Abstract
Background: Today, cancer is the leading cause of death. It appears that using large amounts of natural resources reduces the damaging consequences of cancer therapy. Over the last decade, phytoconstituents in food have shown potential as anticancer agents. Cinnamaldehyde and its congeners have shown their ability to act against several cancers. Objective: This article’s purpose is to examine the cellular and molecular mechanisms that entail cinnamaldehyde’s potential for use in the treatment and prevention of cancer. Methods: The anticancer effects of cinnamaldehydes were researched by searching a variety of academic databases (such as Scopus, PubMed, Science Direct, Medline, and Google scholar) in accordance with a predetermined set of criteria. Results: Studies were conducted in order to investigate the mechanism(s) by which cinnamaldehyde causes cancer cells to undergo apoptosis. Additionally, research has shown that cinnamaldehydes have an effect that inhibits the invasion and metastasis of cancer cells. This class of compounds was investigated for their possible application in the treatment of cancers, such as leukaemia, colon, hepatocellular carcinoma, prostate, mouth, and breast cancers. Conclusion: According to an in-depth examination of the relevant published research, cinnamaldehyde and its analogs demonstrate several signalling pathways that are effective against cancers. This review provides a summary of recent research on cinnamaldehyde and its congeners as potential candidates for anticancer drugs.
1. Introduction
The development of tumours is one of the cardinal features of cancer, which is defined as the uncontrolled division of cells. Cancerous tumours are hefty menaces and difficult to treat [1]. Tumours have the capacity to create new blood vessels, metastasis (spread to other organs), and resist apoptosis, all of which are hallmarks of malignant growths. A huge freak over Western food and the sedentary lifestyle, with the ever-increasing population of elderly people around the globe, are contributing towards raising the number of cancer patients [2]. Cancer is one of the main causes of death, and this is particularly true in areas of the country that are economically considered poor. This is mostly due to the fact that cancer is often misdiagnosed and that patients in these areas have limited access to treatment and support services [3]. In view of the exponential rise in the number of new cases and fatalities, unarguably, cancer will continue to be a serious concern for the health of people all over the world [4]. The management and treatment of cancer may include the use of radiation therapy, chemotherapy, surgery, or any combination of these. Immunotherapy and drug therapy are the two widely accepted ways to treat cancer. Radiation therapy, surgery, and other forms of cancer treatment may often be excruciating for patients, yet they are generally essential. Chemotherapy is not successful against many types of tumours, and some patients may experience severe side effects [5]. The fight against cancer has been elevated to a top priority for the nation’s health system, and one of the primary strategies for this fight is the research and development of highly effective cancer treatments that are lethal only to cancer cells and not to healthy cells [6]. Investigating the possibility of naturally occurring compounds being selectively hazardous to cancer cells is an interesting line of research to pursue. There is reason to be hopeful about the utilization of natural compounds derived from plants and marine life as potential anticancer drugs since recent research has shown promising outcomes [7]. This is due to the fact that these substances have an effect on a variety of cellular processes, such as proliferation, differentiation, apoptosis, metastasis, DNA damaging, and repairing [8]. Herbal medicine has a long history of use in the treatment of cancer, and it has been used in a broad range of cultures around the world [9,10]. This is due to the fact that natural components are less poisonous and have a higher dose tolerance in humans. It is estimated that there are more than five thousand phytochemicals with antineoplastic activities [11]. These components have the potential to provide crucial resources for the development of novel anticancer medications and are a safer alternative to a variety of synthetic medicines that are currently used in clinical therapies [12].
There is a natural class of substances that shows a lot of promise for the treatment of cancer, and one of those classes is essential oils. Essential oils may be extracted from culinary plants and fragrant herbs [13]. Cinnamaldehyde and its analogs are the principal chemical components of the Cinnamomum bark/twig. The Cinnamomum genus is a subset of the Lauraceae family, and it was not recognized as such until the year 1760 [14]. Cinnamomum, along with Laurus and Persea, is considered to be the most important and valuable of the three major genera that make up the Lauraceae family. The genus Cinnamomum is comprised of approximately 250 different species, the majority of which are located in tropical and subtropical regions of Asia, Australia, and the Pacific Islands [15]. Cinnamaldehyde is a naturally occurring flavonoid that is responsible for cinnamon’s flavour and aroma. Cinnamaldehyde has been found to prevent or lessen the severity of a variety of illnesses, including but not limited to diabetes, atherosclerosis, cancer, inflammation, and cardiovascular disease [14]. It has been found that cinnamaldehyde and its natural derivatives have an antiproliferative effect against cancers that originate in various parts of the body [16]. Studies are being conducted both in vitro and in vivo to investigate the molecular and mechanistic pathways of cinnamaldehyde’s antiproliferative activity. Some of these pathways include the induction of apoptosis and the arrest of the cell cycle [17].
Even though there is a significant amount of evidence on the anticancer effects of cinnamaldehyde from in vivo and in vitro studies, a comprehensive review of cinnamaldehyde and its natural analog’s anticancer properties has not yet been carried out in recent times. In light of this, the study that is provided here provides information about the anticancer potential of cinnamaldehyde and its analogs across a variety of cancer types, sheds light on the mechanisms that are at work, and makes some tentative conclusions.
2. Anticancer Studies and Conducting Literature Searches
All of the systematic investigations were carried out in accordance with the guidelines outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses document [18]. Electronic databases and search engines, such as Science Direct, Google Scholar, PubMed, Medline and Scopus, were used in order to collect the research that was conducted on cinnamaldehyde’s ability to inhibit the growth of cancerous cells. This search was not constrained by any time constraints, and the most current one was carried out in December 2022. Combinations of the following terms were used to search for articles on the following topics: cinnamaldehyde; cinnamaldehyde analogs; anticancer; liver cancer; breast cancer; stomach cancer; colon cancer; prostate cancer; lung cancer; skin cancer; leukaemia; antitumor; apoptosis; prevention; in vivo; in vitro. The information that was retrieved was recorded in the order that it was found. This information, which describes the molecular mechanisms that are behind cinnamaldehyde’s antiproliferative activity against various cancers, was recorded. The search for and selection of relevant material are organized according to the diagram in Figure 1.
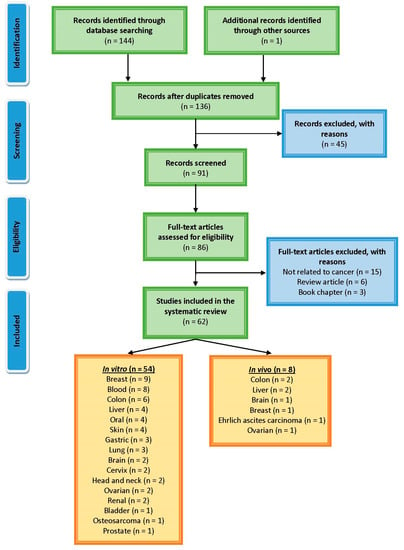
Figure 1.
PRISMA flowchart illustrates the process of selecting and retrieving papers on the molecular pathways that cinnamaldehyde plays in cancer.
3. Description of Cinnamaldehyde
Cinnamon (Cinnamomum genus) contains a significant amount of an α-, β-unsaturated aldehyde, commonly referred to as cinnamaldehyde (3-phenylprop-2-enal; C9H8O). Cinnamaldehyde is a viscous liquid that ranges in colour from yellow to a greenish yellow and smells strongly of cinnamon [19]. The percentage of cinnamaldehyde found in cinnamon oil can range anywhere from 65 to 90%. It is possible for cinnamaldehyde to oxidize into styrene if it is not transported or kept appropriately [20]. It has been proven to be rapidly absorbed from the stomach, and it can also be absorbed by the skin (52%). Cinnamaldehyde is metabolized and excreted primarily in the urine and, to a minor extent, in the faeces. After oral or intraperitoneal administration to rats and mice, 69–98% of the dose of cinnamaldehyde was recovered in the urine and faeces within 24 h [21]. Several diseases, including diabetes, atherosclerosis, cancer, inflammation, and cardiovascular disease, may be avoided or have their severity reduced when exposed to cinnamaldehyde [14]. Cinnamaldehyde is one of the standardized allergens which has an effect on the body that may be seen in the form of increased histamine release and cell-mediated immunity [22].
Pharmacokinetics data reveal that cinnamaldehyde is widely distributed in the body. It undergoes both oxidative and reductive metabolism to produce both acid and alcohol, as evidenced in urine analysis [23]. Apart from these primary metabolites, cinnamaldehyde can also produce methyl cinnamate with the assistance of a transferase enzyme. The biotransformation of cinnamaldehyde, on the other hand, casts doubt on the possibility that the bioactivity of cinnamaldehyde can be attributed to the sum of its metabolites. As a direct consequence of this, it is quite possible that more attempts will be undertaken to conquer the unpredictability [24].
4. Anti-Cancer Potential of Cinnamaldehyde
It is now obvious, based on a review of the relevant literature, that cinnamaldehyde has anti-cancer effects through a number of different mechanisms that are interconnected with one another. These effects reduce some of the most severe symptoms of abnormally rapid cell growth. Some of the mechanistic approaches include the induction of apoptosis, cell cycle arrest, interruption in angiogenesis, free radical scavenging, inhibition of inflammation, and interference with cellular invasion and metastasis. According to the types, doses and duration of treatment of cancer, the mechanisms involved could change. The mechanism with which cinnamaldehyde excels in anticancer activity is shown in Figure 2. In the subsequent subsections, a comprehensive analysis of the anticancer actions of cinnamaldehyde have been reported.
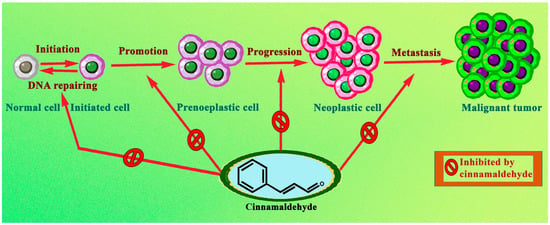
Figure 2.
Biomolecular mechanism through which cinnamaldehyde prevents cancer formation and development.
Normal cells, while being transformed into a malignant tumour, also form several intermediate cells, which do possess cancerous properties. Therefore, cinnamaldehyde, by virtue of its clinical diversity, was largely capable of acting preferentially on several processes which evolve into a malignant tumour.
4.1. Cinnamaldehyde on Bladder Cancer
Researchers are becoming increasingly concerned about the rising global incidence of bladder cancer, and the adverse effects of chemotherapy medications have long been recognized as a primary barrier to developing effective cancer treatments. Bladder cancer, the most common among other forms of cancer, initiates with the lining cells of the bladder (urothelial cells). It is important to note that urothelial cells are not limited to the urinary tract. This can also be found in the kidneys and ureters [25]. Cinnamaldehyde, the active component in cinnamon, has been thought to possess anti-inflammatory and antioxidant properties for a considerable amount of time. In the present study, cinnamaldehyde was taken into account to evaluate its anticancer activity against bladder cancer 5637 cell lines. It has been observed that the exposure of bladder cancer 5637 cells with cinnamaldehyde for 24, 48, and 72 h at concentrations 0.02, 0.04, and 0.08 mg/mL induced apoptosis (confirmed using Annexin V-FITC/PI and Hoechst 33258 staining) in a dose-dependent way. In addition to this, when the scratch test was performed, it had shown to have a significant inhibitory effect on cell migration. Apart from that, cinnamaldehyde also exhibited inhibitory activity on glucose uptake and lactate production (p < 0.05), as well as on Heat Shock Protein Transcription Factor-1 (HSF-1), epidermal growth factor receptor 2 (ErbB2), and lactate dehydrogenase A (LDHA) gene expressions, and finally, HSF1 and LDHA protein levels. Therefore, it concludes that cinnamaldehyde induces apoptosis by suppressing ErbB2-HSF1-LDHA pathway to inhibit 5637 cell lines [26].
4.2. Cinnamaldehyde on Blood Cancer
Blood cancer is a kind of cancer that affects the blood cells. Myeloma, leukaemia, and lymphoma are the three most common types of cancer that originate in the bone marrow or blood and may spread throughout the body [27]. Leukaemia is a kind of cancer that affects both the blood cells and the bone marrow. It manifests itself when the body produces an abnormally high number of white blood cells, which subsequently inhibit the bone marrow’s ability to make healthy red blood cells and platelets [28]. Whereas, lymphoma is of 2 types, viz. Non-Hodgkin lymphoma originates in lymphocytes, a kind of white blood cell that assists the body in its battle against infections and Hodgkin lymphoma is a kind of cancer that affects the blood and originates in lymphocytes, which are the cells that make up the lymphatic system [29]. Hodgkin lymphoma may be diagnosed when an aberrant form of lymphocyte known as a Reed-Sternberg cell is present in the patient’s body. Finally, multiple myeloma may be traced back to a cancer that begins in the plasma cells, a subtype of white blood cell that is produced in the bone marrow [30].
In a study conducted by Moon and Pack in 1983’s cinnamaldehyde was found to have a cytotoxic effect on cancerous cells. Leukaemia L1210 cell lines was taken into consideration where it has been observed that at ED50 of 4.8 µg per mL, cinnamaldehyde in Fischer’s medium reduced the malignant cell growth by half. Therefore, the result revealed that cinnamaldehyde inhibited L1210 cells by blocking protein synthesis via entrapping sulfhydryl-containing amino acids inside the cell [31].
A subsequent study performed in 2003 on human promyelocytic leukaemia, HL-60 cells reveal that cinnamaldehyde at 40 µM acts as a powerful inducer of apoptosis because it causes ROS-mediated mitochondrial permeability transition, which results in the release of cytochrome c into the cytosol. Whereas it has also been noticed that N-acetylcystein, a kind of antioxidant, seemed to be able to inhibit apoptotic cell death in cells previously treated with cinnamaldehyde [32].
In 2004, Fang et al. attempted an experimental study to evaluate anticancer effect of trans-cinnamaldehyde, which is naturally present in Cinnamomum osmophloeum leaves with a yield of 23.79 mg per gram on human Jurkat and U937 cell lines. According to the findings of the study, which utilized IC50 values of 0.057 and 0.076 µM, respectively, trans-cinnamaldehyde displayed a strong inhibitory effect against the viability of both Jurkat and U937 cells. In addition, it is important to note that the concentration of trans-cinnamaldehyde needed to inhibit the growth of Jurkat and U937 cell lines by approximately two-fold with respect to the control was 0.057 µM. trans-cinnamaldehyde causes a nonlinear concentration-dependent rise in the accumulation of Jurkat and U937 cells in the S phase of the cell cycle while simultaneously causing a decline in the percentage of cells in the G0/G1 phase [33].
To examine the effects of trans-cinnamaldehyde on the human leukaemia K562 cell line and the cytotoxicity of cytokine-induced killer (CIK) cells against K562 cells. In order to investigate apoptosis, Fas expression, and mitochondrial transmembrane potential in the K652 cells, flow cytometry was used. Apoptosis seemed to be induced in 8.9% of K562 cells when they were treated with trans-cinnamaldehyde at a concentration of 180 µmol per litre for nine hours. After 24 h of treatment with 120 or 180 µmol per litre trans-cinnamaldehyde, the percentage of apoptotic cells increased to 18.63% and 38.98%, respectively. In K562 cells, trans-cinnamaldehyde has a considerable inducing effect on the expression of Fas and has a suppressive effect on the mitochondrial transmembrane potential. Trans-cinnamaldehyde treatment at 120 and 180 µmol per L for 9 h increased the percentage of K562 cells that were lysed by expanded CIK cells from 34.84% to 48.21% and 64.81% at an E:F ratio of 25:1 and from 49.26% to 57.81% and 73.36% at an E:F ratio of 50:1. Therefore, it was noticed that trans-cinnamaldehyde induces apoptosis in human leukaemia K562 cells, which contributes to the cytotoxic effects that it has on these cells, and it also enhances the cytotoxicity of CIK cells against K562. These advantages of trans-cinnamaldehyde may become particularly helpful for those who have leukaemia and have already had a hematopoietic stem cell transplant (HSCT) [34].
When cinnamaldehyde, a major component of cinnamon bark extract, was taken to investigate its possible antiproliferative effects on myeloma cells by studying its anti-angiogenic and anti-inflammatory effects on the cells. The researchers discovered that cinnamaldehyde suppressed the proliferation of myeloma cells when tested on the human myeloma cell line RPMI 8226. After 24 h of treatment, the IC50 value was discovered to be 72 mg per mL, which resulted in a 50% decrease in cell growth in comparison to untreated controls. The administration of cinnamaldehyde over a prolonged period of time resulted in a significant reduction in the expression of angiogenic factors and cyclooxygenase at the mRNA and protein levels, respectively. The cell cycle of the exposed cells became stopped time dependently at the G0/G1 phase. Cinnamaldehyde has been shown to inhibit cell growth in addition to causing DNA to fragment in a time-dependent way, which ultimately results in cell death. In conclusion, it would seem that there are a few different approaches that may be used in order to eradicate myeloma cells, some of which include inhibiting the proliferation of the cells, lowering inflammatory responses, and triggering cell death. As a result, cinnamaldehyde has the potential to open the door to research that might lead to the development of an effective chemotherapeutic agent or herbal medicine for the treatment of multiple myeloma [35].
In order to find out the mechanistic pathway to suppress canonical IL-1β secretion, Ho et al., in the year 2018 discovered that cinnamaldehyde and 2-methoxy cinnamaldehyde at concentrations 25 to 100 µM exhibited to inhibit IL-1β secretion dose dependently in human monocytic THP-1 cell lines. The tested compounds resulted in the expressions of pro-IL-1β (Interleukin-1β) and NLRP3 (NLR Family Pyrin Domain Containing 3) to bring down to a lower level. Both the cinnamaldehyde and 2-methoxy cinnamaldehyde were found to be able to inhibit the ATP (adenosine triphosphate)-induced, decrease the cytosolic pro-caspase-1, and increase the secreted caspase-1 [36].
Due to the potential to suppress the antitumor immune response by restricting T cell proliferation, cytokine production, and tumour cell death, myeloid-derived suppressor cells have emerged as new therapeutic targets in cancer treatment. Hence, the aim of the authors of this research was to screen the antimyeloid-derived suppressor cell activity of cinnamaldehyde. Therefore, researchers used myeloid-derived suppressor cells isolated from the spleens of TLR4−/− mice that had colon cancer (MC38 tumour) and had exposure to cinnamaldehyde (4 μg/mL) for a certain period of time. They have the increased capacity to inhibit cell proliferations via the TLR (Toll-like receptor)4-dependent pathway and can induce cell death via apoptosis. Additional testing revealed that the treatment with cinnamaldehyde led to an increase in the expression of Bax (Bcl-2-associated X protein) and caspase-9, while the expression of Bcl-2 (B-cell lymphoma 2) was reduced, indicating that cinnamaldehyde induced apoptosis in myeloid-derived suppressor cells by following an intrinsic pathway [37].
The research aims to investigate whether or not cinnamaldehyde, which has been hypothesised to have anticancer properties, has any impact on human HL-60 cell lines. The study showed that cinnamaldehyde, at concentrations ranging from 0 to 0.8 mg/mL, significantly raised the number of cells in the G2/M phase of the cell cycle and revealed significant suppression of the growth of tumour cells. In response to the cinnamaldehyde treatment, the expression of cyclin A, cyclin B1, ERK2 (mitogen-activated protein kinase 1), and p-ERK was shown to be decreased by Western blotting. Cinnamaldehyde, in particular, has been demonstrated to reduce the viability of tumour cells by down-regulating the target molecules that are involved in the control of the cell cycle and mitotic regulation [38].
4.3. Cinnamaldehyde on Brain Cancer
An in vitro and in vivo study was conducted utilizing glioblastoma TS14-15, TS15-88 cells, and the mouse orthotopic xenograft model, respectively, to evaluate the anticancer properties of 2′-hydroxycinnamaldehyde, a natural congener of cinnamaldehyde. Combination treatment with 2′-hydroxycinnamaldehyde at a concentration of 5 μM and commercially available temozolomide at a concentration of 250 μM significantly reduced cellular growth, invasiveness, and cell survival while increasing cytotoxicity. In addition, the combined treatment dramatically decreased the levels of invasiveness, cell differentiation, and mesenchymal transition markers N-cadherin, Zeb1, and β-catenin. Additionally, the combination therapy with 2′-hydroxycinnamaldehyde (50 mg kg−1) and temozolomide (30 mg kg−1) prevented tumour growth in an orthotopic xenograft model carried out on mice. According to the findings of this research, the combination of temozolomide and 2′-hydroxycinnamaldehyde showed a promising effect on glioblastoma [39].
A current study attempted to determine, for the very first time, if cinnamaldehyde could be co-administered with doxorubicin (an anticancer agent) against U87MG glioma cells to enhance doxorubicin’s lethal impact and overcome or lessen its adverse effects. The MTT assay was performed to establish the potential of doxorubicin and cinnamaldehyde as a single performer or in combination using U87MG cells. Several postulations, viz., caspase-3 and -9 activation, and mitochondrial membrane potential, were made to ascertain the etiology of cell death. Additionally, an investigation on the levels of apoptotic gene expression was carried out (Bax and Bcl-2). The fact that the IC50 values for cinnamaldehyde and doxorubicin were 11.6 and 5 µg/mL, respectively, in a cellular toxicity assay suggests that both components may have a detrimental effect on the survival of U87MG cells. Cinnamaldehyde, when combined with doxorubicin, produced a significant increase in the cytotoxic effect of doxorubicin on U87MG cells. Cinnamaldehyde has the potential to induce programmed cell death, i.e., apoptosis in U87MG cells. This was discovered by the use of SUB-G1, MMP, caspase-3, and -9 activity assays, as well as Bcl-2 and Bax gene expressions, where all the levels were enhanced. In addition to this, it has been shown that doxorubicin’s apoptotic effects were amplified in the presence of cinnamaldehyde. Therefore, cinnamaldehyde seemed to have an effect on the level of doxorubicin-induced apoptosis in human glioblastoma cells by reducing cell proliferation. Overall, the data suggested that treating glioblastoma with a combination of doxorubicin and cinnamaldehyde might be effective [40].
It is still unknown how cinnamaldehydes are effective against glioma. Therefore, to investigate the effects of cinnamaldehyde on the viability of temozolomide-treated T98G glioma cells, as well as the expression of chemokine receptors CXCR4 and CXCR7, Chen et al. designed an experiment in 2020 and observed that cell viability was considerably lower following the combination treatment with cinnamaldehyde (75 μM) and temozolomide (300 μM) than treatment with temozolomide or cinnamaldehyde alone. CXCR4 and CXCR7 expressions were evaluated using western blotting. As a consequence of this, it suppresses the expression of CXCR4. Cinnamaldehyde, while co-administering with temozolomide, showed a sharp reduction in the viability of glioma cells, most likely through inhibiting CXCR4 expression [41].
4.4. Cinnamaldehyde on Breast Cancer
Breast cancer is the second most common cancer in women after skin cancer. It is a malignant tumor that starts in the breast tissue and spreads throughout the breast. Although women are more likely to be diagnosed with breast cancer, males are not immune to the disease. The presence of a lump in the breast, a change in the breast’s form or texture, and a bloody discharge from the nipple are all symptoms that can be associated with breast cancer. To get rid of this issue numerous phytocompounds have already been recognized as effective and safe in use, and cinnamaldehyde is one of them. An attempt by Lu et al. was adopted in the year 2010 where cinnamaldehyde, in terms of the cinnamon extract, seemed to be successful in inhibiting the effects of vascular endothelial growth factor (VEGF) on HUVEC and bovine capillary endothelial cell proliferation, migration, and tube formation (32 µg/mL) in vitro and in vivo formation of tumours. Cinnamaldehyde (IC50: 30 ng/mL) possesses those activities by inhibiting the kinase activity of purified VEGFR2 as well as the mitogen-activated protein kinase- and STAT3-mediated signalling cascade in endothelial cells [42].
In a separate study evaluating cinnamaldehyde’s efficacy against the MCF-7 breast cancer cell line, Vangalapati et al., in the year 2013, used an MTT assay to determine whether or not there was an inhibition of cell growth. According to the research, at a dosage of 200 µg/mL, cinnamaldehyde is effective in inhibiting the growth of 32.3% of the studied MCF-7 cancer cell lines [43].
Another study on the same cell lines (MCF-7) showed enhanced cytotoxic action when cinnamaldehyde was tested in a colorimetric MTT assay for 24 and 48 h. The IC50 values for 24 and 48 h after treatment were found to be 58 and 140 µg/mL, respectively, where the tested cell line’s growth was reduced drastically [44].
Protein tyrosine phosphatase 1B (PTP1B) has the potential to be a useful therapeutic target for cancer; thus, cinnamaldehyde’s impact on PTP1B enzyme activity and MCF-7 cancer cell survival is examined. After performing the experiment, it was observed that cinnamaldehyde decreased the activity of PTP1B at a concentration of 500 μM (IC50: 1 mM), which resulted in an inhibition of the growth of MCF-7 cancer cells at 50 μM [45].
One of the most recently discovered adipokines, nicotinamide phosphoribosyl transferase (NAMPT, visfatin), has not only been connected to metabolic syndrome and obesity but it has also been associated to the proliferation of cancer. Therefore, inhibiting NAMPT and reducing the synthesis of nicotinamide adenine dinucleotide might be a viable treatment for cancer. In order to accomplish this, the researchers of this experiment used the breast cancer MDA-MB-231-GFP (GFP: Green fluorescent protein) cell line for an in vitro study and the female Balb/c nude xenograft animal model for an in vivo study, where tumors were implanted by the subcutaneous injection of MDA-MB-231-GFP cells. They treated both models with varying concentrations of visfatin combined with cinnamaldehyde and FK866 (a visfatin inhibitor) to evaluate cellular toxicity. This was the first study to demonstrate that a naturally occurring molecule may block NAMPT both extracellularly and intracellularly. In this experiment, the cinnamaldehyde at concentration 25–100 µM showed significant inhibitory activity against the breast cancer cell line, which led to severe cell death. On the other hand, as compared to the group that had been treated with visfatin, the amount of cinnamaldehyde (75 µM) and FK866 (100 nM) that dramatically decreased cell viability by reducing visfatin-induced proliferative mediated proteins, such as mTOR (mammalian target of rapamycin), p-mTOR, p-PI3K, PCNA, and PI3K. This combination was also found to have reduced intra- and extra-cellular NAMPT protein expressions. However, in the same experiment, the in vivo study revealed that a combination of cinnamaldehyde (100 mg kg−1) and FK866 (4 mg kg−1) results in a tumour with a reduced weight compared to the control. It also demonstrated that the combination reduced the visfatin-induced luminescence signal in tumour luminescence tests. Lower proliferating cell nuclear antigen was also observed in the animal group treated with cinnamaldehyde and FK866 combination [46].
In a different experiment, cinnamaldehyde at 2.5, 5, 10, 20, and 40 μg/mL concentration resulted in a suppression of the proliferation of MDA-MB-231 cells. After 24 h, the IC50 concentration of cinnamaldehyde was measured to be 16.9 μg/mL, and after 48 h, it was measured to be 12.23 μg/mL. The effect of cinnamaldehyde on apoptosis was evaluated using flow cytometry at a range of different concentrations. In MDA-MB-231 cells, induction of apoptosis occurred at rates of 9.5, 10.5, and 22.5% when the cells were treated with cinnamaldehyde at doses of 10, 15, and 20 μg/mL, respectively. The results of Trans well testing demonstrated that cinnamaldehyde at doses 15 and 20 μg/mL were adequate to suppress the invasiveness of MDA-MB-231 cells. In a wound healing test, the same concentration of the compound significantly decreased the migration capability of MDA-MB-231 cells. These findings suggest that cinnamaldehyde may be able to significantly inhibit the invasion of MDA-MB-231 breast cancer cells [47].
In another study cinnamaldehyde (100 μM), when tested on MCF-7 cells, showed significant cytotoxicity and inhibition in cellular growth. At the same concentration in clonogenic survival assay, cinnamaldehyde possesses inhibition in the reproductive ability of breast cancer cells [48].
Oncolytic virotherapy, a relatively new approach to cancer treatment, has promise as an anticancer therapy because of its capacity to boost antitumor adaptive immunity. The oncolytic measles virus is ideally suited for targeting breast cancer because of upregulating the measles virus receptor known as nectin-4. Cinnamaldehyde at concentrations of 60 and 80 μM and the measles virus at a MOI (multiplicity of infection) of 0.1 exhibited a greater antitumor activity against breast cancer MCF-7 cells when combined than treated alone. Additional mechanistic research revealed that the combination of the measles virus and cinnamaldehyde has a synergistic antibreast cancer activity. This activity is mediated by enhanced apoptosis in the cancer cells. Cinnamaldehyde, on the other hand, did not have an effect to enhance the infection in MCF-7 cells with the oncolytic measles virus. It was also shown that cinnamaldehyde displayed greater cytotoxicity at concentrations ranging from 60–400 μM, although to a lower degree than the measles viral combinational treatment. As a result, the findings of this research provided the first evidence that using an oncolytic measles virus in conjunction with cinnamaldehyde could be an effective treatment strategy for breast cancer cells that have been marked with the protein nectin-4 [49].
A recent study found that the combination of cinnamaldehyde and chlorogenic acid had more inhibitory effects on breast cancer cells than either component did when administered alone. The MDA-MB-231, MCF-7, and HCC1419 breast cancer cell lines were used during this study. The only treatment that successfully induced cell death in breast cancer cells by decreasing the mitochondrial membrane potential, which also significantly altered cellular and mitochondrial architecture, and significantly increased superoxide generation in mitochondria was a combination of cinnamaldehyde and chlorogenic acid at a concentration of 35 µM and 250 µg/mL. Cinnamaldehyde and chlorogenic acid are both compounds that have been demonstrated to have potent anticancer capabilities on their own. However, it has been shown that the most effective treatment for breast cancer is when these two substances are combined. According to the findings of this study, the use of phytochemicals or mixes offers innovative approaches to the treatment of breast cancer [50].
In malignant cells, the transcription factor known as STAT3 (signal transducer and activator of transcription 3) often has an elevated level of activity. A downstream target of STAT3, the cMyc gene is an important site in treating cancer. As a result, the objective of the study was to establish whether or not cinnamaldehyde has the potential to cause apoptosis in breast cancer cells by the means of the STAT3/cMyc pathway. Cinnamaldehyde (5, 10, 20, 40, and 80 μg/mL) was shown to have a significant inhibitory effect on cell growth and migration in MDA-MB-231, MCF-7, and 4T1 cells when tested over time and in a dose-dependent way. Cinnamaldehyde, at the same dosage as the cMyc inhibitor 10074-G5, was shown to have a promising effect, according to the findings of the research. It was also able to restrict the proliferation and migration of breast cancer cells, which may be connected to the upregulating of the mitochondrial apoptosis in those cells via downregulating apoptosis-mediated proteins through the STAT3/cMyc pathway [51].
4.5. Cinnamaldehyde on Cervical Cancer
The global mortality rate for female cancer patients remains the highest due to cervical cancer. HPV (human papillomavirus), namely HPV16, is the prime accuse in this development. The antitumor effect of a natural congener of cinnamaldehyde, i.e., 4-methoxycinnamaldehyde, was investigated on C-33A human cervical cancer cell lines. By using the MTT (3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide) assay, it was discovered that 4-methoxycinnamaldehyde (IC50: 110 μM) had a dose-dependent impact on these C-33A cells. Flow cytometry indicated that it significantly induced apoptosis with the same IC50. In addition, non-toxic doses of 4-methoxycinnamaldehyde were sufficient to substantially inhibit the invasive capacity of C-33A cells. Cell invasion of HPV16-expressing C-33A cervical cancer cells was considerably decreased by 4-methoxycinnamaldehyde, as demonstrated by a considerable drop in the MMP(Matrix metalloproteinases)14 expression as determined by the real-time polymerase chain reaction (PCR), although MMP9 expression was unaffected by the treatment [52].
To investigate the anticancer effects of cinnamaldehyde on human HeLa cell lines, Xie et al., in the year 2018, noticed that at concentrations ranging from 0 to 0.8 mg per mL, it dramatically increased the number of cells in the G2/M phase of the cell cycle and demonstrated substantial inhibition of tumour cell growth. Western blotting revealed that the expression of cyclin A, cyclin B1, ERK2, and p-ERK was suppressed in response to cinnamaldehyde treatment. Specifically, cinnamaldehyde was shown to decrease tumour cell viability by down regulating target molecules involved in cell cycle control and mitotic regulation [38].
4.6. Cinnamaldehyde on Colon Cancer
When it comes to malignant growth, colorectal cancer is one of the most common reasons for both morbidity and death. A pharmacological intervention that makes use of dietary components that activate the redox-sensitive Nrf2/Keap1-ARE signalling pathway has recently shown a promising method for the chemoprevention of human cancers, including colorectal cancer. In the quest for dietary Nrf2 (nuclear factor erythroid 2–related factor 2) activators that may have chemo-preventive effects targeting colorectal cancer, researchers have been concentrating their attention on trans-cinnamaldehyde since it is the principal flavour molecule found in cinnamon essential oil. The treatment of human colon cancer HT29, HCT116 cells with trans-cinnamaldehyde (10 µM) led to an increase in the cellular protein levels of Nrf2 and the identification of Nrf2 targets, i.e., γ-glutamyl-cysteine synthetase and heme oxygenase 1 (HO-1), which are involved in the antioxidant response. After being pre-treated with trans-cinnamaldehyde when a strong upregulation of cellular glutathione level occurs, the HCT116 cells seemed to have protected activity against the genotoxicity generated by hydrogen peroxide as well as the oxidative stress caused by arsenic. In human epithelial colon culture cells, all of these findings indicate that cinnamaldehyde has a potent stimulating effect on the antioxidant response mediated by Nrf2. Because of this, cinnamaldehyde may be an underappreciated chemo-preventive component in the diet that suppresses the development of colorectal cancer [53].
In continuation to the previous study, another group of researchers contributed their effort to investigate the chemo-preventive potential and the molecular mechanism behind cinnamaldehyde-induced Nrf2 activation in colorectal epithelial HCT116 cells in vitro and the comparison of Nrf2+/+ with Nrf2−/− mice in vivo. In HCT116 cells, the addition of cinnamaldehyde at a concentration of 20 µmol per litre prolonged the half-life of the Nrf2 protein by inhibiting ubiquitination. This resulted in the activation of cytoprotective Nrf2 target genes and an increase in the amount of cellular glutathione. Cinnamaldehyde was demonstrated to minimize the amount of AOM/DSS-induced inflammatory colon carcinogenesis by modifying the molecular markers of colorectal carcinogenesis. Cinnamaldehyde was able to reduce colorectal cancer in Nrf2+/+ mice, but it did not have this effect on Nrf2−/− mice, which shows that the chemo-preventive effects of cinnamaldehyde are reliant on Nrf2 [54].
Yu et al. in 2014 carried out research to investigate the impact that cinnamaldehyde has on chemotherapeutic-associated gene expression and to evaluate the potential advantages of utilizing cinnamaldehyde in conjunction with chemotherapeutic drugs in the treatment of colorectal cancer. Cinnamaldehyde is nowadays routinely being used in the treatment of colorectal cancer. Cinnamaldehyde was utilized to test the effectiveness of chemotherapeutic medications against human LoVo and HT-29 cells, and combination index analysis was done to assess whether or not it increased the efficacy of the treatments. IC50 values of cinnamaldehyde were found to be 9.48 and 9.12 μg/mL for Lovo and HT-29 cells, respectively. The expression of drug-metabolizing genes, such as orotate phosphoribosyl transferase (OPRT), excision repair cross-complementing 1 (ERCC1) breast cancer susceptibility gene 1 (BRCA1), thymidylate synthase (TS), and topoisomerase 1 (TOPO1), was analysed using quantitative polymerase chain reaction (PCR) in LoVo and HT-29 cells. Cinnamaldehyde made both LoVo and HT-29 cells more susceptible to the cytotoxic effects of the chemotherapeutic medicines than they would have been otherwise. In addition, cinnamaldehyde was able to decrease the mRNA expression of TOPO1, BRCA1, TS, and ERCC1, while simultaneously greatly raising the expression of OPRT. According to the findings, the use of cinnamaldehyde as an adjuvant in combination therapy with the chemotherapeutic medicines 5-fluorouracil and oxaliplatin for the treatment of colorectal cancer has considerable potential for success [55].
In another experiment, the effects of cinnamaldehyde were tested on human colon cancer cells HCT116, SW480, and LoVo at concentrations of 20, 40, and 80 µg/mL for 24 h. It was found that when compared with the control group, the proliferation inhibition rate of the cancer cell lines increased in a time- and dose-dependent way. Experiments using Transwells and cell-matrix adhesion showed that the cells’ ability to invade and adhere to surfaces was significantly reduced. Cinnamaldehyde was found to induce the expression of E-cadherin while simultaneously having a suppressive effect on the expression of MMP-2 and -9. It was also found to increase the rate of apoptosis. Cinnamaldehyde was found to have a pro-apoptotic effect too, which was further validated by the upregulation of genes that contribute to pro-apoptosis and the downregulation of genes that inhibit apoptosis. To investigate how cinnamaldehyde causes apoptosis, a PI3K inhibitor (LY294002) and insulin-like growth factor-1 (IGF-1) were employed to regulate the phosphoinositide 3-kinase (PI3K)/AKT pathway. Cinnamaldehyde, in addition to IGF-1, which acts as an anti-apoptotic agent, significantly decreased the amount of PI3K/AKT transcription activity. Cinnamaldehyde seemed to have mechanisms of action by inhibiting the PI3K/Akt signalling pathway, which regulated the expression of genes involved in processes, such as apoptosis, invasion, and adhesion [56].
BAG3, also known as B-cell lymphoma 2-associated anthanogene, is a protein that belongs to the BAG co-chaperone family. This protein has been investigated for the possibility that it plays a role in the adaptive responses of normal cells and cancer cells to stressful events. It was shown that 2′-hydroxycinnamaldehyde (50 µM) was responsible for the death of SW620 and SW480 colon cancer cells by the activation of caspase-7, -9, and poly (ADP-ribose) polymerase (PARP), as well as the confirmation of apoptosis by staining with Annexin V. Notably, 2′-Hydroxycinnamaldehyde was able to considerably raise BAG3 mRNA and protein levels in both time- and dose-dependent way. Through its ability to stimulate the activity of HSF1, 2′-hydroxycinnamaldehyde is responsible for the enhanced expression of BAG3. When the expression of BAG3 was suppressed using siRNA, more evidence that BAG3 was directly involved in 2′-Hydroxycinnamaldehyde-induced cancer cell death was discovered. In light of the fact that the body’s stress response system contributes to the development of cancer, the findings strongly suggest that BAG3 might be an effective therapeutic target for cancer therapy [57].
Cinnamaldehyde (100 μg/mL) was found to decrease cyclin D1 protein level through cyclin D1 degradation via GSK3β-dependent threonine-286 (T286) phosphorylation of cyclin D1. These findings suggest that cyclin D1 degradation may contribute to cinnamaldehyde-mediated decrease of cyclin D1 protein level in human colorectal cancer LoVo, HCT116, HT-29, and SW480 cells. In addition, cinnamaldehyde was able to lower the amount of cyclin D1 mRNA and prevent the activation of the Wnt pathway by inhibiting β-catenin and TCF4 production. Additionally, it was shown that apoptosis might be caused by cinnamaldehyde via ROS-dependent DNA damage. Cinnamaldehyde was responsible for the production of ROS, which in turn increased the activity of the transcription factors nuclear factor-kappa B (NF-κB) and activating transcription factor 3 (ATF3). As a result, the findings suggest that cinnamaldehyde may limit cell proliferation by inhibiting the expression of cyclin D1 through proteasomal degradation and transcriptional inhibition, as well as by activating NF-κB and activating transcription factor ATF3 in a ROS-dependent manner to cause apoptosis. It is possible that the effects of cinnamaldehyde are to blame for the reduced viability of human colorectal cancer cells [58].
Using the MTT test, the effects of cinnamaldehyde (0.4 µg/mL) on the proliferation of the HCT 116 colon cancer cell line were determined. Fluorescent labelling procedures Rhodamine-123 and MitoSOX were used to analyse the impact of cinnamaldehyde on mitochondrial membrane potential, ROS, and superoxide generation, respectively. Even at low doses, cinnamaldehyde exhibited cytotoxicity. Superoxide anion and ROS generation increased while mitochondrial membrane potential dropped, which inhibited the proliferation of cells. Based on these findings, cinnamaldehyde could be helpful for people with colon cancer [59].
A poor prognosis and resistance to radio-chemotherapy are both associated with hypoxia in the surrounding microenvironment of solid tumours, which is one of the most important pathological markers of solid tumours. According to the findings of a study, hypoxia makes colorectal cancer cells more stem-like and causes them to undergo an epithelial-mesenchymal transition, both of which reduce the cells’ susceptibility to oxaliplatin. On the other hand, cinnamaldehyde at a concentration of 40 μg/mL increased the rate of apoptosis in colon cancer HCT116 and SW480 cells both in vitro and in vivo, using a tumour xenograft model in BALB/c/nu/nu nude mice. This had the effect of enhancing the curative effect of oxaliplatin. Cinnamaldehyde and oxaliplatin were able to effectively upregulate Bax, cleave caspase-3 and PARP, and downregulate Bcl-2. An in vivo investigation showed that the combination of cinnamaldehyde (50 mg kg−1 with oxaliplatin (7.5 mg kg−1)) resulted in antitumor action. This activity was shown by a decrease in Bcl-2 expression and an increase in the expression of the pro-apoptotic marker Bax. Immunohistochemistry performed on tumour tissue demonstrated increasing amounts of the protein Bax and decreasing levels of the protein β-catenin. Cinnamaldehyde and oxaliplatin worked synergistically to inhibit cell proliferation in colorectal cancer cells by reversing hypoxia-induced epithelial-mesenchymal transition and stemness in these cells. This was accomplished on a mechanistic level. These findings not only demonstrated the potential therapeutic benefit of cinnamaldehyde but also offered novel recommendations for increasing the sensitivity of oxaliplatin in the treatment of colorectal cancer [60].
4.7. Cinnamaldehyde on Ehrlich Ascites Carcinoma
Cinnamaldehyde, at a dose of 50 mg kg−1 of body weight, delayed the progression of Ehrlich ascites carcinoma tumours in mice by reducing the number of tumour cells by 45 percent, their viability by 53 percent, and their rate of proliferation by 53 percent. In addition to this, a significant arrest in the G0/G1 phase of the cell cycle was seen. After treatment with cinnamaldehyde, there was an increase in the percentage of splenic T helper cells (CD3+CD4+) and T cytotoxic cells (CD3+CD8+), both of which are indicators that cinnamaldehyde successfully initiated an immune response against cancer. In light of these data, it would seem that cinnamaldehyde has a potent anticancer effect against Ehrlich ascites carcinoma in vivo [16].
4.8. Cinnamaldehyde on Gastric Cancer
Gastric cancer is a term that refers to cancer that develops in the lining of the stomach. It is possible for stomach cancer to grow in any of the tissues that make up the stomach. In the majority of instances, the first sign of cancer will show up in the primary chamber of the stomach, which is also referred to as the stomach body. An experiment was carried out by Milani and colleagues to investigate the effects of trans-cinnamaldehyde on AGS cells. After the study was completed, it was shown that trans-cinnamaldehyde, at a concentration of 2 mg/mL, was able to significantly inhibit the development of AGS cells in a dose-dependent way. According to the findings of this study, trans-cinnamaldehyde produced profound impacts on the AGS cell line [61].
Lee and Jung investigated the antiproliferative effects of trans-cinnamaldehyde in AGS cells derived from stomach cancer and the mechanisms behind these effects. trans-cinnamaldehyde inhibits the viability of AGS cells dose dependently. According to the results, trans-cinnamaldehyde (100 µM) has the potential to trigger apoptosis by modifying the structure of cells. In order to get a better understanding of the process of apoptosis, researchers investigated the quantities of proteins that were involved. trans-cinnamaldehyde was able to increase the amount of cleaved caspase-9 as well as the amount of cleaved PARP. Additionally, it was able to stimulate the synthesis of p53 and Bax proteins. According to these findings, apoptosis was triggered in AGS cells by trans-cinnamaldehyde via a mechanism including p53. According to the findings, trans-cinnamaldehyde showed potential as an alternative for an anticancer pharmaceutical treatment in the case of stomach cancer [62].
Cinnamaldehyde, at a concentration of 50 µg/mL, was shown in studies to induce ER stress and cell death in gastric cancer, including SNU-216, SNU-638, AGS, MKN-45, NCI-N87, and MKN-74 cells. This was accomplished via the PERK-CHOP axis and the release of calcium ions. Intriguingly, autophagy inhibition decreased cinnamaldehyde-induced cell death, while cinnamaldehyde treatment resulted in autophagic cell death by increasing ATG5, Beclin-1, and LC3B expressions and by reducing p62 expression. Additionally, cinnamaldehyde was shown to activate LC3B while simultaneously inhibiting G9a function. In addition, cinnamaldehyde was able to prevent G9a from binding to the promoters of both LC3B and Beclin-1. The combination of these studies demonstrated that cinnamaldehyde regulates PERK–CHOP signalling and that suppression of G9a promotes autophagic cell death in gastric cancer cells through ER stress [63].
4.9. Cinnamaldehyde on Head and Neck Cancer
Cancers of the head and neck may occur in a variety of places, including the oral cavity, the pharynx, and other areas. The most common symptom is a painful and scratchy throat. p53-mutant (YD-10B) and p53-wild (SGT) human head and neck cancer cells were used to evaluate the apoptosis-inducing effects of the cinnamaldehyde derivative 2′-hydroxycinnamaldehyde and the signalling pathways that were involved. It was discovered that YD-10B and SGT cells are more sensitive to the antiproliferative effects of 2′-hydroxycinnamaldehyde. The apoptotic effect that was caused by 2′-hydroxycinnamaldehyde (50 μM) was verified by double staining with annexin V-FITC and PI, and this result was supported by the activation of caspase-3, -7, and -9 in addition to PARP. Following treatment with 2′-hydroxycinnamaldehyde, p21 expression was shown to be significantly elevated in both SGT and YD-10B cells. Furthermore, 2′-hydroxycinnamaldehyde boosted the cell death pathway in a p53-independent manner, as shown by the fact that it raised the expression of pro-apoptotic Bak1 and lowered the expression of antiapoptotic Bcl-2 in both of the cell lines tested. In addition to that, it led to an increase in the expression of LC3B in both SGT and YD-10B cells. When compared to YD-10B cells, SGT cells showed a significant increase in 2′-hydroxycinnamaldehyde-induced apoptosis after pre-incubation with the autophagy inhibitor 3-MA. This suggests that autophagy may actively contribute to 2′-hydroxycinnamaldehyde-induced apoptosis. YD-10B cells did not show this significant increase. In general, the findings of this study suggest that 2′-hydroxycinnamaldehyde, regardless of its impact on the p53 gene, has potential as a therapeutic agent for the treatment of head and neck cancer [64].
Another study conducted by Kang et al., in the year 2018, revealed the effect of bone morphogenetic protein 7 (BMP7) on the antimigration and anti-invasion properties of 2′-hydroxycinnamaldehyde by using FaDU cells obtained from head and neck squamous cell carcinoma. These cells were cultured in the presence of 2′-hydroxycinnamaldehyde. 2′-hydroxycinnamaldehyde, at concentrations ranging from 250 to 500 nM, was able to inhibit FaDU cell motility and spheroids’ invasion of Matrigel without causing cytotoxicity. The administration of 2′-hydroxycinnamaldehyde led to an increase in the amount of mRNA as well as protein that was produced by the BMP7 gene. An increase in the expression of exogenous BMP7 was shown to impede cell penetration through Matrigel in the absence of treatment with 2′-hydroxycinnamaldehyde. siRNA was utilized to knock down BMP7 expression in FaDU cells to further prove that BMP7 is responsible for the antimigration action of 2′-hydroxycinnamaldehyde in FaDU cells. Thus, it was found that this reduced the inhibitory effect of 2′-hydroxycinnamaldehyde on the cell’s invasion into Matrigel. This provided further evidence that BMP7 is responsible for the antimigration action. Therefore, treatment with 2′-hydroxycinnamaldehyde dramatically up regulated BMP7, which resulted in a considerable reduction in the invasion of FaDU cells originating from head and neck squamous cell carcinomas [65].
4.10. Cinnamaldehyde on Liver Cancer
Primary liver cancer is a significant public health problem and a subtype of cancer that is growing at an increasing rate in the United States. Both cancer of the liver and cancer of the bile ducts are considered to be the main forms of liver cancer. The underlying causes, risk factors, symptoms, and treatments for cancers that affect both sexes are the same. Medical practitioners focus on identifying those individuals who are at an increased risk in order to diagnose and treat primary liver cancer in its earlier stages when it is more treatable. Cinnamaldehyde was investigated in a work with the intention of determining whether or not it was able to decrease the growth of human hepatoma Hep G2 cells. Cinnamaldehyde exhibited antiproliferative action with an IC50 value of 9.76 μM, which was equivalent to that of the cancer drug 5-fluorouracil (IC50: 9.57 μM). Additional study into the apoptotic pathways of cinnamaldehyde found that it cleaves PARP and, over time, upregulates CD95 (APO-1), p53, and Bax proteins while concurrently downregulating the expression of Bcl-XL. Cinnamaldehyde effectively prevented CD95 (APO-1), p53 expression and PARP cleavage in cells. On the basis of these findings, it would seem that the apoptotic mechanism induced by cinnamaldehyde in Hep G2 cells is perhaps mediated via the p53 activation and CD95 (APO-1) signalling pathways [66].
In search of the antiapoptotic property of cinnamaldehyde, Lin et al., in the year 2013, conducted a study on PLC/PRF/5 cells. Cinnamaldehyde was found to stimulate the activation of caspase-3, which in turn, led to the cleavage of PARP. This was accomplished by increasing the production of ROS, disrupting the mitochondria membrane potential, and releasing cytochrome c and Smac/DIABLO from the mitochondria into the cytosol. Cinnamaldehyde treatment also resulted in a decrease in the levels of the anti-apoptotic proteins XIAP and Bcl-2, whilst the levels of the pro-apoptotic protein Bax increased in a time-dependent way. Consequently, on the basis of these data, cinnamaldehyde seems to be a mitochondrial death pathway apoptosis inducer in PLC/PRF/5 cells [67].
Considering the importance of the Wnt/β-catenin pathway in aggravating cancer, Abd El Salam et al. in the year of 2022, introduced cinnamaldehyde in thioacetamide-induced hepatocellular carcinoma in male Sprague Dawley rats. For the purpose of analysing the Wnt/β-catenin pathway, the protein concentrations of β-catenin, Wnt-3a, MMP-9, cyclin D, and VEGF were assessed in the liver. Cinnamaldehyde (70 mg kg−1) exhibited an intense inhibitory effect on the Wnt/β-catenin pathway by lowering the levels of hepatic β-catenin, Wnt-3a, MMP-9, cyclin D, and VEGF, thus proven to be a potential candidate in hepatocellular carcinoma [68].
Cinnamaldehyde, according to research that was only recently released, was able to limit the proliferation of HepG2 cells in a dose-dependent way. Researchers observed that after being exposed to cinnamaldehyde, IL-1 levels surged while IL-10 levels dropped. Additionally, after being exposed to cinnamaldehyde, the activity of caspase-3 was shown to be significantly increased in HepG2 cells. As a consequence of this, the findings of the present experiment suggested that cinnamaldehyde exhibited potentially useful antitumor action against hepatocellular carcinoma cells [69].
4.11. Cinnamaldehyde on Lung Cancer
Lung cancer is the term used to describe the development of a tumour in the lining cells of the pulmonary airways of the lungs. It is responsible for the vast majority of fatal instances of cancer in people of both sexes. The most frequent types of lung cancer are small-cell and non-small-cell varieties. These two cultivars have different requirements, and their growth occurs at different speeds because of those requirements. The non-small cell subtype of lung cancer accounts for the vast majority of diagnosed cases. In a study, human lung adenocarcinoma A549 cells were utilized as the subject of an investigation into the anticancer properties of 2-methoxycinnamaldehyde. The findings show that 2-methoxycinnamaldehyde at a concentration of 32 μM inhibited cell proliferation and induced apoptosis. This is demonstrated by the upregulation of pro-apoptotic Bax and Bak genes and the downregulation of anti-apoptotic Bcl-2 and Bcl-XL genes; the loss of mitochondrial membrane potential; the release of cytochrome c; and the activation of caspase-3 and -9. Additionally, 2-methoxycinnamaldehyde was shown to induce lysosomal vacuolation, which was followed by an increase in the volume of the acidic compartment, a downregulation of NF-κB, and a reduction in the activity of topoisomerases I and II. The data suggest that 2-methoxycinnamaldehyde’s growth-inhibiting effect on A549 cells is accompanied by downregulations of NF-κB binding activity and proliferative control involving apoptosis and both topoisomerase I and II activities, as well as upregulations of lysosomal vacuolation and volume of the acidic compartment. Additional studies have shown that an A549 xenograft BALB/c nude mouse model is likewise susceptible to the tumour growth-inhibiting effects of 2-methoxycinnamaldehyde (20 mg kg−1). According to the results of our research, 2-methoxycinnamaldehyde has characteristics that are desirable in an anticancer drug [70].
An investigation carried out by Meng et al., in 2017 aimed at establishing the synergistic effect of berberine and cinnamaldehyde on lung carcinogenesis via the starving of tumor cells. Berberine, in combination with cinnamaldehyde at a dose of 105 mg kg−1 (at a ratio of 20:1), significantly reduced the susceptibility of female ICR mice to urethane-induced lung carcinogenesis in vivo. This was accomplished by upregulating the expression patterns of AMPK and mTOR, as well as suppressing the expression of aquaporin-1 (AQP-1) and NF-κB. Apoptosis was triggered time dependently in A549 cells in vitro by a combination of berberine and cinnamaldehyde. Both the permeability of substances into A549 cells and the amount of ATP produced inside the cells were blocked by the berberine/cinnamaldehyde combination. In addition, the data suggest that the previously described combination was responsible for the induction of an AMPK upregulation as well as an AQP-1 downregulation. These results suggest that berberine and cinnamaldehyde, when employed jointly, starved lung cancer cells by inhibiting primary, and adaptive nutrition intake by lung malignancies through AMPK-reduced AQP-1 expression [71].
Two separate studies investigated the antiproliferative effects of 2-methoxycinnamaldehyde on human lung squamous cell carcinoma NCI-H520 cells in vitro and in a nude mice xenograft tumour model in vivo. As evidenced by the loss of mitochondrial membrane potential, activation of caspases-3 and -9, and the appearance of apoptotic morphology, 2-methoxycinnamaldehyde, at a concentration of 20 μM, was found to inhibit the proliferation of NCI-H520 cells and to promote their progression toward apoptosis. 2-Methoxycinnamaldehyde was shown to inhibit both topoisomerase-I and -II activities. Additionally, the substance was found to generate lysosomal vacuolation along with an increased volume of acidic compartment and cytotoxicity. An additional study conducted using a mouse tumour model revealed that 2-methoxycinnamaldehyde, when administered at a dose of 20 mg kg−1, prevented the formation of tumours. These data imply that the in vitro antiproliferative effect of 2-methoxycinnamaldehyde is related to the overexpression of proapoptotic molecules, the downregulation of cell growth markers (topoisomerase-I and -II), and greater lysosomal vacuolation. In vivo studies showed that 2-methoxycinnamaldehyde reduced the growth of tumours, which may have had significant repercussions in clinical practice [72].
Cinnamaldehyde and hyperthermia are two complementing cancer treatments, and research conducted by scientists has revealed that the A549 non-small cell lung cancer cell line reacts well to both of them. The A549 cells were subjected to a hyperthermia treatment at 43 °C, which resulted in an increase in the cytotoxicity of cinnamaldehyde (200 µM). Cinnamaldehyde with high temperatures had a synergistic effect that resulted in increased generation of ROS and phosphorylation of MAPK. Cell cycle arrest at the G2/M phase was also observed in the combinational therapy at the same concentration. As a result of this, the use of cinnamaldehyde in conjunction with hyperthermia may prove to be an effective therapeutic strategy for the management of non-small cell lung cancer [73].
4.12. Cinnamaldehyde on Oral Cancer
Oral cancer gives the appearance of a chronic sore or growth inside the mouth. It affects approximately 50,000 people each year in the United States, the majority of whom are men (nearly 70% of all cases). Oral cancer refers to a group of diseases that can affect the lips, tongue, cheeks, floor of the mouth, both the hard and soft palates, sinuses, and pharynx (the throat). It is possible that death will result from a delay in diagnosis and treatment. Therefore, cinnamaldehyde’s potential anticancer effects were investigated in a study using HSC-3 cells, which are derived from human oral squamous cell carcinoma. Cinnamaldehyde at a concentration of 10 µg/mL was shown to arrest the cell cycle at the G2/M phase as well as a significant reduction in the viability of HSC-3 cells was observed. In HSC-3 cells that had been treated with cinnamaldehyde, both DNA damage and apoptotic characteristics (DNA laddering and chromatin condensation) were found. Additionally, cinnamaldehyde caused the mitochondrial malfunction, activated cytochrome c release, and raised cytosolic Ca21 levels in addition to these effects. The amount of cellular glutathione and the activity of glutathione peroxidase was discovered to be significantly decreased in HSC-3 cells. On the other hand, the formation of reactive oxygen species and the levels of thiobarbituric acid reactive material were found to be increased. As a result of these observations, it could be concluded that cinnamaldehyde might inhibit the growth of oral cancer in HSC-3 cells [74].
In the year 2020, Varadarajan et al. subjected cinnamaldehyde to the oral squamous cell carcinoma SCC25 cell line. The phytochemical with an IC50 20.21 μM demonstrated anticancer activity via a variety of mechanisms, including the induction of apoptosis enhancing the cytotoxic activity and the progression of cell cycle arrest at the S-phase. By decreasing the potential of the mitochondria, cinnamaldehyde brought about an increase in the rate of apoptosis [75].
In the year 2022, Ahmed et al. determined the cytotoxicity and apoptotic activity of cinnamaldehyde, scorpion venom, and their combination on the oral squamous cell carcinoma SCC25 cell line. In the experiment, the IC50 values of cinnamaldehyde were found to be 90.40 (at 24 h) and 42.95 µg/mL (at 48 h), which indicates that it has a powerful effect on cancer. Whereas the combination group had the lowest nuclear area factor, up regulated the pro-apoptotic genes p53 and Bax, and down regulated the anti-apoptotic gene Bcl-2 with IC50 values of 4.93 (at 24 h) and 4.40 µg/mL (at 48 h). The combination of cinnamaldehyde and scorpion venom demonstrated potential cytotoxicity and enhanced cytotoxic activity on oral squamous cell carcinoma [76].
In the same year, Aggarwal et al. tried to explore the title compound’s potential against oral cancer. Several in vitro studies were conducted on oral cancer SCC-25, SCC-9, and SCC-4 cells. After treatment with 80 µM cinnamaldehyde, it was shown that there was a dose-dependent reduction of the development and proliferation of oral cancer cells. These therapies further increased apoptosis and cytotoxicity as well as the arresting of the cell cycle at the G2/M phase and autophagy. Cinnamaldehyde was able to prevent the invasion of these cell lines as well as the translocation of NF-κB into the cytoplasm. Therefore, in the cancer cells, there was a reduction in the expression of genes that were implicated in COX-2, VEGF, Bcl-2, and NF-κB [77].
4.13. Cinnamaldehyde on Osteosarcoma Cancer
Osteosarcoma is a kind of cancer that begins in the bone cells and spreads throughout the skeleton. Even while osteosarcoma most often starts in the long bones of the body, such as the legs or arms, it may also start in other parts of the skeleton. Extremely infrequently does it disseminate to non-skeletal soft tissue. According to the findings of a study, cinnamaldehyde significantly inhibited cell growth and induced apoptosis in a concentration-dependent manner by increasing the expression of the Bad gene and decreasing the expression of the Bcl-2 and PARP genes, respectively. It was found that it inhibited the migration and invasion of osteosarcoma cell lines 143B (IC50: 67.95 µM) and MG63 (IC50: 56.68 µM), respectively. Cinnamaldehyde was found to cause 143B cells to be arrested at the G2/M phase, whereas MG63 were cells arrested at G0/G1 phase. In an in vivo Balb/c-nude female mouse xenograft model, the administration of cinnamaldehyde at a dose of 100 mg kg−1 delayed the growth of osteosarcoma. Cinnamaldehyde’s suggested approaches include lowering the amount of transcriptional activity associated with Wnt/β-catenin and PI3K/Akt, with the goal of preventing osteosarcoma from developing. Through its effects on the Wnt/β-catenin and PI3K/Akt signalling pathways, cinnamaldehyde may reduce osteosarcoma cell proliferation, migration, and invasion while simultaneously promoting their death [78].
4.14. Cinnamaldehyde on Ovarian Cancer
Ovarian cancer is by far the most dangerous kind of cancer that may affect a woman’s reproductive system. It is seen in the tissues of the uterus that are responsible for egg production (ovaries). In many cases, the disorder is not recognized until it has already spread across the pelvis and into the belly. At this late stage, ovarian cancer is more difficult to treat, and it has a greater chance of becoming fatal. Ovarian cancer often does not produce any symptoms in its early stages. In later stages, patients may have symptoms that are not always clear, such as a loss of appetite and a reduction in their overall body weight. In most cases, chemotherapy and surgery are used in conjunction with one another to treat ovarian cancer. Cinnamaldehyde’s potential uses were studied in a study that used human ovarian cancer SKOV3 cells in vitro and a SCID mice model in vivo. Cinnamaldehyde was shown to have an anticancer impact in vitro by lowering the expression and phosphorylation of AKT and STAT3, crucial factors in the control of HIF-1α production, when it was utilized at a concentration of 10 mg/mL. This resulted in a significant decrease in the angiogenesis capacity of SKOV3 cells. Mice treated with cinnamaldehyde (300 mg kg−1) exhibited significant suppression of VEGF synthesis, blood vessel formation, and tumour growth in a human ovarian tumour model. The findings, considered overall, provide insight into the mechanisms by which cinnamaldehyde exerts its anti-angiogenic and antitumor actions and offer legitimacy to the possibility that the phytocomponent may be used in the treatment or prevention of cancer [79].
It was discovered by researchers that cinnamaldehyde and cisplatin combinedly demonstrated to have a synergistic effect on ovarian cancer cells, leading to an increase in ROS-mediated apoptosis and autophagy. The cell lines that will be studied are cisplatin-resistant A2780/cis cells and cisplatin-sensitive A2780/s cells. Throughout the course of the tests, cinnamaldehyde at IC50 43 μM reduced cell growth for A2780/s. On the other hand, a dose of cisplatin as high as 10 µM did not have any effect on A2780/cis. Therefore, cisplatin combined with cinnamaldehyde (51 μM) was applied to the A2780/cis cells, which resulted in elevated synergistic growth-inhibiting action and ROS-mediated apoptosis and autophagy activity. As a result of this, it is possible to propose that the combination of cinnamaldehyde and cisplatin may induce an excessive amount of ROS as a strategy for overcoming chemoresistance in ovarian cancer [80].
To explore cinnamaldehyde’s ability to act against ovarian cancer, Boggiti et al., in the year 2021 administered cinnamaldehyde to female Wistar rats with the stated pathology. Cinnamaldehyde at a dose of 50 mg kg−1 decrease the levels of estrogen, luteinizing hormone, and follicle-stimulating hormone in a dose-dependent manner. It also raises the level of progesterone. The results indicate that cinnamaldehyde could be a promising candidate in the near future to act against ovarian cancer [81].
4.15. Cinnamaldehyde on Prostate Cancer
One man in every six is diagnosed with prostate cancer in the United States, making it the second leading cause of death due to cancer among males. Due to the gradual nature of prostate cancer, diagnosing, and treating it before symptoms appear may not benefit men’s health or lengthen their lives. In addition, recent studies have shown that fibroblasts that are present in malignancies assist in a variety of ways in the progression of cancer. Cinnamaldehyde’s effect on cancer-associated fibroblasts hasn’t been studied yet. Therefore, to address this cinnamaldehyde’s effects on prostate cancer-associated fibroblasts hTERT PF179T cell line, as well as the method by which it exerts its effects, were the subject of an investigation. In fibroblasts taken from prostate cancer patients, it was shown that cinnamaldehyde (150 μΜ)-induced apoptosis and stopped the cell cycle at the G2/M phase via an inherent mechanism. One of the causes of lowering the proliferation was an increase in the amount of calcium ions as well as intracellular ROS. The examination of protein expression also demonstrated a decrease in mitochondrial membrane potential as well as in the expression levels of Bcl-2, caspase-9, PARP, and DEF-45, and, simultaneously, an increase in the expression of cytochrome c, Bax, cleaved caspase-3 and cleaved PARP. Thus, cinnamaldehyde seems to has the potential to be utilized as a treatment for cancer based on the findings of this analysis [82].
4.16. Cinnamaldehyde on Renal Cancer
A study of the anti-angiogenic activity of cinnamaldehyde and its effect on tumour progression was conducted by Bae et al., in the year of 2015. Cinnamaldehyde, when administered in vivo to BALB/c mice at a dose of 10 mg kg−1, significantly restricted the growth of tumours by preventing the new blood vessels in tumours. The expression of the HIF-1a protein and VEGF was suppressed in mouse tumours in vivo on treatment with cinnamaldehyde. On the other hand, the above two protein expressions were repressed in vitro in hypoxic Renca cells at a concentration of 100 µM. Interestingly, the treatment of cinnamaldehyde had no impact on the stability of HIF-1a that was coupled with the von Hippel-Lindau protein (pVHL), and it also reduced the activation of the mTOR pathway. Cinnamaldehyde’s anti-angiogenic effect is presumed to be mediated in part by the mTOR pathway’s inhibition, resulted in ceasation of HIF-1a protein production. This revelation clearly indicates the pharmacological importance of cinnamaldehyde in cancer treatment [83].
Ahn et al., in the year 2020, presented the effectiveness of the combination of hyperthermia and cinnamaldehyde in the treatment of cancer. Following treatment with cinnamaldehyde, ACHN cell lines derived from patients with renal cell cancer were put through hyperthermia at a temperature of 43 °C. According to the results of Western blot experiments, both cinnamaldehyde (90 µM) and hyperthermia reduced the amount of heat shock protein 70. These treatments also increased the signalling of apoptosis with a sharp fall in the signalling of proliferation and metastasis. Flow cytometry revealed that ACHN cells underwent an arrest in the cell cycle at the G2/M phase. It was also observed that it induces apoptosis with a rise in mitochondrial membrane potential. It was also shown that the formation of ROS may be considerably boosted by combining cinnamaldehyde with hyperthermia at a temperature of 43 °C. In conclusion, it is reasonable to recommend cinnamaldehyde and hyperthermia combination treatment as a viable alternative option for anticancer medicines for renal cell carcinoma patients to consider [84].
4.17. Cinnamaldehyde on Skin Cancer
The incidence of skin cancer is by far the most common form of cancer. Melanoma, squamous cell carcinoma, and basal cell carcinoma are the three basic subtypes of skin cancer that may be distinguished from one another. Melanoma is a kind of skin cancer that develops far less often than other types of skin cancer; nonetheless, it has a significantly greater tendency to invade neighbouring tissue and move to other parts of the body. Melanoma is the kind of skin cancer that has the highest mortality rate. A research paper that was released in 2009 found that trans-cinnamaldehyde suppresses the proliferation of melanoma cells as well as the formation of tumours. In a human A375 melanoma SCID mice xenograft model, it was shown that the therapeutic effectiveness of trans-cinnamaldehyde could be achieved at high doses (120 mg kg−1, daily oral dose for ten days). The human metastatic melanoma cell lines A375, G361, and LOX all inhibited their proliferation by trans-cinnamaldehyde, which resulted in G1 cell-cycle arrest, higher intracellular ROS, and decreased invasiveness. The IC50 values for these three cell lines are as follows: 6.3 µM, 8.1 µM, and 3.4 µM, respectively. trans-cinnamaldehyde was shown to have caused an oxidative stress response in A375 cells by up regulating genes, such as HO-1, sulfiredoxin 1 homolog, thioredoxin reductase 1, and the G1-arresting tumour-suppressor gene cyclin-dependent kinase inhibitor 1A (CDKN1A), as shown by an expression array. A reduction in TNF-induced IL-8 production and NF-κB transcriptional activity was seen in A375 cells when trans-cinnamaldehyde was present [85].
Cinnamaldehyde was examined against mouse melanoma cell lines Clone M3 and B16F10 (in vitro), as well as the C57BL/6 melanoma mice model in vivo. Cinnamaldehyde was shown, in both melanoma cell lines and an experimental melanoma mice model, to effectively suppress the production of pro-angiogenic factors (VEGF-α, EGF, FGF, and TGF-β) and master regulators of tumour growth (HIF-1 and Cox-2) at a dosage of 0.5 mg/mL. Cinnamaldehyde treatment resulted in an increase in both the cytotoxic activity of CD8+ T lymphocytes as well as their production of cytolytic molecules, including interferon gamma and tumour necrosis factor alpha (TNF-α) [86].
In 2010, Kwon et al. conducted an experiment in which they demonstrated that cinnamaldehyde (0.5 mg/mL) effectively suppressed the growth of mouse melanoma Clone M3 and B16F10 cell lines in vitro and triggered the active cell death of tumour cells. This was accomplished by up regulating pro-apoptotic molecules (Bim, Bad, Bak and Bax) and suppressing the activity of NF-κB and activator protein 1 (AP-1) as well as its target genes, which included BcL-xL, surviving and Bcl-2. Cinnamaldehyde at the same dose was able to successfully reduce the formation of tumours in melanoma in vivo C57BL/6 mice model that was transplanted using the same mechanism of action as was seen in vitro. Therefore, the findings of the current study indicate that the antitumor effect of cinnamaldehyde is directly linked with increased pro-apoptotic activity as well as reduction of NF-κB and AP-1 activities and their target genes in vivo and in vitro [87].
Cinnamaldehyde’s effect on hypoxia-induced angiogenesis and metastasis was investigated utilizing in vitro experiments with melanoma B16F10 cell lines and in vivo investigations using melanoma C57BL/6 mice models. Utilization of cinnamaldehyde (100 µM for in vitro and 30 mg kg−1 for in vivo) seems to inhibit tumour angiogenesis, epithelial–mesenchymal transition, and metastasis, as shown by the findings of the study. This was followed by a drop in epithelial–mesenchymal transition-related markers TWIST and ZEB1, as well as a decrease in VEGF secretion, VEGF receptor phosphorylation, and MMP expression. The next part of the investigation focused on the ways in which cinnamaldehyde influences HIF-1α. The findings demonstrated that cinnamaldehyde decreased the HIF-1α protein level by inhibiting its synthesis while having no effect on the rate at which it was degraded by the proteasome. Cinnamaldehyde was also able to obstruct the PI3K/Akt/m-TOR pathway, which is essential for the transcription and translation of HIF-1α both in vivo and in vitro. In conclusion, cinnamaldehyde suppressed the formation of HIF-1α protein in tumour cells, most likely by targeting the PI3K/Akt/mTOR pathway As a result, angiogenesis and metastasis were both reduced [88]. An illustration of anticancer studies have been shown in Table 1 and Table 2.

Table 1.
In vitro anticancer studies of cinnamaldehyde or its natural congeners.

Table 2.
In vivo anti-cancer studies of cinnamaldehyde or its natural congeners.
5. AntiCancer Mechanisms of Cinnamaldehydes
Cinnamaldehydes demonstrated anticancer effects via three separate apoptotic mechanisms, regulated by mitogen-activated protein kinase (MAPK), mitochondria along with the members of Bcl-2 proteins, and death receptors. Apart from the above three, metastasis and cell cycle arrest might be another mechanism for cinnamaldehyde to exhibit anticancer effects.
5.1. Apoptotic Effect of Cinnamaldehyde on MAPK Regulated Cell Death
Extracellular signal-regulated kinases (ERKs), c-Jun N-terminal kinases (JNKs), and p-38 are all members of the superfamily of proline-directed serine/threonine protein kinases known as the mitogen-activated protein kinases (MAPKs), which is found in human cells [89]. As their downstream targets could be pro-inflammatory or mitogenic enzymes as well as nuclear transcriptional factors, MAPKs play an important part in inflammations, cell proliferations, differentiations, and cellular death. The activation of JNKs and p-38 has been found to be associated with the process of apoptosis, whilst the activation of ERKs has been demonstrated to promote cell proliferation and differentiations [90,91,92].
It has been shown that a large number of naturally occurring substances may trigger apoptosis by activating MAPKs. There is evidence to suggest that the activation of ERKs in human breast cancer cells by RRR-α-tocopheryl succinate results in the death of those cells [93]. Phosphorylation is a process that can be used to activate p-38, JNKs, and ERKs in many different types of cancer cells [94,95,96].
Cinnamaldehyde treatment was proven to substantially trigger apoptosis in human hepatoma PLC/PRF/5 cells, as revealed by the phosphorylated state of p-38, JNKs, and ERKs (Figure 3). This was demonstrated by the fact that the cells underwent apoptosis. In addition, the p-38 inhibitor (SB203580) and the inhibitor of JNKs (SP600125) effectively restored the liver cancer PLC/PRF/5 cells from the apoptosis produced by cinnamaldehyde, although the ERK inhibitor (PD98059) has a less significant influence [97].
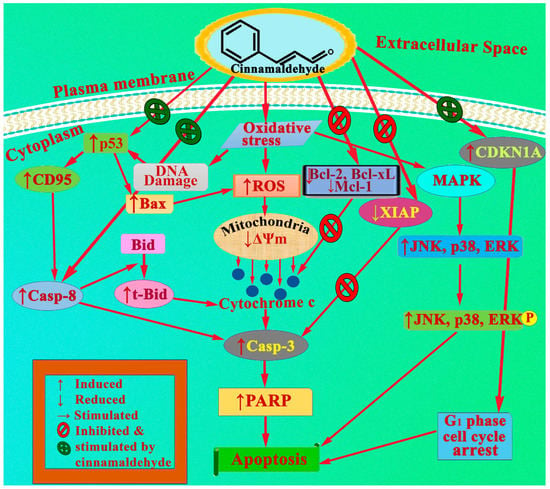
Figure 3.
Inhibition of cell growth and induction of apoptosis by cinnamaldehyde.
Cinnamaldehyde inhibits the activity of NF-κB and activator protein (AP)-1 in cancer cells, which results in an increase in the rate of apoptosis in these cells [85,98]. In addition to that, it is capable of reducing inflammation. Cinnamaldehyde produces a dose-dependent decrease in the activation of NF-κB in lipopolysaccharide-stimulated THP1 cells and human peripheral blood mononuclear cells; however, low dosages led to a slight increase in NF-κB activation. This was seen in both peripheral blood mononuclear cell types. In primary and immortalized immune cells, treatment with cinnamaldehyde reduces the synthesis of inflammatory cytokines and chemokines, such as interleukin 10 (IL-10) and TNF-α [99,100]. This includes the creation of nitric oxide (NO). In addition, cinnamaldehyde inhibits the survival of immunological cells, as well as their proliferation and death [101]. In conclusion, cinnamaldehyde has an influence on immune cells; this fact deserves more examination since it may be a factor in the development of cancer in a roundabout way.
In a similar pattern, one of the naturally obtained cinnamaldehyde congener 2′-hydroxycinnamaldehyde also showed apoptotic action. The induction of apoptosis in colon cancer cells by 2′-hydroxycinnamaldehyde is accompanied by a number of different mechanisms, including the downregulation of c-Fos and c-Jun expressions and the reduction of the DNA-binding ability of AP-1 [98] and also by inactivating ERK-dependent NF-κB [102].
5.2. Apoptotic Effect of Cinnamaldehyde on Mitochondria and Members of the Bcl-2 Proteins
Apoptosis is not only a crucial step in the process of becoming a multicellular organism, but it also plays a vital role in the overall maintenance of cellular equilibrium. The abnormal regulation of the apoptotic programme has been linked to a wide variety of diseases, including autoimmune diseases, cancer, neurodegenerative disorders, and stroke, amongst others [103,104]. It is now generally accepted that mitochondria play a pivotal role in the induction of apoptosis, which can be mediated by a wide variety of apoptotic stimuli, including DNA damage, chemotherapeutic drugs, reactive oxygen species [52], UV irradiation, and other cellular stress factors. This role is thought to be induced by a wide variety of apoptotic stimuli, such as those listed above [105,106]. The Bcl-2 protein family is responsible for the regulation of mitochondrial death. This is accomplished through the actions of pro-apoptotic molecules (Bax, Bak, Bid, and Bad) and anti-apoptotic molecules (Bcl-xL, Bcl-2, Mcl-1, and Bcl-w), which result in a decrease in the mitochondrial membrane potential (Ψm) and the production of ROS [107]. Caspase-8 (CASP-8) is responsible for the cleavage of the Bid protein into the truncated form (tBid) that is required for apoptosis to occur in cancerous cells, which is caused by many chemotherapeutic treatments. This causes the release of cytochrome c from the mitochondria into the cytosol. Additionally, in conjunction with an appropriate balance of pro- to anti-apoptotic Bcl-2 family members, this causes the formation of a complex between cytochrome c and the apoptotic protease activating factor 1 (Apaf-1) that activates CASP-9 and, in turn, CASP-3 ultimately results in cellular death [108]. Apoptosis results in the degradation and release of mitochondrial DNA, as well as cytochrome c and the second mitochondria-derived activator of caspase (Smac/DIABLO) and/or Omi/HtrA2. These molecules help to activate caspase by inhibiting the effect that is caused by a class of proteins known as inhibitors of apoptosis (IAP), which is done by impairing their influence [109,110].
It has been shown that cinnamaldehyde may trigger apoptosis in HCC cells and that this process is mediated in part by the mitochondria and the Bcl-2 family of proteins. In particular, treatment with cinnamaldehyde results in an accumulation of PLC/PRF/5 hepatoma cells in the S phase of the cell cycle, as well as a loss of Ψm, an increase in the generation of ROS, and the expression of Bax [97,111]. Activating CASP-8 and -3 produces an increase in the amount of mitochondrial cytochrome c that releases into the cytosol. This occurs because these two proteins, such as Bid and PARP, can effectively cleave the biomolecular targets. The levels of Bcl-2, Mcl-1, and X-linked inhibitor of apoptosis protein (XIAP) in hepatoma cells drastically fell in exposure to cinnamaldehyde (Figure 3) [97,111]. Additionally, a pre-treatment with the cyclosporin A (CsA), the general caspase inhibitor (z-VAD-fmk), and mitochondrial permeability transition (MPT) pore inhibitor, may decrease the apoptotic effects produced by cinnamaldehyde. This indicates that the mitochondria are involved in the process [67]. It is also important to point out that cinnamaldehyde, in combination with an anti-oxidant such as vitamin E, will prevent the release of apoptotic factors caused by mitochondria in hepatoma cells [111].
5.3. Apoptotic Effect of Cinnamaldehyde on Death Receptors
The CD95 (APO-1/Fas) receptor is particularly crucial in immune and liver cells. This is because it is one of the many signalling pathways that are involved in the control of apoptosis [112,113]. CD95 is a type I transmembrane receptor that is expressed in activated lymphocytes as well as in various tissues of non-lymphoid or lymphoid origin, as well as on cancer cells. It is also expressed on activated lymphocytes. It belongs to the TNF-R superfamily, which stands for the tumour necrosis factor receptor superfamily. Initiation of the death receptor pathway is caused by the cross-linking of CD95 to CD95L. This, in turn, causes the formation of the death-inducing signalling complex (DISC), the sequential activation of activator caspases (CASP-8 and -10), and executioner caspases (CASP-3, -6, and -7), as well as the production of death substrates [114]. Through the CD95/CD95L- CASP-8 signalling pathway, it has been demonstrated that apoptosis can be induced by several naturally occurring anticancer agents [115,116,117]. When anticancer agents cause DNA damage, the protein that acts as a tumour suppressor, p-53, immediately goes to work on the promoter of the CD95 gene. Wild-type p-53 (HepG2) cells show up regulation of the CD95 death receptor, in contrast to cells with mutant (PLC/PRF/5) or null p-53 (HepG2) [118,119]. p-53 activation is known to alter the transcription of a number of genes, some of which are involved in cellular metabolisms, the regulation of apoptosis, and cell cycles, to name just a few examples. By inhibiting p53-mediated transactivation and the p-53 inhibitor pifithrinalpha (PFT) can significantly lower the level of p-53 expression in cells that have wildtype p-53 but not in cells that have mutant p-53 or p53-deficient cells [120,121].
HepG2 cell proliferation can be inhibited by cinnamaldehyde, the degree of which depends on both the concentration of the compound and the length of time it is exposed to it. Following treatment with cinnamaldehyde, expression of Bcl-xL is decreased, but an expression of CD95, Bax, and p-53 is increased. In that very event, PARP is cleaved off in a time-dependent manner (Figure 3). These effects can be reversed by treatment with PFT, which also protects HepG2 cells from the apoptosis caused by cinnamaldehyde and leads to lower levels of cleavaged p-53, Bax, CD95, and PARP [66].
5.4. Cinnamaldehyde on Cancer Metastasis
There is no conclusive evidence to suggest that cinnamaldehyde or any of its congeners may impede the invasion or metastasis of cancer cells. According to Koppikar et al., the principle component cinnamaldehyde acts by blocking the transcription of MMP-2 mRNA, which led to a reduction in the migration of human cervical cancer SiHa cells [122]. Cinnamon extract at a concentration of 30 µg/mL showed inhibition of new blood vessel growth (angiogenesis) and the migration of cells [42]. Treatment with cinnamaldehyde suppressed the development of melanoma and decreased blood vessel production. This was accomplished by lowering the expression of vascular endothelial growth factor (VEGF) and hypoxia-inducible factor-1α, respectively [123]. Additionally, in a mouse model of renal adenocarcinoma, cinnamaldehyde inhibits both the growth of tumours and the formation of new blood vessels. According to the findings of this study, treatment with cinnamaldehyde decreased the expression levels of HIF-1α and VEGF after being exposed to hypoxic environments in a manner that was reliant on mTOR [83]. Cinnamaldehyde has the effect of inhibiting neutrophil chemotaxis as well [124]. Cinnamaldehyde, on the other hand, elevates CXCL12 levels, which in turn promotes migration of Langerhans cells [125]. These contradictory results underscore the need for more study into the regulatory effects that cinnamaldehyde has on cell migration, especially research that seeks to understand the underlying molecular pathways that are responsible for these effects. Inhibition of cancer cell migration is shown Figure 4. This could definitely add additional feathers to its sleeves.
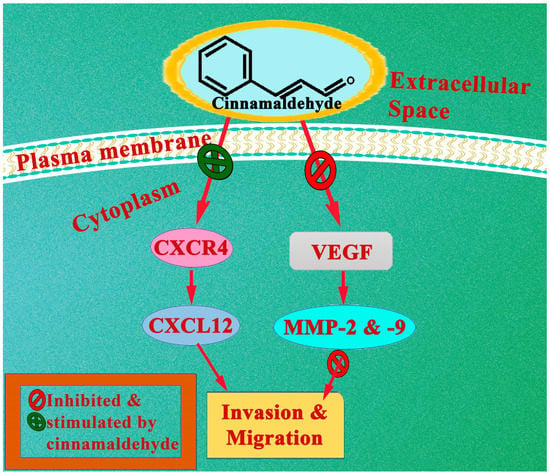
Figure 4.
Inhibition of cancer cell migration by cinnamaldehyde shown in a schematic form.
5.5. Cinnamaldehyde on Cell Cycle Arrest
Investigating with microarrays and Western blots, Cabello et al. demonstrated that melanoma cells treated with 25 µM cinnamaldehyde upregulate the expression of the CDKN1A. CDKN1A is a redox-sensitive cell cycle regulator and stress-responsive tumor suppressor gene. In addition, under oxidative stress, the level of cyclin-dependent kinase inhibitor p21 (encoded by CDKN1A) in HT-29 colon cancer cells significantly increased, which ultimately resulted in G1 arrest (Figure 3) [85].
6. Safety Profile of Cinnamaldehyde
Cinnamaldehyde is regarded to be a risk-free natural component that is well absorbed by both animals and human beings [126,127]. Both the Council of Europe and the Food and Drug Administration are on board with the concept, and both organizations suggest that the acceptable daily intake be set at 1.25 mg per kg. In this article, we present a synopsis of the research that has been conducted over the course of many decades on the harmful effects of this compound [24].
6.1. Acute Toxicological Study
Despite the fact that the molecule was administered at a dose that was twenty times higher than the effective dosage (20 mg/kg), the researchers did not observe any negative behavioural symptoms or anomalies in blood cells [128]. These findings suggest that cinnamaldehyde has a large margin of safety. The oral median lethal dose (LD50) values for cinnamaldehyde range from 600 mg per kg body weight to 3400 mg per kg b.w. among numerous species. These results suggest that the molecule is not particularly dangerous in the near term [24].
6.2. Chronic Toxicological Study
Multiple studies have been performed on female and male F344/N rats, and B6C3F1 mice fed diets containing cinnamaldehyde for either three months (4100, 8200, 16,500, or 33,000 ppm of micro-encapsulated cinnamaldehyde) or two years (1000, 2100, or 4100 ppm of micro-encapsulated cinnamaldehyde) in order to investigate the possibility of long-term toxicity [129]. During the course of the study which lasted for three months, researchers found that the body weight of female rats exposed to 16,500 or 33,000 ppm and female mice exposed to 8200 ppm or higher had considerable weight loss. In addition, for all exposed groups of rats and the highest dose group of mice, food consumption was largely reduced. The occurrence of squamous epithelial hyperplasia of the forestomach is observed in rats when they are exposed to cinnamaldehyde at levels of 8200 ppm or higher and in female mice at levels of 33,000 ppm. There is an increased risk of olfactory epithelium degeneration in female mice when they are exposed to cinnamaldehyde at a concentration of 33,000 ppm, but there is an increased risk in male mice when they are exposed to cinnamaldehyde at a concentration of 16,500 ppm. During the course of the study that lasted for three months, not a single rat died.
The study that lasted for two years found that when rats and mice were given cinnamaldehyde at a concentration of 4100 ppm, it was observed that they were unable to gain body weight. Survival rates of male rats and male B6C3F1 mice were lower at a level of 4100 ppm compared to those of the controls. Cinnamaldehyde exposure doesn’t cause tumours in rats or B6C3F1 mice [24].
From an overall perusal of the literature, it is observed that cinnamaldehyde and its natural congeners have shown their dominancy against a diverse range of cancers both in in vivo and in vitro as presented in the form of a pie chart in Figure 5.
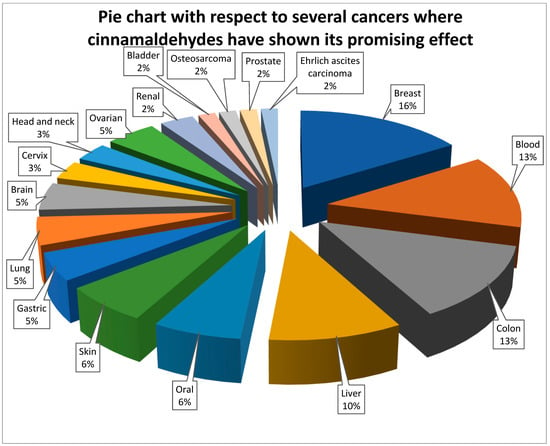
Figure 5.
Pie chart with respect to several cancers where cinnamaldehydes have shown its promising effect.
7. Results and Conclusions
The purpose of this review is to compile the information that is currently available from the scientific literature that points to cinnamaldehyde as a leading natural phytochemical with anticancer potential in various cancers. A particular emphasis was placed on the intervened molecular mechanisms. There is absolutely no need to argue for the remarkable effectiveness of cinnamaldehyde in preventing and treating a wide range of cancers. It is possible that the anticancer and antimetastatic effects of cinnamaldehyde are the result of a number of different molecular mechanisms; this suggests that cinnamaldehyde is a compound with multiple targets. It is necessary to assess the differential responsiveness of various cancers to cinnamaldehyde or its various congeners in order to select the most effective compound for treating each type of cancer. Only then can one choose the most effective compound.
According to the findings of this review, cinnamaldehyde and its congeners have the potential as chemotherapeutic agents because they inhibit the spread of cancer and cell invasion. Combination therapies that make use of conventional anticancer drugs and the nanomolar amounts of cinnamaldehyde required for them to exert their anti-invasion effects would be especially beneficial for cancer patients. The in vivo anti-invasion efficacy of cinnamaldehyde and its congeners on various types of cancer, as well as the molecular mechanisms by which cinnamaldehyde inhibits cell invasion, still require additional research.
Author Contributions
S.B. (Sabyasachi Banerjee) and S.B. (Subhasis Banerjee) contributed equally in making the manuscript, starting from conceptualization, data collection, original draft preparation, review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Since it’s a review article, no such data is used.
Acknowledgments
Both the authors would like to express their deepest gratitude to the researchers from all around the globe who have made important contributions to our knowledge of the role that cinnamaldehyde may play role in the treatment and prevention of cancer.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mondal, A.; Banerjee, S.; Bose, S.; Das, P.P.; Sandberg, E.N.; Atanasov, A.G.; Bishayee, A.J. Cancer preventive and therapeutic potential of banana and its bioactive constituents: A systematic, comprehensive, and mechanistic review. Front. Oncol. 2021, 11, 697143. [Google Scholar] [CrossRef]
- Morounke, S.G.; Ayorinde, J.B.; Benedict, A.O.; Adedayo, F.F.; Adewale, F.O.; Oluwadamilare, I.; Sokunle, S.S.; Benjamin, A. Epidemiology and incidence of common cancers in Nigeria. Population 2017, 84, 166–629. [Google Scholar]
- India State-Level Disease Burden Initiative Cancer, C. The burden of cancers and their variations across the states of India: The Global Burden of Disease Study 1990–2016. Lancet Oncol. 2018, 19, 1289–1306. [Google Scholar] [CrossRef]
- Smittenaar, C.; Petersen, K.; Stewart, K.; Moitt, N. Cancer incidence and mortality projections in the UK until 2035. Br. J. Cancer 2016, 115, 1147–1155. [Google Scholar] [CrossRef]
- Schirrmacher, V. From chemotherapy to biological therapy: A review of novel concepts to reduce the side effects of systemic cancer treatment. Int. J. Oncol. 2019, 54, 407–419. [Google Scholar]
- Sedighi, M.; Zahedi Bialvaei, A.; Hamblin, M.R.; Ohadi, E.; Asadi, A.; Halajzadeh, M.; Lohrasbi, V.; Mohammadzadeh, N.; Amiriani, T.; Krutova, M. Therapeutic bacteria to combat cancer; current advances, challenges, and opportunities. Cancer Med. 2019, 8, 3167–3181. [Google Scholar] [CrossRef]
- Lichota, A.; Gwozdzinski, K. Anticancer Activity of Natural Compounds from Plant and Marine Environment. Int. J. Mol. Sci. 2018, 19, 3533. [Google Scholar] [CrossRef] [PubMed]
- A Aljuffali, I.; Fang, C.-L.; Chen, C.-H.; Fang, J.-Y. Nanomedicine as a strategy for natural compound delivery to prevent and treat cancers. Curr. Pharm. Des. 2016, 22, 4219–4231. [Google Scholar] [CrossRef]
- Abubakar, I.B.; Ukwuani-Kwaja, A.N.; Garba, A.D.; Singh, D.; Malami, I.; Salihu, T.S.; Muhammad, A.; Yahaya, Y.; Sule, S.M.; Ahmed, S.J. Ethnobotanical study of medicinal plants used for cancer treatment in Kebbi state, North-west Nigeria. Acta Ecol. Sin. 2020, 40, 306–314. [Google Scholar] [CrossRef]
- Adewole, K.E. Nigerian antimalarial plants and their anticancer potential: A review. J. Integr. Med. 2020, 18, 92–113. [Google Scholar] [CrossRef] [PubMed]
- Rizeq, B.; Gupta, I.; Ilesanmi, J.; AlSafran, M.; Rahman, M.; Ouhtit, A. The power of phytochemicals combination in cancer chemoprevention. J. Cancer 2020, 11, 4521–4533. [Google Scholar] [CrossRef]
- Choudhari, A.S.; Mandave, P.C.; Deshpande, M.; Ranjekar, P.; Prakash, O. Phytochemicals in cancer treatment: From preclinical studies to clinical practice. Front. Pharmacol. 2020, 10, 1614. [Google Scholar] [CrossRef] [PubMed]
- Padhy, I.; Paul, P.; Sharma, T.; Banerjee, S.; Mondal, A. Molecular Mechanisms of Action of Eugenol in Cancer: Recent Trends and Advancement. Life 2022, 12, 1795. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Su, B.; Jiang, H.; Cui, N.; Yu, Z.; Yang, Y.; Sun, Y. Traditional uses, phytochemistry and pharmacological activities of the genus Cinnamomum (Lauraceae): A review. Fitoterapia 2020, 146, 104675. [Google Scholar] [CrossRef]
- Guangfu, T. Cinnamomum (Lauraceae) resources in Hubei Province, China. Wuhan Zhi Wu Xue Yan Jiu Wuhan Bot. Res. 2001, 19, 489–496. [Google Scholar]
- Morsi, D.S.; El-Nabi, S.H.; Elmaghraby, M.A.; Abu Ali, O.A.; Fayad, E.; Khalifa, S.A.; El-Seedi, H.R.; El-Garawani, I.M. Anti-proliferative and immunomodulatory potencies of cinnamon oil on Ehrlich ascites carcinoma bearing mice. Sci. Rep. 2022, 12, 11839. [Google Scholar] [CrossRef]
- Sadeghi, S.; Davoodvandi, A.; Pourhanifeh, M.H.; Sharifi, N.; ArefNezhad, R.; Sahebnasagh, R.; Moghadam, S.A.; Sahebkar, A.; Mirzaei, H. Anti-cancer effects of cinnamon: Insights into its apoptosis effects. Eur. J. Med. Chem. 2019, 178, 131–140. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef]
- Ibi, A.A.; Kyuka, C.K. Sources, extraction and biological activities of cinnamaldehyde. Trends Pharm. Sci. 2022, 8, 263–282. [Google Scholar]
- Rao, P.V.; Gan, S.H. Cinnamon: A multifaceted medicinal plant. Evid. Based Complement. Altern. Med. 2014, 2014, 642942. [Google Scholar] [CrossRef]
- Panel, T.R.E.; Belsito, D.; Bickers, D.; Bruze, M.; Calow, P.; Greim, H.; Hanifin, J.; Rogers, A.; Saurat, J.; Sipes, I.J.F.; et al. A toxicologic and dermatologic assessment of related esters and alcohols of cinnamic acid and cinnamyl alcohol when used as fragrance ingredients. Food Chem. Toxicol. 2007, 45, S1–S23. [Google Scholar]
- Walter, A.; Seegräber, M.; Wollenberg, A. Food-related contact dermatitis, contact urticaria, and atopy patch test with food. Clin. Rev. Allergy Immunol. 2019, 56, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Xie, Y.; Yang, Q.; Cao, Y.; Tu, H.; Cao, W.; Wang, S. Pharmacokinetic study of cinnamaldehyde in rats by GC–MS after oral and intravenous administration. J. Pharm. Biomed. Anal. 2014, 89, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Liu, H.; Liu, C.; Wang, L.; Ma, R.; Chen, B.; Li, L.; Niu, J.; Fu, M.; Zhang, D. Cinnamaldehyde in diabetes: A review of pharmacology, pharmacokinetics and safety. Pharmacol. Res. 2017, 122, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Kamat, A.M.; Hahn, N.M.; Efstathiou, J.A.; Lerner, S.P.; Malmström, P.-U.; Choi, W.; Guo, C.C.; Lotan, Y.; Kassouf, W. Bladder cancer. Lancet 2016, 388, 2796–2810. [Google Scholar] [CrossRef]
- Aminzadeh, Z.; Ziamajidi, N.; Abbasalipourkabir, R. Anticancer Effects of Cinnamaldehyde Through Inhibition of ErbB2/HSF1/LDHA Pathway in 5637 Cell Line of Bladder Cancer. Anticancer Agents Med. Chem. 2022, 22, 1139–1148. [Google Scholar] [CrossRef]
- Rosmarin, A. Leukemia, Lymphoma, and Myeloma; Wiley Online Library: Hoboken, NJ, USA, 2019; pp. 299–316. [Google Scholar]
- Greim, H.; Kaden, D.A.; Larson, R.A.; Palermo, C.M.; Rice, J.M.; Ross, D.; Snyder, R. The bone marrow niche, stem cells, and leukemia: Impact of drugs, chemicals, and the environment. Ann. N. Y. Acad. Sci. 2014, 1310, 7–31. [Google Scholar] [CrossRef] [PubMed]
- Anand, U.; Dey, A.; Chandel, A.K.S.; Sanyal, R.; Mishra, A.; Pandey, D.K.; De Falco, V.; Upadhyay, A.; Kandimalla, R.; Chaudhary, A.J.G.; et al. Cancer chemotherapy and beyond: Current status, drug candidates, associated risks and progress in targeted therapeutics. Genes Dis. 2022, in press. [Google Scholar] [CrossRef]
- McCabe, B.; Liberante, F.; Mills, K.I. Repurposing medicinal compounds for blood cancer treatment. Ann. Hematol. 2015, 94, 1267–1276. [Google Scholar] [CrossRef]
- Moon, K.; Pack, M.Y. Cytotoxicity of cinnamic aldehyde on leukemia L1210 cells. Drug Chem. Toxicol. 1983, 6, 521–535. [Google Scholar] [CrossRef]
- Ka, H.; Park, H.-J.; Jung, H.-J.; Choi, J.-W.; Cho, K.-S.; Ha, J.; Lee, K.-T. Cinnamaldehyde induces apoptosis by ROS-mediated mitochondrial permeability transition in human promyelocytic leukemia HL-60 cells. Cancer Lett. 2003, 196, 143–152. [Google Scholar] [CrossRef]
- Fang, S.-H.; Rao, Y.K.; Tzeng, Y.-M. Cytotoxic effect of trans-cinnamaldehyde from cinnamomum osmophloeum leaves on Human cancer cell lines. Int. J. Appl. Sci. Eng. 2004, 2, 136–147. [Google Scholar]
- Zhang, J.-H.; Liu, L.-Q.; He, Y.-L.; Kong, W.-J.; Huang, S.-A. Cytotoxic effect of trans-cinnamaldehyde on human leukemia K562 cells. Acta Pharmacol. Sin. 2010, 31, 861–866. [Google Scholar] [CrossRef]
- Khan, R.; Sharma, M.; Kumar, L.; Husain, S.A.; Sharma, A. Cinnamon extract exhibits potent anti-proliferative activity by modulating angiogenesis and cyclooxygenase in myeloma cells. J. Herb. Med. 2016, 6, 149–156. [Google Scholar] [CrossRef]
- Ho, S.-C.; Chang, Y.-H.; Chang, K.-S. Structural moieties required for cinnamaldehyde-related compounds to inhibit canonical IL-1β secretion. Molecules 2018, 23, 3241. [Google Scholar] [CrossRef]
- He, W.; Zhang, W.; Zheng, Q.; Wei, Z.; Wang, Y.; Hu, M.; Ma, F.; Tao, N.; Luo, C. Cinnamaldehyde causes apoptosis of myeloid-derived suppressor cells through the activation of TLR4. Oncol. Lett. 2019, 18, 2420–2426. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Ma, J.; Guan, L.; Liu, X.; Wang, A.; Hu, C.-H. Proliferation effects of cinnamon extract on human HeLa and HL-60 tumor cell lines. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 5347–5354. [Google Scholar] [PubMed]
- Jeong, H.; Park, J.; Shim, J.-K.; Lee, J.E.; Kim, N.H.; Kim, H.S.; Chang, J.H.; Yook, J.I.; Kang, S.-G. Combined treatment with 2′-hydroxycinnamaldehyde and temozolomide suppresses glioblastoma tumorspheres by decreasing stemness and invasiveness. J. Neuro-Oncol. 2019, 143, 69–77. [Google Scholar] [CrossRef]
- Abbasi, A.; Hajialyani, M.; Hosseinzadeh, L.; Jalilian, F.; Yaghmaei, P.; Navid, S.J.; Motamed, H. Evaluation of the cytotoxic and apoptogenic effects of cinnamaldehyde on U87MG cells alone and in combination with doxorubicin. Res. Pharm. Sci. 2020, 15, 26. [Google Scholar]
- Chen, J.-C.; Hsieh, P.-S.; Chen, S.-M.; Hwang, J.-H. Effects of Cinnamaldehyde on the viability and expression of chemokine receptor genes in Temozolomide-treated Glioma cells. In Vivo 2020, 34, 595–599. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, K.; Nam, S.; Anderson, R.A.; Jove, R.; Wen, W. Novel angiogenesis inhibitory activity in cinnamon extract blocks VEGFR2 kinase and downstream signaling. Carcinogenesis 2010, 31, 481–488. [Google Scholar] [CrossRef]
- Vangalapati, M.; Prakash, D. In-vitro anti-cancer studies of Cinnamaldehyde on breast cancer cell line (MCF-7). BioTechnology 2013, 7, 81–84. [Google Scholar]
- Abd Wahab, W.; Adzmi, A.N. The investigation of cytotoxic effect of Cinnamomum zeylanicum extracts on human breast cancer cell line (MCF7). Sci. Herit. J. 2017, 1, 23–28. [Google Scholar] [CrossRef]
- Kostrzewa, T.; Przychodzen, P.; Gorska-Ponikowska, M.; Kuban-Jankowska, A. Curcumin and cinnamaldehyde as PTP1B inhibitors with antidiabetic and anticancer potential. Anticancer Res. 2019, 39, 745–749. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.-F.; Chen, H.-Y.; Huang, K.-C.; Lin, P.-H.; Hsia, S.-M. Dietary antioxidant trans-cinnamaldehyde reduced visfatin-induced breast cancer progression: In vivo and in vitro study. Antioxidants 2019, 8, 625. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; An, T.; Wan, D.; Yu, B.; Fan, Y.; Pei, X. Targets and mechanism used by cinnamaldehyde, the main active ingredient in cinnamon, in the treatment of breast cancer. Front. Pharmacol. 2020, 11, 582719. [Google Scholar] [CrossRef]
- Poornima, B.; Deeba, F. Activities of cinnamaldehyde from Boswellia serrata on MCF-7 breast cancer cell line. J. Innov. Dev. Pharm. Tech. Sci. 2020, 3, 35–43. [Google Scholar]
- Kuo, Y.-T.; Liu, C.-H.; Wong, S.H.; Pan, Y.-C.; Lin, L.-T. Small molecules baicalein and cinnamaldehyde are potentiators of measles virus-induced breast cancer oncolysis. Phytomedicine 2021, 89, 153611. [Google Scholar] [CrossRef]
- Schuster, C.; Wolpert, N.; Moustaid-Moussa, N.; Gollahon, L.S. Combinatorial Effects of the Natural Products Arctigenin, Chlorogenic Acid, and Cinnamaldehyde Commit Oxidation Assassination on Breast Cancer Cells. Antioxidants 2022, 11, 591. [Google Scholar] [CrossRef]
- Yi, X.; Hou, L.; Tan, X.; Pei, T.; Li, S.; Huang, L. Cinnamic Aldehyde Induces Apoptosis of Breast Cancer Cells via STAT3/cMyc Pathway. 2022. preprint. [Google Scholar] [CrossRef]
- Jaisamak, K.; Iawsipo, P.; Ponglikitmongkol, M. Anti-invasion activity of trans-4-methoxy-cinnamaldehyde (4-MCA) in cervical cancer cells. In Proceedings of the 6th International Conference on Biochemistry and Molecular Biology (BMB 2018), Tokyo, Japan, 18–20 January 2018. [Google Scholar]
- GT, W.; Villeneuve, N.F.; Lamore, S.D.; Bause, A.S.; Jiang, T.; Zhang, D.D. The cinnamon-derived dietary factor cinnamic aldehyde activates the Nrf2-dependent antioxidant response in human epithelial colon cells. Molecules 2010, 15, 3338–3355. [Google Scholar]
- Long, M.; Tao, S.; Rojo de la Vega, M.; Jiang, T.; Wen, Q.; Park, S.L.; Zhang, D.D.; Wondrak, G.T. Nrf2-Dependent Suppression of Azoxymethane/Dextran Sulfate Sodium–Induced Colon Carcinogenesis by the Cinnamon-Derived Dietary Factor CinnamaldehydeNrf2-Directed Colon Cancer Chemoprevention by Cinnamaldehyde. Cancer Prev. Res. 2015, 8, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Liu, S.-L.; Qi, M.-H.; Zou, X. Cinnamaldehyde/chemotherapeutic agents interaction and drug-metabolizing genes in colorectal cancer. Mol. Med. Rep. 2014, 9, 669–676. [Google Scholar] [CrossRef]
- Li, J.; Teng, Y.; Liu, S.; Wang, Z.; Chen, Y.; Zhang, Y.; Xi, S.; Xu, S.; Wang, R.; Zou, X. Cinnamaldehyde affects the biological behavior of human colorectal cancer cells and induces apoptosis via inhibition of the PI3K/Akt signaling pathway. Oncol. Rep. 2016, 35, 1501–1510. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.-A.; Kim, S.-A. 2′-Hydroxycinnamaldehyde induces apoptosis through HSF1-mediated BAG3 expression. Int. J. Oncol. 2017, 50, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Park, G.H.; Song, H.M.; Park, S.B.; Son, H.-J.; Um, Y.; Kim, H.-S.; Jeong, J.B. Cytotoxic activity of the twigs of Cinnamomum cassia through the suppression of cell proliferation and the induction of apoptosis in human colorectal cancer cells. BMC Complement. Altern. Med. 2018, 18, 28. [Google Scholar] [CrossRef]
- Anju, R.; Sunitha, M.; Nevin, K.G. Cinnamon extract enhances the mitochondrial reactive oxygen species production and arrests the proliferation of human colon cancer cell line, HCT-116. J. Herbs Spices Med. Plants 2018, 24, 293–301. [Google Scholar] [CrossRef]
- Wu, C.-E.; Zhuang, Y.-W.; Zhou, J.-Y.; Liu, S.-L.; Wang, R.-P.; Shu, P. Cinnamaldehyde enhances apoptotic effect of oxaliplatin and reverses epithelial-mesenchymal transition and stemnness in hypoxic colorectal cancer cells. Exp. Cell Res. 2019, 383, 111500. [Google Scholar] [CrossRef]
- Milani, A.T.; Rashidi, S.; Mahmoudi, R.; Douna, B.K. Cytotoxic activity of epigallocatechin and trans-cinnamaldehyde in gastric cancer cell line. Asian Pac. J. Cancer Biol. 2019, 4, 71–74. [Google Scholar] [CrossRef]
- Lee, S.; Jung, J. trans-Cinnamaldehyde-Induced Apoptosis in AGS Cells. J. Food Hyg. Saf. 2021, 36, 100–104. [Google Scholar] [CrossRef]
- Kim, T.W. Cinnamaldehyde induces autophagy-mediated cell death through ER stress and epigenetic modification in gastric cancer cells. Acta Pharmacol. Sin. 2022, 43, 712–723. [Google Scholar] [CrossRef]
- Ahn, S.-G.; Jin, Y.-H.; Yoon, J.-H.; Kim, S.-A. The anticancer mechanism of 2′-hydroxycinnamaldehyde in human head and neck cancer cells. Int. J. Oncol. 2015, 47, 1793–1800. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-M.; Kim, J.; Kang, S.H.; Oh, S.Y.; Lee, H.-J.; Kwon, B.-M.; Hong, S.-H. Up-regulation of Bone Morphogenetic Protein 7 by 2-Hydroxycinnamaldehyde Attenuates HNSCC Cell Invasion. Antican. Res. 2018, 38, 5747–5757. [Google Scholar] [CrossRef]
- Ng, L.-T.; Wu, S.-J. Antiproliferative activity of Cinnamomum cassia constituents and effects of pifithrin-alpha on their apoptotic signaling pathways in Hep G2 cells. Evid. Based Complement. Altern. Med. 2011, 2011, 492148. [Google Scholar] [CrossRef]
- Lin, L.-T.; Tai, C.-J.; Chang, S.-P.; Chen, J.-L.; Wu, S.-J.; Lin, C.-C. Cinnamaldehyde-induced apoptosis in human hepatoma PLC/PRF/5 cells involves the mitochondrial death pathway and is sensitive to inhibition by cyclosporin A and z-VAD-fmk. Anti-Cancer Agents Med. Chem. 2013, 13, 1565–1574. [Google Scholar] [CrossRef] [PubMed]
- Abd El Salam, A.S.G.; Samra, Y.A.; El-Shishtawy, M.M. Cinnamaldehyde Relieves Induced Hepatocellular Carcinoma in Rat Model via Targeting Wnt/β-Catenin Pathway. Sci. Pharm. 2022, 90, 22. [Google Scholar] [CrossRef]
- Haripriya, D.; Santhosh, A.; Bupesh, G.; Roshini, P.; Nivedha, S.; Padmashree, S.; Srivatsan, P.; Bhaskar, M.; Saravanan, K.M. Immunomodulatory and Apoptotic Effect of Cinnamaldehyde in HepG2 Cells. Biointerface Res. Appl. Chem. 2022, 13. [Google Scholar]
- Wong, H.Y.; Tsai, K.d.; Liu, Y.H.; Yang, S.m.; Chen, T.W.; Cherng, J.; Chou, K.S.; Chang, C.M.; Yao, B.T.; Cherng, J.M. Cinnamomum verum component 2-methoxycinnamaldehyde: A novel anticancer agent with both anti-topoisomerase I and II activities in human lung adenocarcinoma a549 cells in vitro and in vivo. Phytother. Res. 2016, 30, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Meng, M.; Geng, S.; Du, Z.; Yao, J.; Zheng, Y.; Li, Z.; Zhang, Z.; Li, J.; Duan, Y.; Du, G. Berberine and cinnamaldehyde together prevent lung carcinogenesis. Oncotarget 2017, 8, 76385. [Google Scholar] [CrossRef]
- Liu, Y.-H.; Tsai, K.-D.; Yang, S.-M.; Wong, H.-Y.; Chen, T.-W.; Cherng, J.; Cherng, J.-M. Cinnamomum verum ingredient 2-methoxycinnamaldehyde: A new antiproliferative drug targeting topoisomerase I and II in human lung squamous cell carcinoma NCI-H520 cells. Eur. J. Cancer Prev. 2017, 26, 314–323. [Google Scholar] [CrossRef]
- Park, J.; Baek, S.H. Combination therapy with cinnamaldehyde and hyperthermia induces apoptosis of A549 non-small cell lung carcinoma cells via regulation of reactive oxygen species and mitogen-activated protein kinase family. Int. J. Mol. Sci. 2020, 21, 6229. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.L.; Cheng, F.C.; Wang, S.P.; Chou, S.T.; Shih, Y. Cinnamomum cassia essential oil and its major constituent cinnamaldehyde induced cell cycle arrest and apoptosis in human oral squamous cell carcinoma HSC-3 cells. Environ. Toxicol. 2017, 32, 456–468. [Google Scholar] [CrossRef] [PubMed]
- Varadarajan, S.; Narasimhan, M.; Balaji, T.M.; Chamundeeswari, D.P.; Sakthisekaran, D. In vitro anticancer effects of Cinnamomum verum J. Presl, Cinnamaldehyde, 4 Hydroxycinnamic acid and Eugenol on an oral squamous cell carcinoma cell line. J. Contemp. Dent. Pr. 2020, 21, 1027–1033. [Google Scholar]
- Ahmed, B. Effect of Cinnamon Oil and Scorpion Venom on Oral Squamous Cell Carcinoma Cell Line (In vitro study). Egypt. Dent. J. 2022, 68, 533–541. [Google Scholar] [CrossRef]
- Aggarwal, S.; Bhadana, K.; Singh, B.; Rawat, M.; Mohammad, T.; Al-Keridis, L.A.; Alshammari, N.; Hassan, M.I.; Das, S.N. Cinnamomum zeylanicum Extract and its Bioactive Component Cinnamaldehyde Show Anti-Tumor Effects via Inhibition of Multiple Cellular Pathways. Front. Pharmacol. 2022, 13, 918479. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Chen, J.; Yang, S.; Tan, T.; Wang, N.; Wang, Y.; Zhang, L.; Yang, C.; Huang, H.; Luo, J.; et al. Cinnamaldehyde inhibits the function of osteosarcoma by suppressing the Wnt/β-catenin and PI3K/Akt signaling pathways. Drug Des. Dev. Ther. 2020, 14, 4625. [Google Scholar] [CrossRef]
- Zhang, K.; Han, E.S.; Dellinger, T.H.; Lu, J.; Nam, S.; Anderson, R.A.; Yim, J.H.; Wen, W. Cinnamon extract reduces VEGF expression via suppressing HIF-1α gene expression and inhibits tumor growth in mice. Mol. Carcinog. 2017, 56, 436–446. [Google Scholar] [CrossRef]
- Jae-young, J. Cinnamaldehyde Enhances Cytotoxic Effect of Cisplatin by ROS-Induced Autophagy in Cisplatin-Resistant Ovarian Cancer Cells; Seoul National University Graduate School: Seoul, Republic of Korea, 2017. [Google Scholar]
- Boggiti, M.; Penchalaneni, J. Effect of Cinnamaldehyde on Female Reproductive Hormones in 7, 12 Dimethyl Benzanthracene Induced Ovarian Cancer Rats. Int. J. Res. Appl. Sci. Eng. Technol. 2021, 9, 346–350. [Google Scholar] [CrossRef]
- Han, L.; Mei, J.; Ma, J.; Wang, F.; Gu, Z.; Li, J.; Zhang, Z.; Zeng, Y.; Lou, X.; Yao, X.; et al. Cinnamaldehyde induces endogenous apoptosis of the prostate cancer-associated fibroblasts via interfering the Glutathione-associated mitochondria function. Med. Oncol. 2020, 37, 91. [Google Scholar] [CrossRef]
- Bae, W.-Y.; Choi, J.-S.; Kim, J.-E.; Jeong, J.-W. Cinnamic aldehyde suppresses hypoxia-induced angiogenesis via inhibition of hypoxia-inducible factor-1α expression during tumor progression. Biochem. Pharmacol. 2015, 98, 41–50. [Google Scholar] [CrossRef]
- Ahn, C.R.; Park, J.; Kim, J.-E.; Ahn, K.S.; Kim, Y.W.; Jeong, M.; Kim, H.J.; Park, S.H.; Baek, S.H. Cinnamaldehyde and hyperthermia co-treatment synergistically induces ROS-mediated apoptosis in ACHN renal cell carcinoma cells. Biomedicines 2020, 8, 357. [Google Scholar] [CrossRef] [PubMed]
- Cabello, C.M.; Bair III, W.B.; Lamore, S.D.; Ley, S.; Bause, A.S.; Azimian, S.; Wondrak, G.T. The cinnamon-derived Michael acceptor cinnamic aldehyde impairs melanoma cell proliferation, invasiveness, and tumor growth. Free. Radic. Biol. Med. 2009, 46, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.-K.; Jeon, W.K.; Hwang, J.-S.; Lee, C.-G.; So, J.-S.; Park, J.-A.; Ko, B.S.; Im, S.-H. Cinnamon extract suppresses tumor progression by modulating angiogenesis and the effector function of CD8+ T cells. Cancer Lett. 2009, 278, 174–182. [Google Scholar] [CrossRef]
- Kwon, H.-K.; Hwang, J.-S.; So, J.-S.; Lee, C.-G.; Sahoo, A.; Ryu, J.-H.; Jeon, W.K.; Ko, B.S.; Im, C.-R.; Lee, S.H. Cinnamon extract induces tumor cell death through inhibition of NFκB and AP1. BMC Cancer 2010, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Patra, K.; Jana, S.; Sarkar, A.; Mandal, D.P.; Bhattacharjee, S. The inhibition of hypoxia-induced angiogenesis and metastasis by cinnamaldehyde is mediated by decreasing HIF-1α protein synthesis via PI3K/Akt pathway. Biofactors 2019, 45, 401–415. [Google Scholar] [CrossRef]
- Johnson, G.L.; Lapadat, R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 2002, 298, 1911–1912. [Google Scholar] [CrossRef] [PubMed]
- Geest, C.R.; Coffer, P.J. MAPK signaling pathways in the regulation of hematopoiesis. J. Leukoc. Biol. 2009, 86, 237–250. [Google Scholar] [CrossRef]
- Harper, S.J.; LoGrasso, P. Signalling for survival and death in neurones: The role of stress-activated kinases, JNK and p38. Cell. Signal. 2001, 13, 299–310. [Google Scholar] [CrossRef]
- Wang, Z.; Cao, N.; Nantajit, D.; Fan, M.; Liu, Y.; Li, J.J. Mitogen-activated protein kinase phosphatase-1 represses c-Jun NH2-terminal kinase-mediated apoptosis via NF-κB regulation. J. Biol. Chem. 2008, 283, 21011–21023. [Google Scholar] [CrossRef]
- Yu, W.; Liao, Q.Y.; Hantash, F.M.; Sanders, B.G.; Kline, K. Activation of extracellular signal-regulated kinase and c-Jun-NH2-terminal kinase but not p38 mitogen-activated protein kinases is required for RRR-α-tocopheryl succinate-induced apoptosis of human breast cancer cells. Cancer Res. 2001, 61, 6569–6576. [Google Scholar]
- Chen, C.; Shen, G.; Hebbar, V.; Hu, R.; Owuor, E.D.; Kong, A.-N. Epigallocatechin-3-gallate-induced stress signals in HT-29 human colon adenocarcinoma cells. Carcinogenesis 2003, 24, 1369–1378. [Google Scholar] [CrossRef]
- Hu, R.; Kim, B.R.; Chen, C.; Hebbar, V.; Kong, A.-N. The roles of JNK and apoptotic signaling pathways in PEITC-mediated responses in human HT-29 colon adenocarcinoma cells. Carcinogenesis 2003, 24, 1361–1367. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Kuo, H.-C.; Chu, C.-Y.; Wang, C.-J.; Lin, W.-C.; Tseng, T.-H. Involvement of tumor suppressor protein p53 and p38 MAPK in caffeic acid phenethyl ester-induced apoptosis of C6 glioma cells. Biochem. Pharmacol. 2003, 66, 2281–2289. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-J.; Ng, L.-T.; Lin, C.-C. Cinnamaldehyde-induced apoptosis in human PLC/PRF/5 cells through activation of the proapoptotic Bcl-2 family proteins and MAPK pathway. Life Sci. 2005, 77, 938–951. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.W.; Lee, S.H.; Lee, J.W.; Ban, J.O.; Lee, S.Y.; Yoo, H.S.; Jung, J.K.; Moon, D.C.; Oh, K.W.; Hong, J.T. 2-hydroxycinnamaldehyde inhibits SW620 colon cancer cell growth through AP-1 inactivation. J. Pharmacol. Sci. 2007, 104, 19–28. [Google Scholar] [CrossRef]
- Balkwill, F. Tumour necrosis factor and cancer. Nat. Rev. Cancer 2009, 9, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.W.; de Waal Malefyt, R.; Coffman, R.L.; O’Garra, A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001, 19, 683. [Google Scholar] [CrossRef]
- Roth-Walter, F.; Moskovskich, A.; Gomez-Casado, C.; Diaz-Perales, A.; Oida, K.; Singer, J.; Kinaciyan, T.; Fuchs, H.C.; Jensen-Jarolim, E. Immune suppressive effect of cinnamaldehyde due to inhibition of proliferation and induction of apoptosis in immune cells: Implications in cancer. PLoS ONE 2014, 9, e108402. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, C.W.; Lee, J.W.; Choi, M.S.; Son, D.J.; Chung, Y.B.; Lee, C.K.; Oh, K.W.; Moon, D.C.; Kwon, B.M.; et al. Induction of apoptotic cell death by 2′-hydroxycinnamaldehyde is involved with ERK-dependent inactivation of NF-κB in TNF-α-treated SW620 colon cancer cells. Biochem. Pharmacol. 2005, 70, 1147–1157. [Google Scholar] [CrossRef]
- Thompson, C.B. Apoptosis in the pathogenesis and treatment of disease. Science 1995, 267, 1456–1462. [Google Scholar] [CrossRef]
- Kroemer, G.; Reed, J.C. Mitochondrial control of cell death. Nat. Med. 2000, 6, 513–519. [Google Scholar] [CrossRef]
- Wang, X. The expanding role of mitochondria in apoptosis. Genes Dev. 2001, 15, 2922–2933. [Google Scholar]
- Desagher, S.; Martinou, J.-C. Mitochondria as the central control point of apoptosis. Trends Cell Biol. 2000, 10, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Galluzzi, L.; Brenner, C. Mitochondrial membrane permeabilization in cell death. Physiol. Rev. 2007, 87, 99–163. [Google Scholar] [CrossRef] [PubMed]
- Danial, N.N.; Korsmeyer, S.J. Cell death: Critical control points. Cell 2004, 116, 205–219. [Google Scholar] [CrossRef]
- Zhang, X.D.; Zhang, X.Y.; Gray, C.P.; Nguyen, T.; Hersey, P. Tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis of human melanoma is regulated by smac/DIABLO release from mitochondria. Cancer Res. 2001, 61, 7339–7348. [Google Scholar]
- Arnt, C.R.; Chiorean, M.V.; Heldebrant, M.P.; Gores, G.J.; Kaufmann, S.H. Synthetic Smac/DIABLO peptides enhance the effects of chemotherapeutic agents by binding XIAP and cIAP1 in situ. J. Biol. Chem. 2002, 277, 44236–44243. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.J.; Ng, L.T.; Lin, C.C. Effects of vitamin E on the cinnamaldehyde-induced apoptotic mechanism in human PLC/PRF/5 cells. Clin. Exp. Pharmacol. Physiol. 2004, 31, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Walczak, H.; Krammer, P.H. The CD95 (APO-1/Fas) and the TRAIL (APO-2L) apoptosis systems. Exp. Cell Res. 2000, 256, 58–66. [Google Scholar] [CrossRef]
- Krammer, P.H. CD95’s deadly mission in the immune system. Nature 2000, 407, 789–795. [Google Scholar] [CrossRef]
- Rowinsky, E.K. Targeted induction of apoptosis in cancer management: The emerging role of tumor necrosis factor–related apoptosis-inducing ligand receptor activating agents. J. Clin. Oncol. 2005, 23, 9394–9407. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.-C.; Kuo, Y.-H.; Kuo, C.-C.; Chen, L.-T.; Cheung, C.-H.A.; Chao, T.-Y.; Lin, C.-H.; Pan, W.-Y.; Chang, C.-Y.; Chien, S.-C.; et al. Chamaecypanone C, a novel skeleton microtubule inhibitor, with anticancer activity by trigger caspase 8-Fas/FasL dependent apoptotic pathway in human cancer cells. Biochem. Pharmacol. 2010, 79, 1261–1271. [Google Scholar] [CrossRef]
- Hsu, Y.-L.; Kuo, P.-L.; Lin, C.-C. Acacetin inhibits the proliferation of Hep G2 by blocking cell cycle progression and inducing apoptosis. Biochem. Pharmacol. 2004, 67, 823–829. [Google Scholar] [CrossRef]
- Singh, N.P.; Singh, U.P.; Hegde, V.L.; Guan, H.; Hofseth, L.; Nagarkatti, M.; Nagarkatti, P.S. Resveratrol (trans-3, 5, 4′-trihydroxystilbene) suppresses EL4 tumor growth by induction of apoptosis involving reciprocal regulation of SIRT1 and NF-κB. Mol. Nutr. Food Res. 2011, 55, 1207–1218. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Wilder, S.; Bannasch, D.; Israeli, D.; Lehlbach, K.; Li-Weber, M.; Friedman, S.L.; Galle, P.R.; Stremmel, W.; Oren, M. p53 activates the CD95 (APO-1/Fas) gene in response to DNA damage by anticancer drugs. J. Exp. Med. 1998, 188, 2033–2045. [Google Scholar] [CrossRef]
- Shin, E.-C.; Shin, W.-C.; Choi, Y.; Kim, H.; Park, J.H.; Kim, S.J. Effect of interferon-γ on the susceptibility to Fas (CD95/APO-1)-mediated cell death in human hepatoma cells. Cancer Immunol. Immunother. 2001, 50, 23–30. [Google Scholar] [CrossRef]
- Komarov, P.G.; Komarova, E.A.; Kondratov, R.V.; Christov-Tselkov, K.; Coon, J.S.; Chernov, M.V.; Gudkov, A.V. A chemical inhibitor of p53 that protects mice from the side effects of cancer therapy. Science 1999, 285, 1733–1737. [Google Scholar] [CrossRef] [PubMed]
- Charlot, J.; Nicolier, M.; Pretet, J.; Mougin, C. Modulation of p53 transcriptional activity by PRIMA-1 and Pifithrin-α on staurosporine-induced apoptosis of wild-type and mutated p53 epithelial cells. Apoptosis 2006, 11, 813–827. [Google Scholar] [CrossRef]
- Koppikar, S.J.; Choudhari, A.S.; Suryavanshi, S.A.; Kumari, S.; Chattopadhyay, S.; Kaul-Ghanekar, R. Aqueous Cinnamon Extract (ACE-c) from the bark of Cinnamomum cassiacauses apoptosis in human cervical cancer cell line (SiHa) through loss of mitochondrial membrane potential. BMC Cancer 2010, 10, 210. [Google Scholar] [CrossRef]
- Zhou, L.; Lu, Y.; Yang, G.; Wu, J. Research on tumorigenicity of cinnamaldehyde in melanoma cell lines and its mechanism. Tumor Biol. 2014, 35, 5717–5722. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Kang, H.S.; Park, B.E.; Moon, H.J.; Sim, S.S.; Kim, C.J. Inhibitory effects of Geijigajakyak-Tang on trinitrobenzene sulfonic acid-induced colitis. J. Ethnopharmacol. 2009, 126, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Ouwehand, K.; Santegoets, S.J.; Bruynzeel, D.P.; Scheper, R.J.; de Gruijl, T.D.; Gibbs, S. CXCL12 is essential for migration of activated Langerhans cells from epidermis to dermis. Eur. J. Immunol. 2008, 38, 3050–3059. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Wang, S.-M.; Lu, Q.; Luo, J.; Cheng, Y.-X. Identification of compounds from the water soluble extract of Cinnamomum cassia barks and their inhibitory effects against high-glucose-induced mesangial cells. Molecules 2013, 18, 10930–10943. [Google Scholar] [CrossRef]
- Dugoua, J.-J.; Seely, D.; Perri, D.; Cooley, K.; Forelli, T.; Mills, E.; Koren, G. From type 2 diabetes to antioxidant activity: A systematic review of the safety and efficacy of common and cassia cinnamon bark. Can. J. Physiol. Pharmacol. 2007, 85, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Murali, K.; Tandon, V.; Murthy, P.; Chandra, R. Insulinotropic effect of cinnamaldehyde on transcriptional regulation of pyruvate kinase, phosphoenolpyruvate carboxykinase, and GLUT4 translocation in experimental diabetic rats. Chem. Interact. 2010, 186, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Hooth, M.; Sills, R.; Burka, L.; Haseman, J.; Witt, K.; Orzech, D.; Fuciarelli, A.; Graves, S.; Johnson, J.; Bucher, J.J.F.; et al. Toxicology and carcinogenesis studies of microencapsulated trans-cinnamaldehyde in rats and mice. Food Chem. Toxicol. 2004, 42, 1757–1768. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
