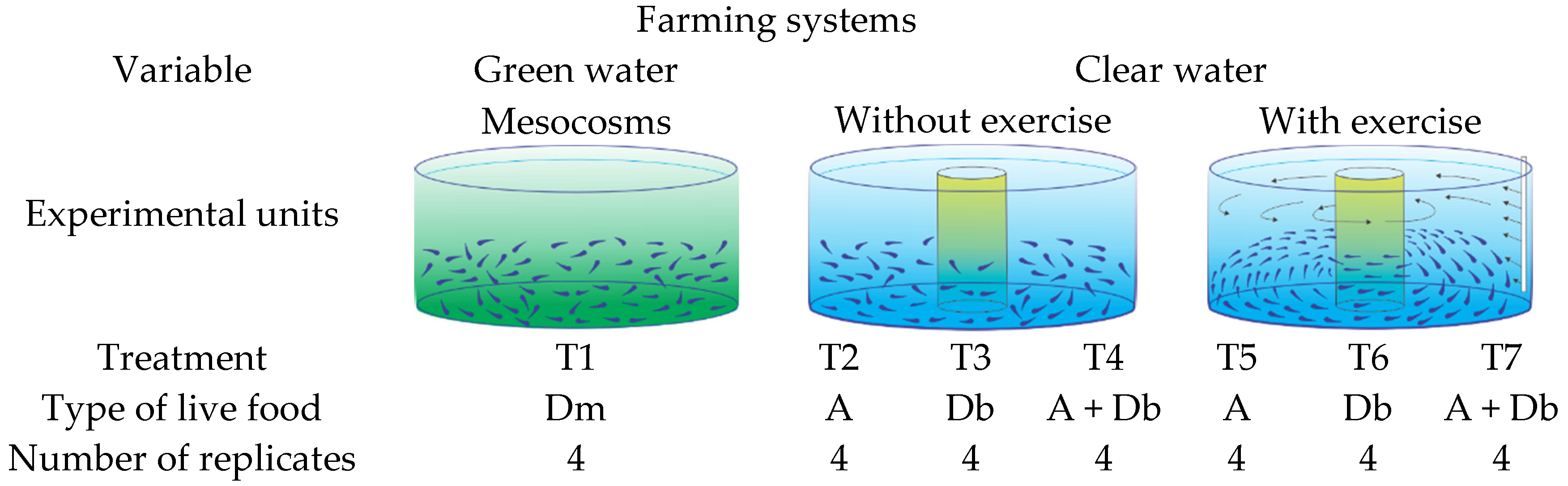
2. Materials and Methods
2.1. Place of Experiment and Biological Material
2.2. Installations and Experimental Conditions
2.3. Semi-Intensive Mesocosm System
2.4. Clear Water System
2.5. Production of nauplii of Dendrocephalus brasiliensis
2.6. Larva Swimming Exercise System Protocol
2.7. Assessed Parameters
2.7.1. Evaluation of Larvae Growth and Survival
2.7.2. Evaluation of Total and Free Amino Acids
2.7.3. Analysis of Total Lipids and Fatty Acids
2.8. Statistical Data Analysis
3. Results
3.1. Larvae Growth
3.2. Biochemical Composition of Matrinxã (Brycon amazonicus) Larvae Reared under Different Production Systems and Food
3.2.1. Composition of Amino Acids in Larvae of Brycon amazonicus
3.2.2. Composition of Fatty Acids in Larvae of Matrinxã, Brycon amazonicus
4. Discussion
5. Conclusions
- −
- The clear water system with sustained swimming exercise is the most adequate among the rearing systems for matrinxã larviculture tested in this work.
- −
- Adopting moderate currents in clear water systems enables us to minimize the occurrence of cannibalism and offers better survival rates for the larvae.
- −
- The nutritional quality of live foods, tested through the performance responses of Brycon amazonicus larvae, is appropriate.
- −
- The composition of free amino acids in young animals of D. brasiliensis consists mainly of arginine.
- −
- In practical terms, for efficient production and based on the feed rates practiced in Brycon amazonicus larviculture, it is recommended that the feed rates be at least 300% of the larva’s live weight.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tingman, W.; Jian, Z.; Xiaoshuan, Z. Fish product quality evaluation based on temperature monitoring in cold chain. Afr. J. Biotechnol. 2010, 9, 6146–6151. [Google Scholar]
- Fernandes, A.C.; Medeiros, C.O.; Bernardo, G.L.; Ebone, M.V.; Di Pietro, P.F.; Assis, M.A.A.D.; Vasconcelos, F.D.A.G.D. Benefits and risks of fish consumption for the human health. Rev. Nutr. 2012, 25, 283–295. [Google Scholar] [CrossRef]
- Liao, I.C.; Su, H.M.; Chang, E.Y. Techniques in finfish larviculture in Taiwan. Aquaculture 2001, 200, 1–31. [Google Scholar] [CrossRef]
- Conceição, L.E.; Yúfera, M.; Makridis, P.; Morais, S.; Dinis, M.T. Live feeds for early stages of fish rearing. Aquac. Res. 2010, 41, 613–640. [Google Scholar] [CrossRef]
- Portella, M.C.; Leitão, N.D.J.; Takata, R.; Lopes, T.S. Alimentação e nutrição de larvas. In Nutriaqua: Nutrição e Alimentação de Espécies de Interesse Para a Aquicultura Brasileira; Fracalossi, D.M., Cyrino, J.E.P., Eds.; Sociedade Brasileira de Aquicultura e Biologia Aquática: Florianópolis, Brazil, 2012; pp. 185–216. [Google Scholar]
- Gandra, A.L. O Mercado do Pescado da Região Metropolitana de Manaus; INFOPESCA: Montevideo, Uruguay, 2010; 84p. [Google Scholar]
- Cestarolli, M.A. Larvicultura do Pintado Pseudoplatystoma coruscans (Agassiz, 1829): Aspectos da Alimentação Inicial e do Desenvolvimento de Estruturas Sensoriais. Ph.D. Thesis, Aquaculture Center, São Paulo State University, Jaboticabal, Brazil, 2005. Available online: https://repositorio.unesp.br/bitstream/handle/11449/144162/000330182.pdf?sequence=1&isAllowed=y (accessed on 15 September 2023).
- Tesser, M.B.; Portella, M.C. Ingestão de ração e comportamento de larvas de pacu em resposta a estímulos químicos e visuais. Rev. Bras. Zootec. 2006, 35, 1887–1892. [Google Scholar] [CrossRef]
- Kolkovski, S. Digestive enzymes in fish larvae and juveniles—Implications and applications to formulated diets. Aquaculture 2001, 200, 181–201. [Google Scholar] [CrossRef]
- Cahu, C.; Infante, J.Z. Substitution of live food by formulated diets in marine fish larvae. Aquaculture 2001, 200, 161–180. [Google Scholar] [CrossRef]
- Cahu, C.; Infante, J.Z.; Akeuchi, T. Nutritional components affecting skeletal development in fish larvae. Aquaculture 2003, 227, 245–258. [Google Scholar] [CrossRef]
- de Mello, P.H.; Lundstedt, L.M.; Moraes, G.; Cavalheiro Araújo, B.; Leite Venturieri, R.L.; Guimarães Moreira, R. Ontogeny of the digestive system and the profile of proteases in larvae of cachara (Pseudoplatystoma reticulatum Siluriformes: Pimelodidae) and its hybrid (Pseudoplatystoma corruscans × Pseudoplatystoma reticulatum). J. Fish Biol. 2021, 99, 1135–1139. [Google Scholar] [CrossRef]
- Tesser, M.B.; Carneiro, D.J.; Portella, M.C. Co-feeding of pacu, Piaractus mesopotamicus Holmberg (1887), larvae with Artemia nauplii and a microencapsulated diet. J. Appl. Aquac. 2005, 17, 47–59. [Google Scholar] [CrossRef]
- Jomori, R.K.; Carneiro, D.J.; Martins, M.I.E.G.; Portella, M.C. Economic evaluation of Piaractus mesopotamicus juvenile production in different rearing systems. Aquaculture 2005, 243, 175–183. [Google Scholar] [CrossRef]
- Jomori, R.K.; Ducatti, C.; Carneiro, D.J.; Portella, M.C. Stable carbon (δ13C) and nitrogen (δ15N) isotopes as natural indicators of live and dry food in Piaractus mesopotamicus (Holmberg, 1887) larval tissue. Aquac. Res. 2008, 39, 370–381. [Google Scholar] [CrossRef]
- Alvarado-Castillo, J.D. Substituição Precoce do Alimento vivo pelo Alimento Inerte na Larvicultura de Acará Bandeira (Pterophyllum scalare). 2010. Available online: https://repositorio.unesp.br/bitstream/handle/11449/86685/alvaradocastillo_jd_me_jabo.pdf?sequence=1&isAllowed=y (accessed on 15 September 2023).
- Howes, G. Review of the genus Brycon (Teleostei: Characoidei). Bull. Br. Mus. (Nat. Hist.) Zool. 1982, 43, 1–47. [Google Scholar]
- Arbeláez-Rojas, G.A.; Moraes, G. Sustained swimming and stocking density interaction in the performance and body composition of matrinxã Brycon amazonicus juveniles. Ciênc. Rural. 2009, 39, 201–208. [Google Scholar] [CrossRef]
- Gomes, L.C.; Urbinati, E.C. Matrinxã (Brycon amazonicus). In Espécies Nativas para Piscicultura no Brasil; Balsisserotto, B., Gomes, L.C., Eds.; UFSM: Santa Maria, Brasil, 2005; pp. 149–174. [Google Scholar]
- Senhorini, J.A.; Mantelatto, F.L.M.; Casanova, S.M.C. Growth and survival of larvae the amazon species “matrinxã”, Brycon cephalus (Pisces Characidade), in larvicultura ponds. Bol. Técnico CEPTA Pirassununga 1998, 11, 13–28. Available online: https://www.icmbio.gov.br/cepta/images/stories/producao_cientifica/growth_1998_01.pdf (accessed on 15 September 2023).
- Zaniboni Filho, E.; Reynalte-Tataje, D.; Weingartner, M. Potencialidad del género Brycon en la piscicultura brasileña. Rev. Colomb. Cienc. Pecu. 2006, 19, 233–240. [Google Scholar]
- Smith, C.; Reay, P. Cannibalism in teleost fish. Rev. Fish Biol. Fish. 1991, 1, 41–64. [Google Scholar] [CrossRef]
- Pereira, L.S.; Agostinho, A.A.; Winemiller, K.O. Revisiting cannibalism in fishes. Rev. Fish Biol. Fish. 2017, 27, 499–513. [Google Scholar] [CrossRef]
- Souza, E.C.M.; Silva, J.P.; Villacorta-Correa, M.A.; Carvalho, T.B. Aggressiveness and locomotion activity related to hatching time in Matrinxã, Brycon amazonicus (Spix and Agassiz, 1829). Appl. Anim. Behav. Sci. 2014, 157, 146–151. [Google Scholar] [CrossRef]
- Atencio García, V.J.; Pertuz Buelvas, V.M.; Pérez Espitia, F.; Ortiz Mestra, R.; Pardo Carrasco, S.C. Manejo de la primera alimentación de dorada Brycon sinuensis ofreciendo larvas de bocachico Prochilodus magdalenae. Rev. Colomb. Cienc. Pecu. 2010, 23, 317–324. [Google Scholar]
- Gomes, L.C.; Baldisserotto, B.; Senhorini, J.A. Effect of stocking density on water quality, survival, and growth of larvae of matrinxã, Brycon cephalus (Characidae), in ponds. Aquaculture 2000, 183, 73–81. [Google Scholar] [CrossRef]
- Leonardo, A.F.G.; Hoshiba, M.A.; Urbinati, E.C.; Senhorini, J.A. Improvement of matrinxã, Brycon amazonicus, larviculture by exposing eggs to triiodothyronine. J. World Aquac. Soc. 2013, 44, 141–147. [Google Scholar] [CrossRef]
- Hoshiba, M.A. Enriquecimento da Alimentação das Larvas de Matrinxã (Brycon amazonicus) com Aminoácidos: Influência no Crescimento Inicial e Sobrevivência das Larvas. 2007. Available online: https://repositorio.unesp.br/bitstream/handle/11449/96566/hoshiba_ma_me_jabo.pdf?sequence=1&isAllowed=y (accessed on 15 September 2023).
- Dias, D.D.C.; Corrêa, C.F.; Leonardo, A.F.G.; Tachibana, L.; Romagosa, E.; Ranzani-Paiva, M.J.T. Probiótico na larvicultura de matrinxã, Brycon amazonicus. Acta Sci. Anim. Sci. 2011, 33, 365–368. [Google Scholar] [CrossRef][Green Version]
- Carvalho, T.B.; Souza, E.C.M.D.; Pinheiro-Da-Silva, J.; Villacorta-Correa, M.A. Effect of body size heterogeneity on the aggressive behavior of larvae of matrinxã, Brycon amazonicus (Characiformes, Bryconidae). Acta Amaz. 2018, 48, 304–310. [Google Scholar] [CrossRef]
- Jomori, R.K.; Carneiro, D.J.; Malheiros, E.B.; Portella, M.C. Growth and survival of pacu Piaractus mesopotamicus (Holmberg, 1887) juveniles reared in ponds or at different initial larviculture periods indoors. Aquaculture 2003, 221, 277–287. [Google Scholar] [CrossRef]
- Mai, M.G.; Zaniboni Filho, E. The effect of storage age in external tanks in the larviculture performance of Salminus brasiliensis (Osteichthyes, Characidae). Acta Sci.-Anim. Sci. 2005, 27, 287–296. [Google Scholar]
- Bernardino, G.; Senhorini, J.A.; Fontes, N.A.; Bock, C.L.; Mendonça, J.O.J. Propagação artificial do matrinchã Brycon cephalus (Günther, 1869) (Teleostei, Characidae). Bol. Técnico CEPTA 1993, 6, 1–9. [Google Scholar]
- Christiansen, J.S.; Ringo, E.; Jobling, J. Effects of Sustained Exercise on Growth and Body Composition of First-Feeding Fry of Arctic Charr, Salvelinus alpinus (L.). Aquaculture 1989, 79, 329–335. [Google Scholar] [CrossRef]
- Grünbaum, T.; Cloutier, R.; Le Francois, N.R. Positive effects of exposure to increased water velocity on growth of newly hatched Arctic charr, Salvelinus alpinus L. Aquac. Res. 2008, 39, 106–110. [Google Scholar] [CrossRef]
- Bagatto, B.; Pelster, B.; Burggren, W.W. Growth and metabolism of larval zebrafish: Effects of swim training. J. Exp. Biol. 2001, 204, 4335–4343. [Google Scholar] [CrossRef]
- Andrade, C.; Nogueira, N.; Silva, P.; Dinis, M.T.; Narciso, L. Mesocosm hatcheries using semi-intensive methodologies and species diversification in aquaculture. J. Agric. Sci. Technol. 2012, 2, 428. [Google Scholar]
- Divanach, P.; Kentouri, M. Hatchery techniques for specific diversification in Mediterranean finfish larviculture. Cah. Opt. Medit. 2000, 47, 75–87. [Google Scholar]
- Robin, J.; Gatesoupe, F.J. Feeding fish larvae with live prey. In Nutrition and Feeding of Fish and Crustaceans; Guillaume, J., Kaushik, S., Bergot, P., Metailler, R., Eds.; Springer Praxis Books/Food Sciences [Hardcover]; 2001; pp. 213–228. [Google Scholar]
- Arbeláez-Rojas, G.A.; Melão, M.D.G.G. Production performance and nutritional quality of the fairy shrimp Dendrocephalus brasiliensis Pesta, 1921 (Crustacea, Anostraca) cultured with fish effluent in recirculation system. Aquaculture 2022, 548, 737692. [Google Scholar] [CrossRef]
- Lopes, J.P.; Hélio de Castro, B.G.; Gálvez, A.O.; Pontes, C.S. Produção de cistos de “branchoneta” Dendrocephalus brasiliensis (Crustacea: Anostraca). Biotemas 2007, 20, 33–39. [Google Scholar]
- National Research Council. Nutrient Requirements of Fish and Shrimp; National Academies Press: Washington, DC, USA, 2011.
- Jomori, R.K. Estudos Sobre a Alimentação de Larvas de Pacu, Piaractus Mesopotamicus (Holmberg, 1887) com Náuplios de Artemia e sua Substituição por Dieta Artificial; Monography Faculdade de Ciências Agrárias e Veterinárias, Universidade Estadual Paulista: São Paulo, Brazil, 1999. [Google Scholar]
- Hopkins, K.D. Reporting fish growth: A review of the basics. World Aquac. Soc. 1992, 23, 173–179. [Google Scholar] [CrossRef]
- Ceccarelli, P.S. Canibalismo em larvas de matrinxã Brycon cephalus (Günther, 1869). J. Aquac. Trop. 1997, 34, 88–90. [Google Scholar]
- AOAC. Official methods of the Association of Official Analytical Chemists, 16th ed.; Association of Official Analytical Chemists: Arlington, TX, USA, 2005. [Google Scholar]
- Tavares, L.H.S. Limnologia Aplicada à Aquicultura; Funep: Jaboticabal, Brazil, 1995; Volume 1, 72p. [Google Scholar]
- Carter, C.G.; Houlihan, D.F. Protein synthesis. Fish Physiol. 2001, 20, 31–75. [Google Scholar]
- Schnaittacher, G.; King, W.; Berlinsky, D.L. The effects of feeding frequency on growth of juvenile Atlantic halibut, Hippoglossus hippoglossus L. Aquac. Res. 2005, 36, 370–377. [Google Scholar] [CrossRef]
- Jomori, R.K.; Luz, R.K.; Takata, R.; Fabregat, T.E.H.P.; Portella, M.C. Água levemente salinizada aumenta a eficiência da larvicultura de peixes neotropicais. Pesqui. Agropecu. Bras. 2013, 48, 809–815. [Google Scholar] [CrossRef]
- Beux, L.F.; Zaniboni Filho, E. Influência da baixa salinidade na sobrevivência de náuplios de Artemia sp. Bol. Do Inst. Pesca 2006, 32, 73–77. [Google Scholar]
- Baras, E. Minimización del canibalismo en especies de peces con larvas piscívoras: Estrategias y éxitos con el carácido Brycon moorei. In Biología de las Poblaciones de Peces de la Amazonía y Piscicultura. Comunicaciones del Primer Coloquio de la Red de Investigación Sobre la Ictiofauna Amazónica, Iquitos, Perú; Renno, J.F., García, C., Duponchelle, F., Nuñez, J., Eds.; IIAP–IQUITOS/IRD–PARIS, 2005; pp. 227–233. Available online: https://repositorio.iiap.gob.pe/bitstream/20.500.12921/144/1/Renno_libro_2005.pdf (accessed on 14 June 2023).
- Schipp, G.; Bosmans, J.; Humphrey, J. Barramundi Farming Handbook; Department of Primary Industry, Fisheries and Mines, Northern Territory Government: Darwin, NT, Australia, 2007; pp. 1–81.
- Sornsupharp, S.; Dahms, H.U.; Sanoamuang, L. Nutrient composition of fairy shrimp Streptocephalus sirindhornae nauplii as live food and growth performance of giant freshwater prawn postlarvae. Aquac. Nutr. 2013, 19, 349–359. [Google Scholar] [CrossRef]
- Bogut, I.; Adamek, Z.; Puškadija, Z.; Galović, D. Nutritional value of planktonic cladoceran Daphnia magna for common carp (Cyprinus carpio) fry feeding. Croat. J. Fish. Ribar. 2010, 68, 1–10. [Google Scholar]
- Yuslan, A.; Najuwa, S.; Hagiwara, A.; Ghaffar, M.A.; Suhaimi, H.; Rasdi, N.W. Production performance of Moina macrocopa (Straus 1820) (Crustacea, cladocera) cultured in different salinities: The effect on growth, survival, reproduction, and fatty acid composition of the neonates. Diversity 2021, 13, 105. [Google Scholar] [CrossRef]



| Interval in Days | Estimated Live Weight (LW) of Larvae of Matrinxã (mg) | Amount of Food Offered Considering that the Matrinxã Larva Consumes between 70 to 150% of Its Own Weight in Food (mg) | Number of Artemia nauplii/Larva/Day |
|---|---|---|---|
| 1–3 | 1.5–2.7 | 2.3–4.1 (150% LW) | 260–465 |
| 4–6 | 3.5–17.0 | 3.5–17.0 (100% LW) | 400–2000 |
| 7–10 | 20.3–110.0 | 14.2–77.0 (70% LW) | 1600–8850 |
| Production Systems | |||||||
|---|---|---|---|---|---|---|---|
| Clear Water | Green Water | ||||||
| Performance Parameters | Larvae Raised in Traditional Standing Water System | Larvae Reared in Sustained Swimming Exercise System | Larvae Reared in Mesocosm System | ||||
| A | B | A + B | A | B | A + B | Cladocerans | |
| Initial length (il—mm) | 6.22 ± 0.30 | 6.22 0.30 | 6.22 ± 0.30 | 6.22 ± 0.30 | 6.22 ± 0.30 | 6.22 ± 0.30 | 6.22 ± 0.30 |
| Initial weight (iw—mg) | 1.79 ± 0.42 | 1.79 ± 0.42 | 1.79 ± 0.42 | 1.79 ± 0.42 | 1.79 ± 0.42 | 1.79 ± 0.42 | 1.79 ± 0.42 |
| Final length (fl—mm) | 16.6 ± 1.15 a | 15.6 ± 1.12 a | 16.1 ± 1.06 a | 17.6 ± 1.29 a | 17.3 ± 1.09 a | 17.0 ± 1.06 a | 16.32 ± 1.02 a |
| Final weight (fw—mg) | 30.1 ± 5.73 b | 27.7 ± 7.59 cd | 29.2 ± 6.81 de | 42.9 ± 2.58 a | 32.3 ± 4.10 a | 36.7 ± 3.05 a | 29.38 ± 5.30 be |
| Specific growth rate (SGR—%) | 28.1 ± 1.89 ab | 27.1 ± 2.93 b | 27.7 ± 2.57 ab | 31.7 ± 0.60 a | 28.9 ± 1.21 ab | 30.2 ± 0.80 ab | 27.85 ±1.92 ab |
| Survival (%) | 18.2 ± 2.11 b | 10.6± 2.21 c | 12.8 ± 2.45 d | 25.7 ± 1.70 a | 20.9 ± 2.46 a | 22.2 ± 2.36 a | 15.00 ± 2.94 e |
| Cannibalism (%) | 81.8 | 89.3 | 87.2 | 74.2 | 79.1 | 77.7 | 85.00 |
| Production Systems | |||||||
|---|---|---|---|---|---|---|---|
| Clear Water | Green Water | ||||||
| Traditional System | Exercise System | Mesocosm | |||||
| Amino Acids | A | A + B | B | A | A + B | B | Cladocerans |
| EAA 1 | |||||||
| Arg | 45.3 ± 0.9 bc | 49.8 ± 0.9 b | 47.7 ± 0.9 b | 46.6 ± 0.9 b | 48.0 ± 0.9 b | 58.0 ± 1.2 a | 45.1 ± 0.9 bc |
| Ile | 26. 9 ± 0.5 | 29.2 ± 0.6 | 29.9 ± 0.4 | 27.5 ± 0.5 | 28.5 ± 0.5 | 36.1 ± 0.7 | 26.5 ± 0.5 |
| Phe | 27.9 ± 0.5 | 30.2 ± 0.6 | 30.2 ± 0.6 | 28.4 ± 0.6 | 29.5 ± 0.6 | 3.6 ± 0.7 | 27.4 ± 0.5 |
| His | 14.5 ± 0.4 | 16.6 ± 0.3 | 15.9 ± 0.3 | 15.2 ± 0.3 | 15.6 ± 0.3 | 19.6 ± 0.4 | 16.6 ± 0.3 |
| Leu | 49.3 ± 0.9 bc | 54.3 ± 1.0 b | 56.5 ± 1.1 b | 51.9 ± 1.0 bc | 54.8 ± 1.1 b | 68.0 ± 1.4 a | 50.6 ± 1.0 bc |
| Lys | 49.2 ± 0.9 bcd | 54.9 ± 1.0 b | 55.1 ± 1.1 b | 52.0 ± 1.0 bd | 55.4 ± 1.1 b | 67.6 ± 1.3 a | 50.7 ± 1.0 bd |
| Met | 16.6 ± 0.3 | 18.3 ± 0.4 | 17.4 ± 0.3 | 17.0 ± 0.3 | 17.4 ± 0.3 | 20.4 ± 0.4 | 16.6 ± 0.3 |
| Thr | 26.4 ± 0.5 | 28.9 ± 0.6 | 29.4 ± 0.6 | 27.8 ± 0.5 | 29.3 ± 0.6 | 35.7 ± 0.7 | 26.1 ± 0.5 |
| Val | 36.7 ± 0.7 | 39.0 ± 0.8 | 40.9 ± 0.8 | 37.7 ± 0.7 | 38.5 ± 0.8 | 49.0 ± 0.9 | 36.5 ±0.7 |
| NEAA 2 | |||||||
| Asp | 52.2 ± 1.0 | 61.5 ± 1.2 | 61.2 ± 1.2 | 47.2 ± 0.9 | 62.4 ± 1.2 | 83.3 ± 1.7 | 51.9 ± 1.0 |
| Glut | 91.5 ± 1.8 | 101.7 ± 2.0 | 106.0 ± 2.1 | 91.1 ± 1.8 | 103.9 ± 2.0 | 130.0 ± 2.6 | 93.1 ± 1.8 |
| Ala | 41.0 ± 0.8 | 44.7 ± 0.9 | 44.8 ± 0.9 | 43.2 ± 0.9 | 45.2 ± 0.9 | 54.8 ± 1.1 | 43.1 ± 0.8 |
| Cis | 15.5 ± 0.3 | 15.9 ± 0.3 | 17.5 ± 0.3 | 14.2 ± 0.3 | 17.7 ± 0.3 | 21.7 ± 0.4 | 12.8 ± 0.2 |
| Gly | 45.7 ± 0.9 | 49.3 ± 0.9 | 44.6 ± 0.9 | 49.0± 0.9 | 49.2 ± 0.9 | 55.4 ± 1.1 | 52.3 ± 1.0 |
| Pro | 30.3 ± 0.6 | 32.9 ± 0.6 | 30.3 ± 0.6 | 31.6 ± 0.6 | 31.9 ± 0.6 | 37.2 ± 0.7 | 33.7 ± 0.6 |
| Ser | 24.7 ± 0.5 | 26.6 ± 0.5 | 27.0 ± 0.5 | 24.9 ± 0.5 | 27.5 ± 0.5 | 32.4 ± 0.6 | 24.6 ± 0.5 |
| Tau | 10.5 ± 0.2 | 9.5 ± 0.2 | 7.2 ± 0.1 | 11.7 ± 0.2 | 11.7 ± 0.2 | 9.0 ± 0.2 | 9.1 ± 0.2 |
| Tyr | 24.9 ± 0.5 | 26.7 ± 0.5 | 23.6 ± 0.5 | 24.1 ± 0.4 | 22.7 ± 0.4 | 31.5 ± 0.6 | 22.7 ± 0.4 |
| TOTAL AA | 629.0± 12.6 bd | 689.5 ± 13.8 b | 685.2 ± 12.7 b | 642.0± 12.84 bc | 689.7 ± 13.8 b | 846.8 ± 16.9 a | 640.1 ± 12.8 bc |
| Traditional System | Exercise System | Mesocosms | |||||
|---|---|---|---|---|---|---|---|
| Fatty Acids | Artemia | Artemia + D. brasiliensis | D. brasiliensis | Artemia | Artemia + D. brasiliensis a | D. brasiliensis | Cladocerans |
| Saturated fatty acid | |||||||
| Laurelic acid (C12:0) | 0.01 ± 0.0 | 0.02 ± 0.0 | 0.01 ± 0.0 | 0.03 ± 0.0 | 0.01 ± 0.0 | 0.02 ± 0.0 | |
| Miristic acid (C14:0) | 0.14 ± 0.0 | 0.14 ± 0.0 | 0.15 ± 0.0 | 0.11 ± 0.0 | 0.08 ± 0.0 | 0.15 ± 0.0 | 0.07 ± 0.0 |
| Pentadecanoic acid (C15:0) | 0.12 ± 0.0 | 0.10 ± 0.0 | 0.12 ± 0.0 | 0.09 ± 0.0 | 0.09 ± 0.0 | 0.12 ± 0.0 | 0.06 ± 0.0 |
| Palmitic acid (C16:0) | 3.18 ± 0.3 | 3.03 ± 0.3 | 3.30 ± 0.3 | 3.11 ± 0.0 | 2.77 ± 0.4 | 3.36 ± 0.1 | 2.03 ± 0.2 |
| Margaric acid (C17:0) | 0.26 ± 0.0 | 0.21 ± 0.0 | 0.27 ± 0.0 | 0.23 ± 0.0 | 0.14 ± 0.0 | 0.28 ± 0.0 | 0.15 ± 0.0 |
| Stearic acid (C18:0) | 1.68 ± 0.2 | 1.79 ± 0.2 | 1.74 ± 0.3 | 2.16 ± 0.2 | 2.01 ± 0.2 | 1.77 ± 0.1 | 1.41 ± 0.1 |
| Araquic acid (C20:0) | 0.19 ± 0.0 | 0.04 ± 0.0 | 0.20 ± 0.0 | 0.06 ± 0.0 | 0.04 ± 0.0 | 0.20 ± 0.0 | 0.04 ± 0.0 |
| Heneicosanoic acid (C21:0) | 0.02 ± 0.0 | 0.02 ± 0.0 | 0.00 | 0.02 ± 0.0 | |||
| Behenic acid (C22:0) | 0.05 ± 0.0 | 0.03 ± 0.0 | 0.05 ± 0.0 | 0.08 ± 0.0 | 0.05 ± 0.0 | 0.05 ± 0.0 | 0.05 ± 0.0 |
| Lignoceric acid (C24:0) | 0.03 ± 0.0 | 0.03 ± 0.0 | 0.03 ± 0.0 | 0.06 ± 0.0 | 0.05 ± 0.0 | 0.03 ± 0.0 | 0.04 ± 0.0 |
| Total saturated fatty acid | 5.68 ± 0.6 | 5.39 ± 0.5 | 5.90 ± 0.5 | 5.91 ± 0.5 | 5.22 ± 0.5 | 6.01 ± 0.2 | 3.86 ± 0.3 |
| Unsaturated fatty acid | |||||||
| Palmitoleic acid (C16:1) | 0.90 ± 0.0 | 0.44 ± 0.0 | 0.93 ± 0.1 | 0.28 ± 0.0 | 0.22 ± 0.0 | 0.95 ± 0.0 | 0.18 ± 0.0 |
| Oleic acid (C18:1n9c) | 2.55 ± 0.3 | 2.27 ± 0.2 | 2.65 ± 0.2 | 2.30 ± 0.2 | 2.03 ± 0.2 | 2.70 ± 0.1 | 1.50 ± 0.1 |
| Cis-eicosenoic acid (C20:1) | 0.06 ± 0.0 | 0.05 ± 0.0 | 0.06 ± 0.0 | 0.05 ± 0.0 | 0.03 ± 0.0 | 0.06 ± 0.0 | 0.03 ± 0.0 |
| Erucic (C22:1n9) | 0.04 ± 0.0 | 0.04 ± 0.0 | 0.06 ± 0.0 | 0.03 ± 0.0 | 0.04 ± 0.0 | 0.04 ± 0.0 | |
| Total monosaturated fatty acids | 3.64 ± 0.4 | 2.83 ± 0.3 | 3.78 ± 0.3 | 2.74 ± 0.2 | 2.35 ± 0.2 | 3.85 ± 0.1 | 1.79 ± 0.2 |
| Linoleic acid (C18:2n6c) | 0.77 ± 0.1 | 0.74 ± 0.1 | 0.80 ± 0.1 | 1.25 ± 0.11 | 0.76 ± 0.1 | 0.82 ± 0.0 | 0.82 ± 0.1 |
| Gamma linolenic acid (C18:3n6) | 0.03 ± 0.0 | 0.02 ± 0.0 | 0.03 ± 0.0 | 0.35 ± 0.0 | 0.03 ± 0.0 | ||
| Linolenic acid (C18:3n3) | 0.27 ± 0.0 a | 0.12 ± 0.0 d | 0.28 ± 0.0 a | 0.03 ± 0.0 e | 0.18 ± 0.0 c | 0.29 ± 0.0 a | 0.23 ± 0.0 b |
| Cis-eicosadienoic acid (C20:2) | 0.02 ± 0.0 | 0.02 ± 0.0 | 0.02 ± 0.0 | 0.00 | 0.02 ± 0.0 | 0.02 ± 0.0 | 0.02 ± 0.0 |
| Cis- eicosatrienoic acid (C20-3n3) | 0.01 ± 0.0 | 0.01 ± 0.0 | 0.05 ± 0.0 | 0.03 ± 0.0 | 0.01 ± 0.0 | 0.03 ± 0.0 | |
| Cis-eicosatrienoic acid (C20:3n6) | 0.09 ± 0.0 | 0.09 ± 0.0 | 0.09 ± 0.0 | 0.08 ± 0.0 | 0.09 ± 0.0 | 0.06 ± 0.0 | |
| Arachidonic acid (C20:4n6) AA 1 | 1.17 ± 0.1 c | 1.16 ± 0.1 c | 1.21 ± 0.6 a | 1.07 ± 0.1 b | 1.21 ± 0.1 a | 1.23 ± 0.0 a | 0.70 ± 0.16 d |
| Cis-eicosapentaenoic acid (C20:5n3) EPA 2 | 0.64 ± 0.1 a | 0.50 ± 0.0 b | 0.66 ± 0.1 a | 0.50 ± 0.0 b | 0.40 ± 0.0 c | 0.67 ± 0.0 a | 0.33 ± 0.0 d |
| Cis-docosahexaenoic acid (C22:6n3) DHA 3 | 1.74 ± 0.2 b | 1.20 ± 0.1 d | 1.81 ± 0.2 b | 1.50 ± 0.1 c | 1.99 ± 0.2 a | 1.84 ± 0.1 b | 0.98 ± 0.1 d |
| Total polyunsaturated fatty acids | 4.69 ± 0.5 a | 3.77 ± 0.4 b | 4.87 ± 0.4 a | 4.82 ± 0.43 a | 4.62 ± 0.5 a | 4.96 ± 0.2 a | 3.15 ± 03 c |
| Elaidic acid (C18:1n9t) | 0.03 ± 0.0 | 0.03 ± 0.0 | 0.03 ± 0.0 | 0.03 ± 0.0 | 0.03 ± 0.0 | 0.02 ± 0.0 | |
| Total unsaturated fatty acids | 8.32 ± 0.8 a | 6.61 ± 0.7 c | 8.64 ± 0.8 a | 7.56 ± 0.7 b | 6.98 ± 0.7 c | 8.80 ± 0.3 a | 4.94 ± 0.4 d |
| PUFA 4 | 4.72 | 3.8 | 4.9 | 4.82 | 4.65 | 4.99 | 3.15 |
| EPA + DHA | 2.38 | 1.70 | 2.47 | 2.00 | 2.39 | 2.51 | 1.31 |
| Omega–3 5 | 2.65 | 1.83 | 2.75 | 2.40 | 2.60 | 2.80 | 1.57 |
| Omega–6 | 2.07 | 1.97 | 2.15 | 2.42 | 2.05 | 2.19 | 1.58 |
| Total fatty acids | 14.01 ± 1.4 a | 12.00 ± 1.2 a | 14.54 ± 1.3 a | 13.5 ± 1.2 a | 12.20 ± 1.1 a | 14.82 ± 0.6 a | 8.80 ± 0.8 b |
| Treatments | Temperature | pH | Dissolved Oxygen | Conductivity (µS cm −1) | Salinity (%) | |
|---|---|---|---|---|---|---|
| mg L−1 | (% Sat.) | |||||
| Mesocosms Cladocerans | 28.5 ± 1.90 | 7.59 ± 0.10 | 7.21 ± 0.42 | 104 ± 2.40 | 141.7 ± 17 | 0.05 ± 0.03 |
| Exercise + Artemia | 28.4 ± 1.91 | 7.62 ± 0.11 | 7.57 ± 0.68 | 99.2 ± 2.75 | 6065.6 ± 527 | 3.0 ± 0.04 |
| Exercise + D. brasiliensis | 29.9 ± 1.57 | 7.6 ± 0.08 | 7.71 ± 0.78 | 100 ± 3.46 | 5932.1 ± 390 | 3.06 ± 0.02 |
| Exercise + mixture (A + Db) | 28.1 ± 1.95 | 7.56 ± 0.04 | 7.72 ± 0.41 | 99.0 ±1.00 | 6038.3 ± 309. | 3.08 ±0.02 |
| Traditional + Artemia | 28.8 ± 1.78 | 7.64 ± 0.04 | 7.44 ± 0.52 | 97.1± 4.00 | 6131.3 ± 256 | 3.07 ± 0.08 |
| Traditional + D. brasiliensis | 28.2 ± 1.79 | 7.56 ± 0.10 | 7.12 ± 0.25 | 97.7± 2.69 | 6202.2 ± 374 | 3.02 ± 0.10 |
| Traditional + mixture (A + Db) | 28.7 ± 1.31 | 7.66 ± 0.10 | 7.44 ± 0.51 | 98.7 ±3.09 | 5944.6 ± 244 | 3.12 ± 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arbeláez-Rojas, G.A.; Melão, M.d.G.G. Larviculture of Brycon amazonicus under Different Food and Farming Systems. Aquac. J. 2023, 3, 209-226. https://doi.org/10.3390/aquacj3040017
Arbeláez-Rojas GA, Melão MdGG. Larviculture of Brycon amazonicus under Different Food and Farming Systems. Aquaculture Journal. 2023; 3(4):209-226. https://doi.org/10.3390/aquacj3040017
Chicago/Turabian StyleArbeláez-Rojas, Gustavo Alberto, and Maria da Graça Gama Melão. 2023. "Larviculture of Brycon amazonicus under Different Food and Farming Systems" Aquaculture Journal 3, no. 4: 209-226. https://doi.org/10.3390/aquacj3040017
APA StyleArbeláez-Rojas, G. A., & Melão, M. d. G. G. (2023). Larviculture of Brycon amazonicus under Different Food and Farming Systems. Aquaculture Journal, 3(4), 209-226. https://doi.org/10.3390/aquacj3040017





