Presence of Major Bacterial Foodborne Pathogens in the Domestic Environment and Hygienic Status of Food Cleaning Utensils: A Narrative Review
Abstract
1. Introduction
2. Bacterial Foodborne Pathogens Prevalent in the Domestic Environment
2.1. Campylobacter spp.
2.2. Clostridium Perfringens
2.3. Escherichia coli Pathotypes
2.4. Salmonella spp.
2.5. Staphylococcus aureus
2.6. Listeria monocytogenes
2.7. Other Bacterial Pathogens
3. Household Kitchen Equipment That Harbors Foodborne Pathogenic Bacteria
3.1. Cutting Boards
3.2. Kitchen Countertops and Other Surfaces
3.3. Kitchen Sink and Faucet
3.4. Domestic Refrigerators
3.5. Food Cleaning Utensils
3.6. Other Kitchen Appliances and Utensils
4. Discussion
4.1. Food Consumer Behavior
4.2. Hygienic Status of Food Cleaning Utensils and Kitchen Appliances
4.3. Persistence of Foodborne Bacterial Pathogens in Home Kitchens
4.4. AMR in the Domestic Environment
4.5. Towards Effective Consumer Awareness, Risk Management, and Guidelines for Food Hygiene Implementation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Newell, D.G.; Koopmans, M.; Verhoef, L.; Duizer, E.; Aidara-Kane, A.; Sprong, H.; Opsteegh, M.; Langelaar, M.; Threfall, J.; Scheutz, F.; et al. Food-borne diseases—The challenges of 20 years ago still persist while new ones continue to emerge. Int. J. Food Microbiol. 2010, 139, S3–S15. [Google Scholar] [CrossRef] [PubMed]
- Odeyemi, O. Public health implications of microbial food safety and foodborne diseases in developing countries. Food Nutr. Res. 2016, 60, 29819. [Google Scholar] [CrossRef] [PubMed]
- WHO/FERG (World Health Organization/Foodborne Disease Burden Epidemiology Reference Group). WHO Estimates of the Global Burden of Foodborne Diseases; WHO: Geneva, Switzerland, 2015; pp. 1–254. Available online: https://iris.who.int/bitstream/handle/10665/199350/9789241565165_eng.pdf (accessed on 28 August 2025).
- WHO/FERG (World Health Organization/Foodborne Disease Burden Epidemiology Reference Group). WHO Foodborne Disease Burden Epidemiology Reference Group for 2021–2024: Second Meeting Report, 19 October–2 November 2021; WHO: Geneva, Switzerland, 2024; pp. 1–23. Available online: https://iris.who.int/bitstream/handle/10665/379327/9789240100510-eng.pdf?sequence=1 (accessed on 28 August 2025).
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.-A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne illness acquired in the United States—Major pathogens. Emerg. Inf. Dis. 2011, 17, 7–15. [Google Scholar] [CrossRef]
- Scallan Walter, E.J.; Cui, Z.; Tierney, R.; Griffin, P.M.; Hoekstra, R.M.; Payne, D.C.; Rose, E.B.; Devine, C.; Namwase, A.S.; Mirza, S.A.; et al. Foodborne illness acquired in the United States—Major pathogens, 2019. Emerg. Inf. Dis. 2025, 31, 669–677. [Google Scholar] [CrossRef]
- DG SANTE (Directorate General for Health and Food Safety). 2024 Annual Report Alert & Cooperation Network; European Union: Luxembourg, 2025; pp. 1–31. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority); ECDC (European Centre for Disease Prevention and Control). The European Union One Health 2023 zoonoses report. EFSA J. 2024, 22, e9106. [Google Scholar]
- Data Sheet on Foodborne Biological Hazards: “Domestic Hygiene”. Available online: https://www.anses.fr/en/content/data-sheet-foodborne-biological-hazards-domestic-hygiene (accessed on 2 October 2025).
- FAO (Food and Agriculture Organization of the United Nations); WHO (World Health Organization). General Principles of Food Hygiene; Codex Alimentarius Code of Practice, No. CXC 1-1969; FAO: Rome, Italy; WHO: Rome, Italy, 2023; pp. 1–60. [Google Scholar] [CrossRef]
- FAO (Food and Agriculture Organization of the United Nations); WHO (World Health Organization). Food Hygiene (Basic Texts), 4th ed.; FAO: Rome, Italy; WHO: Rome, Italy, 2009; pp. 1–125. Available online: https://www.fao.org/4/a1552e/a1552e00.htm (accessed on 28 August 2025).
- Jones, M.V. Application of HACCP to identify hygiene risks in the home. Int. Biodeter. Biodegrad. 1998, 41, 191–199. [Google Scholar] [CrossRef]
- Beumer, R.R.; Kusumaningrum, H. Kitchen hygiene in daily life. Int. Biodeter. Bidegrad. 2003, 51, 299–302. [Google Scholar] [CrossRef]
- Byrd-Bredbenner, C.; Berning, J.; Martin-Biggers, J.; Quick, V. Food safety in home kitchens: A synthesis of the literature. Int. J. Environ. Res. Public Health 2013, 10, 4060–4085. [Google Scholar] [CrossRef]
- Taché, J.; Carpentier, B. Hygiene in the home kitchen: Changes in behaviour and impact of key microbiological hazard control measures. Food Control 2014, 14, 392–400. [Google Scholar] [CrossRef]
- Borrusso, P.A.; Quinlan, J.J. Prevalence of pathogens and indicator organisms in home kitchens and correlation with unsafe food handling practices and conditions. J. Food Prot. 2017, 80, 590–597. [Google Scholar] [CrossRef]
- Cardoso, M.J.; Ferreira, V.; Truninger, M.; Maia, R.; Teixeira, P. Cross-contamination events of Campylobacter spp. in domestic kitchens associated with consumer handling practices of raw poultry. Int. J. Food Microbiol. 2021, 338, 108984. [Google Scholar] [CrossRef]
- Gorman, R.; Bloomfield, S.; Adley, C.C. A study of cross-contamination of food-borne pathogens in the domestic kitchen in the Republic of Ireland. Int. J. Food Microbiol. 2002, 76, 143–150. [Google Scholar] [CrossRef]
- Agata, N.; Ohta, M.; Yokoyama, K. Production of Bacillus cereus emetic toxin (cereulide) in various foods. Int. J. Food Microbiol. 2002, 73, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Pirhonen, T.I.; Andersson, M.A.; Jääskeläinen, E.L.; Salkinoja-Salonen, M.S.; Honkanen-Buzalski, T.; Johansson, T.M.L. Biochemical and toxic diversity of Bacillus cereus in a pasta and meat dish associated with a food-poisoning case. Food Microbiol. 2005, 22, 87–91. [Google Scholar] [CrossRef]
- Thirkell, C.E.; Sloan-Gardner, T.S.; Kacmarek, M.C.; Polkinghorne, B. An outbreak of Bacillus cereus toxin-mediated emetic and diarrhoeal syndromes at a restaurant in Canberra, Australia 2018. Commun. Dis. Intell. 2019, 43. [Google Scholar] [CrossRef]
- Yu, S.; Yu, P.; Wang, J.; Li, C.; Guo, H.; Liu, C.; Kong, L.; Yu, L.; Wu, S.; Lei, T.; et al. A study on prevalence and characterization of Bacillus cereus in ready-to-eat foods in China. Front. Microbiol. 2020, 10, 3043. [Google Scholar] [CrossRef]
- Jung, S.-W. A foodborne outbreak of gastroenteritis caused by Vibrio parahaemolyticus associated with cross-contamination from squid in Korea. Epidemiol. Health 2019, 40, e2018056. [Google Scholar] [CrossRef]
- Malcolm, T.T.H.; Chang, W.S.; Loo, Y.Y.; Cheah, Y.K.; Radzi, C.W.J.W.M.; Kantilal, H.K.; Nishibuchi, M.; Son, R. Simulation of improper food hygiene practices: A quantitative assessment of Vibrio parahaemolyticus distribution. Int. J. Food Microbiol. 2018, 284, 112–119. [Google Scholar] [CrossRef]
- Azevedo, I.; Albano, H.; Silva, J.; Teixeira, P. Food safety in the domestic environment. Food Control 2014, 37, 272–276. [Google Scholar] [CrossRef]
- Carrasco, E.; Morales-Rueda, A.; García-Gimeno, R.M. Cross-contamination and recontamination by Salmonella in foods: A review. Food Res. Int. 2012, 45, 545–556. [Google Scholar] [CrossRef]
- Dunn, R.R.; Fierer, N.; Henley, J.B.; Leff, J.W.; Menninger, H.L. Home life: Factors structuring the bacterial diversity found within and between homes. PLoS ONE 2013, 8, e64133. [Google Scholar] [CrossRef]
- Adams, R.; Bateman, A.C.; Bik, H.M.; Meadow, J.F. Microbiota of the indoor environment: A meta-analysis. Microbiome 2015, 3, 49. [Google Scholar] [CrossRef] [PubMed]
- Flores, G.E.; Bates, S.T.; Caporaso, J.G.; Lauber, C.L.; Leff, J.W.; Knight, R.; Fierer, N. Diversity, distribution and sources of bacteria in residential kitchens. Environ. Microbiol. 2013, 15, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Chmielewski, R.A.N.; Frank, J.F. Biofilm formation and control in food processing facilities. Compr. Rev. Food Sci. Food Saf. 2003, 2, 22–32. [Google Scholar] [CrossRef]
- Giaouris, E.; Heir, E.; Hébraud, M.; Chorianopoulos, N.; Langsrud, S.; Møretrø, T.; Habimana, O.; Desvaux, M.; Renier, S.; Nychas, G.-J. Attachment and biofilm formation by foodborne bacteria in meat processing environments: Causes, implications, role of bacterial interactions and control by alternative novel methods. Meat Sci. 2014, 97, 298–309. [Google Scholar] [CrossRef]
- Andritsos, N.D.; Stasinou, V.; Tserolas, D.; Giaouris, E. Temperature distribution and hygienic status of domestic refrigerators in Lemnos island, Greece. Food Control 2021, 127, 108121. [Google Scholar] [CrossRef]
- Kusumaningrum, H.D.; van Putten, M.M.; Rombouts, F.M.; Beumer, R.R. Effects of antibacterial dishwashing liquid on foodborne pathogens and competitive microorganisms in kitchen sponges. J. Food Prot. 2002, 65, 61–65. [Google Scholar] [CrossRef]
- Mattick, K.; Durham, K.; Domingue, G.; Jørgensen, F.; Sen, M.; Schaffner, D.W.; Humphrey, T. The survival of foodborne pathogens during domestic washing-up and subsequent transfer onto washing-up sponges, kitchen surfaces and food. Int. J. Food Microbiol. 2003, 85, 213–226. [Google Scholar] [CrossRef]
- Evans, E.W.; Redmond, E.C. Behavioral observation and microbiological analysis of older adult consumers’ cross-contamination practices in a model domestic kitchen. J. Food Prot. 2018, 81, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Al-Sakkaf, A. Domestic food preparation practices: A review of the reasons for poor home hygiene practices. Health Promot. Int. 2015, 30, 427–437. [Google Scholar] [CrossRef]
- Redmond, E.C.; Griffith, C.J. Consumer food handling in the home: A review of food safety studies. J. Food Prot. 2003, 66, 130–161. [Google Scholar] [CrossRef]
- Møretrø, T.; Martens, L.; Teixeira, P.; Ferreira, V.B.; Maia, R.; Maugesten, T.; Langsrud, S. Is visual motivation for cleaning surfaces in the kitchen consistent with a hygienically clean environment? Food Control 2020, 111, 107077. [Google Scholar] [CrossRef]
- Hilton, A.C.; Austin, E. The kitchen dishcloth as a source of and vehicle for foodborne pathogens in a domestic setting. Int. J. Environ. Health Res. 2000, 10, 257–261. [Google Scholar] [CrossRef]
- Kusumaningrum, H.D.; Riboldi, G.; Hazeleger, W.C.; Beumer, R.R. Survival of foodborne pathogens on stainless steel surfaces and cross-contamination to foods. Int. J. Food Microbiol. 2003, 85, 227–236. [Google Scholar] [CrossRef]
- Møretrø, T.; Moen, B.; Almli, V.L.; Teixeira, P.; Ferreira, V.B.; Asli, A.W.; Nilsen, C.; Langsrud, S. Dishwashing sponges and brushes: Consumer practices and bacterial growth and survival. Int. J. Food Microbiol. 2021, 337, 108928. [Google Scholar] [CrossRef]
- Garrido, V.; Garćia-Jalón, I.; Vitas, A.I. Temperature distribution in Spanish domestic refrigerators and its effect on Listeria monocytogenes growth in sliced ready-to-eat ham. Food Control 2010, 21, 896–901. [Google Scholar] [CrossRef]
- Jackson, V.; Blair, I.S.; McDowell, D.A.; Kennedy, J.; Bolton, D.J. The incidence of significant foodborne pathogens in domestic refrigerators. Food Control 2007, 18, 346–351. [Google Scholar] [CrossRef]
- Roccato, A.; Uyttendaele, M.; Membré, J.-M. Analysis of domestic refrigerator temperatures and home storage time distributions for shelf-life studies and food safety risk assessment. Food Res. Int. 2017, 96, 171–181. [Google Scholar] [CrossRef]
- Webb, M.D.; Barker, G.C.; Goodburn, K.E.; Peck, M.W. Risk presented to minimally processed chilled foods by psychrotrophic Bacillus cereus. Trends Food Sci. Technol. 2019, 93, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Hong, Y.J.; Jo, J.I.; Ha, S.D.; Kim, S.H.; Lee, H.J.; Rhee, M.S. Raw ready-to-eat seafood safety: Microbiological quality of the various seafood species available in fishery, hyper and online markets. Lett. Appl. Microbiol. 2017, 67, 27–34. [Google Scholar] [CrossRef]
- Baek, E.; Lee, D.; Jang, S.; An, H.; Kim, M.; Kim, K.; Lee, K.; Ha, N. Antibiotic resistance and assessment of food-borne pathogenic bacteria in frozen foods. Arch. Pharm. Res. 2009, 32, 1749–1757. [Google Scholar] [CrossRef]
- Lin, W.; Xu, F.; Guo, H.; Cui, L. Domestic refrigerators: An overlooked breeding ground of antibiotic resistance genes and pathogens. Environ. Int. 2022, 170, 107647. [Google Scholar] [CrossRef] [PubMed]
- Marshall, B.M.; Robleto, E.; Dumont, T.; Levy, S.B. The frequency of antibiotic-resistant bacteria in homes differing in their use of surface antibacterial agents. Curr. Microbiol. 2012, 65, 407–415. [Google Scholar] [CrossRef]
- Caniça, M.; Manageiro, V.; Abriouel, H.; Moran-Gilad, J.; Franz, C.M.A.P. Antibiotic resistance in foodborne bacteria. Trends Food Sci. Technol. 2019, 84, 41–44. [Google Scholar] [CrossRef]
- Maillard, J.-Y.; Bloomfield, S.F.; Courvalin, P.; Essack, S.Y.; Gandra, S.; Gerba, C.P.; Rubino, J.R.; Scott, E.A. Reducing antibiotic prescribing and addressing the global problem of antibiotic resistance by targeted hygiene in the home and everyday life settings: A position paper. Am. J. Infect. Control 2020, 48, 1090–1099. [Google Scholar] [CrossRef]
- Borrusso, P.; Quinlan, J.J. Development and piloting of a food safety audit tool for the domestic environment. Foods 2013, 2, 572–584. [Google Scholar] [CrossRef]
- Evans, E.W.; Redmond, E.C. Domestic kitchen microbiological contamination and self-reported food hygiene practices of older adult consumers. J. Food Prot. 2019, 82, 1326–1335. [Google Scholar] [CrossRef]
- Ropkins, K.; Beck, A.J. HACCP in the home: A framework for improving awareness of hygiene and safe food handling with respect to chemical risk. Trends Food Sci. Technol. 2000, 11, 105–114. [Google Scholar] [CrossRef]
- Langsrud, S.; Sørheim, O.; Skuland, S.E.; Almli, V.L.; Jensen, M.R.; Grøvlen, M.S.; Ueland, Ø.; Møretrø, T. Cooking chicken at home: Common or recommended approaches to judge doneness may not assure sufficient inactivation of pathogens. PLoS ONE 2020, 15, e0230928. [Google Scholar] [CrossRef] [PubMed]
- Moen, B.; Langsrud, S.; Berget, I.; Maugesten, T.; Møretrø, T. Mapping the kitchen microbiota in five European counties reveals a set of core bacteria across countries, kitchen surfaces, and cleaning utensils. Appl. Environ. Microbiol. 2023, 89, e0026723. [Google Scholar] [CrossRef] [PubMed]
- De Boer, A.; Hahné, M. Cross-contamination with Campylobacter jejuni and Salmonella spp. from raw chicken products during food preparation. J. Food Prot. 1990, 53, 1067–1068. [Google Scholar] [CrossRef] [PubMed]
- Santos-Ferreira, N.; Alves, A.; Cardoso, M.J.; Langsrud, S.; Malheiro, A.R.; Fernandes, R.; Maia, R.; Truninger, M.; Junqueira, L.; Nicolau, A.I.; et al. Cross-contamination of lettuce with Campylobacter spp. via cooking salt during handling raw poultry. PLoS ONE 2021, 16, e0250980. [Google Scholar] [CrossRef] [PubMed]
- Didier, P.; Nguyen-The, C.; Martens, L.; Foden, M.; Dumitrascu, L.; Mihalache, A.O.; Nicolau, A.I.; Skuland, S.E.; Truninger, M.; Junqueira, L.; et al. Washing hands and risk of cross-contamination during chicken preparation among domestic practitioners in five European countries. Food Control 2021, 127, 108062. [Google Scholar] [CrossRef]
- Hedeen, N.; Smith, K. Restaurant practices for cooling food in Minessota: An interventional study. Foodborne Pathog. Dis. 2020, 17, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Kalinowski, R.M.; Tompkin, R.B.; Bodnaruk, P.W.; Payton Pruett, W., Jr. Impact of cooking, cooling, and subsequent refrigeration on the growth and survival of Clostridium perfringens in cooked meat and poultry products. J. Food Prot. 2003, 66, 1227–1232. [Google Scholar] [CrossRef]
- Li, J.; Sayeed, S.; McClane, B.A. Prevalence of enterotoxigenic Clostridium perfringens isolates in Pittsburgh (Pennsylvania) area soils and home kitchens. Appl. Environ. Microbiol. 2007, 73, 7218–7224. [Google Scholar] [CrossRef]
- Castro-Rosas, J.; Cerna-Cortés, J.F.; Méndez-Reyes, E.; Lopez-Hernandez, D.; Gómez-Aldapa, C.; Estrada-Garcia, T. Presence of faecal coliforms, Escherichia coli and diarrheagenic E. coli pathotypes in ready-to-eat salads, from an area where crops are irrigated with untreated sewage water. Int. J. Food Microbiol. 2012, 156, 176–180. [Google Scholar] [CrossRef]
- Azevedo, I.; Regalo, M.; Mena, C.; Almeida, G.; Carneiro, L.; Teixeira, P.; Hogg, T.; Gibbs, P.A. Incidence of Listeria spp. in domestic refrigerators in Portugal. Food Control 2005, 16, 121–124. [Google Scholar] [CrossRef]
- Jackson, T.C.; Acuff, G.R.; Lucia, L.M.; Prasai, R.K.; Benner, R.A.; Terry, C.T. Survey of residential refrigerators for the presence of Listeria monocytogenes. J. Food Prot. 1993, 56, 874–875. [Google Scholar] [CrossRef]
- Dumitraşcu, L.; Nicolau, A.I.; Neagu, C.; Didier, P.; Maître, I.; Nguyen-The, C.; Skuland, S.E.; Møretrø, T.; Langsrud, S.; Truninger, M.; et al. Time-temperature profiles and Listeria monocytogenes presence in refrigerators from households with vulnerable consumers. Food Control 2020, 111, 107078. [Google Scholar] [CrossRef]
- Sergelidis, D.; Abrahim, A.; Sarimvei, A.; Panoulis, C.; Karaioannoglou, P.; Genigeorgis, C. Temperature distribution and prevalence of Listeria spp. in domestic, retail and industrial refrigerators in Greece. Int. J. Food Microbiol. 1997, 34, 171–177. [Google Scholar] [CrossRef]
- Castillo, A.; Villarruel-López, A.; Navarro-Hidalgo, V.; Martínez-González, N.E.; Torres-Vitela, M.R. Salmonella and Shigella in freshly squeezed orang juice, fresh oranges, and wiping cloths collected from public markets and street booths in Guadalajara, Mexico: Incidence and comparison of analytical routes. J. Food Prot. 2006, 69, 2595–2599. [Google Scholar] [CrossRef] [PubMed]
- Janjic, J.; Ivanovic, J.; Glamoclija, N.; Boskovic, M.; Baltic, T.; Glisic, M.; Baltic, M.Z. The presence of Salmonella spp. in Belgrade domestic refrigerators. Procedia Food Sci. 2015, 5, 125–128. [Google Scholar] [CrossRef]
- Atanassova, V.; Meindl, A.; Ring, C. Prevalence of Staphylococcus aureus and staphylococcal enterotoxins in raw pork and uncooked smoked ham–a comparison of classical culturing detection and RFLP-PCR. Int. J. Food Microbiol. 2001, 68, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Smyth, D.S.; Kennedy, J.; Twohig, J.; Miajlović, H.; Bolton, D.; Smyth, C.J. Staphylococcus aureus isolates from Irish domestic refrigerators possess novel enterotoxin end enterotoxin-like genes and are clonal in nature. J. Food Prot. 2006, 69, 508–515. [Google Scholar] [CrossRef]
- Chaves, R.D.; Pradella, F.; Turatti, M.A.; Amaro, E.C.; da Silva, A.R.; Farias, A.S.; Pereira, J.L.; Khaneghah, A.M. Evaluation of Staphylococcus spp. in food and kitchen premises of Campinas, Brazil. Food Control 2018, 84, 463–470. [Google Scholar] [CrossRef]
- Millman, C.; Rigby, D.; Edward-Jones, G.; Lighton, L.; Jones, D. Perceptions, behaviours and kitchen hygiene of people who have and have not suffered campylobacteriosis: A case control study. Food Control 2014, 41, 82–90. [Google Scholar] [CrossRef]
- Dufrenne, J.; Ritmeester, W.; Delfgou-van Asch, E.; van Leusden, F.; de Jonge, R. Quantification of the contamination of chicken and chicken products in the Netherlands with Salmonella and Campylobacter. J. Food Prot. 2001, 64, 538–541. [Google Scholar] [CrossRef]
- Kennedy, J.; Gibney, S.; Nolan, A.; O’Brien, S.; McMahon, M.A.S.; McDowell, D.; Fanning, S.; Wall, P.G. Identification of critical points during domestic food preparation: An observational study. Br. Food J. 2011, 113, 766–783. [Google Scholar] [CrossRef]
- Wittry, B.C.; Holst, M.M.; Anderberg, J.; Hedeen, N. Operational antecedents associated with Clostridium perfringens outbreaks in retail food establishments, United States, 2015–2018. Foodborne Pathog. Dis. 2022, 19, 209–216. [Google Scholar] [CrossRef]
- Taormina, P.J.; Dorsa, W.J. Growth potential of Clostridium perfringens during cooling of cooked meats. J. Food Prot. 2004, 67, 1537–1547. [Google Scholar] [CrossRef]
- Cornillot, E.; Saint-Joanis, B.; Daube, G.; Katayama, S.-I.; Granum, P.E.; Canard, B.; Cole, S.T. The enterotoxin gene (cpe) of Clostridium perfringens can be chromosomal or plasmid-borne. Mol. Microbiol. 1995, 15, 639–647. [Google Scholar] [CrossRef]
- Miyamoto, K.; Wen, Q.; McClane, B.A. Multiplex PCR genotyping assay that distinguishes between isolates of Clostridium perfringens Type A carrying a chromosomal enterotoxin gene (cpe) locus, a plasmid cpe locus with an IS1470-like sequence, or a plasmid cpe locus with an IS1151 sequence. Appl. Environ. Microbiol. 2004, 42, 1552–1558. [Google Scholar] [CrossRef]
- E. coli Infection. Available online: https://my.clevelandclinic.org/health/diseases/16638-e-coli-infection (accessed on 23 August 2025).
- Bolívar, A.; Saiz-Abajo, M.J.; García-Gimeno, R.M.; Petri-Ortega, E.; Díez-Leturia, M.; González, D.; Vitas, A.I.; Pérez-Rodríguez, F. Cross-contamination of Escherichia coli O157:H7 in fresh-cut leafy vegetables: Derivation of a food safety objective and other risk management metrics. Food Control 2023, 147, 109599. [Google Scholar] [CrossRef]
- Kuan, C.H.; Lim, L.W.K.; Ting, T.W.; Rukayadi, Y.; Ahmad, S.H.; Radzi, C.W.J.W.M.; Thung, T.Y.; Ramzi, O.B.; Chang, W.S.; Loo, Y.Y.; et al. Simulation of decontamination and transmission of Escherichia coli O157:H7, Salmonella Enteritidis, and Listeria monocytogenes during handling of raw vegetables in domestic kitchens. Food Control 2017, 80, 395–400. [Google Scholar] [CrossRef]
- Chavatte, N.; Baré, J.; Lambrecht, E.; Van Damme, I.; Vaerewijck, M.; Sabbe, K.; Houf, K. Co-occurrence of free-living protozoa and foodborne pathogens on dishcloths: Implications for food safety. Int. J. Food Microbiol. 2014, 191, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A. Isolation of Salmonella from liquid whole eggs sold in retail outlets in Egypt, Bangladesh and not in Japan. Adv. Anim. Vet. Sci. 2014, 2, 390–392. [Google Scholar] [CrossRef]
- Márquez, M.L.F.; Burgos, M.J.G.; Pulido, R.P.; Gálvez, A.; López, R.L. Correlations among resistances to different antimicrobial compounds in Salmonella strains from hen eggshells. J. Food Prot. 2018, 81, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Bremer, V.; Bocter, N.; Rehmet, S.; Klein, G.; Breuer, T.; Ammon, A. Consumption, knowledge, and handling of raw meat: A representative cross-sectional survey in Germany, March 2001. J. Food Prot. 2005, 68, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Luber, P. Cross-contamination versus undercooking of poultry meat or eggs—Which risks need to be managed first? Int. J. Food Microbiol. 2009, 134, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.; Sakagami, Y.; Tanaka, M.; Inoue, R.; Jojima, T. Analysis of the relationship of microbial contamination with temperature and cleaning frequency and method of domestic refrigerators in Japan. J. Food Prot. 2020, 83, 1234–1240. [Google Scholar] [CrossRef] [PubMed]
- Lamas, A.; Regal, P.; Vázquez, B.; Miranda, J.M.; Cepeda, A.; Franco, C.M. Salmonella and Campylobacter biofilm formation: A comparative assessment from farm to fork. J. Sci. Food Agric. 2018, 98, 4014–4032. [Google Scholar] [CrossRef]
- Macías-Rodríguez, M.E.; Navarro-Hidalgo, V.; Linares-Morales, J.R.; Olea-Rodríguez, M.A.; Villaruel-López, A.; Castro-Rosas, J.; Gómez-Aldapa, C.A.; Torres-Vitela, M.R. Microbiological safety of domestic refrigerators and the dishcloths used to clean them in Guadalajara, Jalisco, Mexico. J. Food Prot. 2013, 76, 984–990. [Google Scholar] [CrossRef] [PubMed]
- Scott, E.; Duty, S.; Callahan, M. A pilot study to isolate Staphylococcus aureus and methicillin-resistant S. aureus from environmental surfaces in the home. Am. J. Infect. Control 2008, 36, 458–460. [Google Scholar] [CrossRef] [PubMed]
- Todd, E.C.D.; Greig, J.D.; Bartleson, C.A.; Michaels, B.S. Outbreaks where food workers have been implicated in the spread of foodborne disease. Part 3. Factors contributing to outbreaks and description of outbreak categories. J. Food Prot. 2007, 70, 2199–2217. [Google Scholar] [CrossRef] [PubMed]
- Czarnecka, C.; Jensen, M.R.; Astorga, A.; Zaród, M.; Stępień, K.; Gewartowska, M.; Møretrø, T.; Sabała, I.; Heir, E.; Jagielska, E. Staphylococcus spp. eradication from surfaces by the engineered bacteriolytic enzymes. Food Control 2025, 167, 110795. [Google Scholar] [CrossRef]
- Miao, J.; Liang, Y.; Chen, L.; Wang, W.; Wang, J.; Li, B.; Li, L.; Chen, D.; Xu, Z. Formation and development of Staphylococcus biofilm: With focus on food safety. J. Food Saf. 2017, 37, e12358. [Google Scholar] [CrossRef]
- Cox, L.J.; Kleiss, T.; Cordier, J.L.; Cordellana, C.; Konkel, P.; Pedrazzini, C.; Beumer, R.; Siebenga, A. Listeria spp. in food processing, non-food and domestic environments. Food Microbiol. 1989, 6, 49–61. [Google Scholar] [CrossRef]
- Ferreira, V.; Wiedmann, M.; Teixeira, P.; Stasiewicz, M.J. Listeria monocytogenes persistence in food-associated environments: Epidemiology, strain characteristics, and implications for public health. J. Food Prot. 2014, 77, 150–170. [Google Scholar] [CrossRef]
- Lianou, A.; Sofos, J.N. A review of the incidence and transmission of Listeria monocytogenes in ready-to-eat products in retail and food service environments. J. Food Prot. 2007, 70, 2172–2198. [Google Scholar] [CrossRef] [PubMed]
- Ricci, A.; Allende, A.; Bolton, D.; Chemaly, M.; Davies, R.; Escámez, P.S.F.; Girones, R.; Herman, L.; Koutsoumanis, K.; Nørrung, B.; et al. Listeria monocytogenes contamination of ready-to-eat foods and the risk for human health in the EU. EFSA J. 2018, 16, e05134. [Google Scholar] [CrossRef]
- Evans, E.W.; Redmond, E.C. Analysis of older adults’ domestic kitchen storage practices in the United Kingdom: Identification of risk factors associated with listeriosis. J. Food Prot. 2015, 78, 738–745. [Google Scholar] [CrossRef] [PubMed]
- Thaivalappil, A.; Young, I.; Paco, C.; Jeyapalan, A.; Papadopoulos, A. Food safety and the older consumer: A systematic review and meta-regression of their knowledge and practices at home. Food Control 2020, 107, 106782. [Google Scholar] [CrossRef]
- Jessim, A.I.; Fakhry, S.S.; Alwash, S.J. Detection and determination of Bacillus cereus in cooked rice and some types of spices with ribosomal 16SrRNA gene selected from Iraqi public restaurants. Int. J. Bio-Resour. Stress Manag. 2017, 8, 382–387. Available online: https://ojs.pphouse.org/index.php/IJBSM/article/view/1122 (accessed on 23 August 2025). [CrossRef]
- Navaneethan, Y.; Effarizah, M.E. Prevalence, toxigenic profiles, multidrug resistance, and biofilm formation of Bacillus cereus isolated from ready-to-eat cooked rice in Penang, Malaysia. Food Control 2021, 121, 107553. [Google Scholar] [CrossRef]
- Chiu, T.-H.; Duan, J.; Liu, C.; Su, Y.-C. Efficacy of electrolysed oxidizing water in inactivating Vibrio parahaemolyticus on kitchen cutting boards and food contact surfaces. Lett. Appl. Microbiol. 2006, 43, 666–672. [Google Scholar] [CrossRef]
- Álvarez-Contreras, A.K.; Quiñones-Ramírez, E.I.; Vázquez-Salinas, C. Prevalence, detection of virulence genes and antimicrobial susceptibility of pathogen Vibrio species isolated from different types of seafood samples at “La Nueva Viga” market in Mexico City. Antonie Van Leeuwenhoek 2021, 114, 1417–1429. [Google Scholar] [CrossRef]
- Roy, P.K.; Park, S.-H.; Song, M.G.; Park, S.Y. Antimicrobial efficacy of quercetin against Vibrio parahaemolyticus biofilm on food surfaces and downregulation of virulence genes. Polymers 2022, 14, 3847. [Google Scholar] [CrossRef] [PubMed]
- Arslan, S.; Ertürk, H.G. Occurrence, virulence and antimicrobial susceptibility profiles of Cronobacter spp. from ready-to-eat foods. Curr. Microbiol. 2021, 78, 3403–3416. [Google Scholar] [CrossRef]
- Houf, K.; Devriese, L.A.; De Zutter, L.; Van Hoof, J.; Vandamme, P. Development of a new protocol for the isolation and quantification of Arcobacter species from poultry products. Int. J. Food Microbiol. 2001, 71, 189–196. [Google Scholar] [CrossRef]
- Ramees, T.P.; Dhama, K.; Karthik, K.; Rathore, R.S.; Kumar, A.; Saminathan, M.; Tiwari, R.; Malik, Y.S.; Singh, R.K. Arcobacter: An emerging food-borne zoonotic pathogen, its public health concerns and advances in diagnosis and control—A comprehensive review. Vet. Q. 2017, 37, 136–161. [Google Scholar] [CrossRef]
- Carvalheira, A.; Silva, J.; Teixeira, P. Acinetobacter spp. in food and drinking water—A review. Food Microbiol. 2021, 95, 103675. [Google Scholar] [CrossRef]
- Atapoor, S.; Dehkordi, F.S.; Rahimi, E. Detection of Helicobacter pylori in various types of vegetables and salads. Jundishapur J. Microbiol. 2014, 7, e10013. [Google Scholar] [CrossRef]
- Azevedo, N.F.; Almeida, C.; Fernandes, I.; Cerquiera, L.; Dias, S.; Keevil, C.W.; Vieira, M.J. Survival of gastric and enterohepatic Helicobacter spp. in water: Implications for transmission. Appl. Environ. Microbiol. 2008, 74, 1805–1811. [Google Scholar] [CrossRef]
- García-Ferrús, M.; González, A.; Pina-Pérez, M.C.; Ferrús, M.A. Helicobacter pylori is present at quantifiable levels in raw vegetables in the Mediterranean area of Spain. Agriculture 2022, 12, 339. [Google Scholar] [CrossRef]
- Aviat, F.; Gerhards, C.; Rodriguez-Jerez, J.-J.; Michel, V.; Le Bayon, I.; Ismail, R.; Federighi, M. Microbial safety of wood in contact with food: A review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 491–505. [Google Scholar] [CrossRef] [PubMed]
- Biranjia-Hurdoyal, S.; Latouche, M.C. Factors affecting microbial load and profile of potential pathogens and food spoilage bacteria from household kitchen tables. Can. J. Infect. Dis. Med. Microbiol. 2016, 2016, 3574149. [Google Scholar] [CrossRef] [PubMed]
- Gerba, C.P.; Tamimi, A.H.; Maxwell, S.; Sifuentes, L.Y.; Hoffman, D.R.; Koenig, D.W. Bacterial occurrence in kitchen hand towels. Food Prot. Trends 2014, 34, 312–0317. [Google Scholar]
- Møretrø, T.; Almli, V.L.; Åsli, A.W.; Kummen, C.; Galler, M.; Langsrud, S. Kitchen cloths: Consumer practices, drying properties and bacterial growth and survival. Food Control 2022, 143, 109195. [Google Scholar] [CrossRef]
- Sabillón, L.; Stratton, J.; Rose, D.; Bianchini, A. Microbiological survey of equipment and wheat-milled fractions of a milling operation. Cereal Chem. 2021, 98, 44–51. [Google Scholar] [CrossRef]
- Cardinale, M.; Kaiser, D.; Lueders, T.; Schnell, S.; Egert, M. Microbiome analysis and confocal microscopy of used kitchen sponges reveal massive colonization by Acinetobacter, Moraxella and Chryseobacterium species. Sci. Rep. 2017, 7, 5791. [Google Scholar] [CrossRef] [PubMed]
- Møretrø, T.; Ferreira, V.B.; Moen, B.; Almli, V.L.; Teixeira, P.; Kasbo, I.M.; Langsrud, S. Bacterial levels and diversity in kitchen sponges and dishwashing brushes used by consumers. J. Appl. Microbiol. 2022, 133, 1378–1391. [Google Scholar] [CrossRef] [PubMed]
- Ak, N.O.; Cliver, D.O.; Kaspar, C.W. Cutting boards of plastic and wood contaminated experimentally with bacteria. J. Food Prot. 1994, 57, 16–22. [Google Scholar] [CrossRef]
- Ak, N.O.; Cliver, D.O.; Kaspar, C.W. Decontamination of plastic and wooden cutting boards for kitchen use. J. Food Prot. 1994, 57, 23–30. [Google Scholar] [CrossRef]
- De Cesare, A.; Sheldon, B.W.; Smith, K.S.; Jaykus, L.-A. Survival and persistence of Campylobacter and Salmonella species under various organic loads on food contact surfaces. J. Food Prot. 2003, 66, 1587–1594. [Google Scholar] [CrossRef] [PubMed]
- Barmpaki, A.A.; Zavvou, E.E.; Drivas, C.; Papapetros, K.; Sygellou, L.; Andrikopoulos, K.S.; Kennou, S.; Andritsos, N.D.; Giannakas, A.; Salmas, C.E.; et al. Atomic layer deposition of ZnO on PLA/TiO2 bionanocomposites: Evaluation of surface chemistry and physical properties toward food packaging applications. J. Appl. Polym. Sci. 2023, 140, e54465. [Google Scholar] [CrossRef]
- De Carvalho, T.B.; Barbosa, J.B.; Teixeira, P. Effectiveness and durability of a quaternary ammonium compounds-based surface coating to reduce surface contamination. Biology 2023, 12, 669. [Google Scholar] [CrossRef]
- Ilg, Y.; Bruckner, S.; Kreyenschmidt, J. Applicability of surfaces containing silver in domestic refrigerators. Int. J. Consum. Stud. 2011, 35, 221–227. [Google Scholar] [CrossRef]
- Mendes, A.R.; Granadeiro, C.M.; Leite, A.; Geiss, O.; Bianchi, I.; Ponti, J.; Mehn, D.; Pereira, E.; Teixeira, P.; Poças, F. Functional properties and safety considerations of zinc oxide nanoparticles under varying conditions. Nanomaterials 2025, 15, 892. [Google Scholar] [CrossRef]
- Querido, M.M.; Aguiar, L.; Neves, P.; Pereira, C.C.; Teixeira, J.P. Self-disinfecting surfaces and infection control. Colloids Surf. B Biointerfaces 2019, 178, 8–21. [Google Scholar] [CrossRef]
- Eriksson, D.; Råhlén, E.; Bergenkvist, E.; Skarin, M.; Fernström, L.-L.; Rydén, J.; Hansson, I. Survival of Campylobacter jejuni in frozen chicken meat and risks associated with handling contaminated chicken in the kitchen. Food Control 2023, 145, 109471. [Google Scholar] [CrossRef]
- Jensen, D.A.; Friedrich, L.M.; Harris, L.J.; Danyluk, M.D.; Schaffner, D.W. Quantifying transfer rated of Salmonella and Escherichia coli O157:H7 between fresh-cut produce and common kitchen surfaces. J. Food Prot. 2013, 76, 1530–1538. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.; Tang, Y.; Ren, F.; Li, Z.; Li, F.; Cui, C.; Jiao, X.; Huang, J. An investigation into the critical factors influencing the spread of Campylobacter during chicken handling in commercial kitchens in China. Microorganisms 2021, 9, 1164. [Google Scholar] [CrossRef] [PubMed]
- Møretrø, T.; Nguyen-The, C.; Didier, P.; Maître, I.; Izsó, T.; Kasza, G.; Skuland, S.E.; Cardoso, M.J.; Ferreira, V.B.; Teixeira, P.; et al. Consumer practices and prevalence of Campylobacter, Salmonella and norovirus in kitchens from six European countries. Food Control 2021, 347, 109172. [Google Scholar] [CrossRef] [PubMed]
- Garrett, T.R.; Bhakoo, M.; Zhang, Z. Bacterial adhesion and biofilms on surfaces. Prog. Natur. Sci. 2008, 18, 1049–1056. [Google Scholar] [CrossRef]
- Bischoff, A.; Alter, T.; Schoenknecht, A. Hygienic evaluation of wooden cutting boards: Microbiological parameters. J. Food Prot. 2025, 88, 100576. [Google Scholar] [CrossRef]
- Miller, A.J.; Brown, T.; Call, J.E. Comparison of wooden and polyethylene cutting boards: Potential for the attachment and removal of bacteria from ground beef. J. Food Prot. 1996, 59, 854–858. [Google Scholar] [CrossRef]
- Catellani, P.; Scapin, R.M.; Alberghini, L.; Radu, I.L.; Giaccone, V. Levels of microbial contamination of domestic refrigerators in Italy. Food Control 2014, 42, 257–262. [Google Scholar] [CrossRef]
- Kennedy, J.; Jackson, V.; Blair, I.S.; McDowell, D.A.; Cowan, C.; Bolton, D.J. Food safety knowledge of consumers and the microbiological and temperature status of their refrigerators. J. Food Prot. 2005, 68, 1421–1430. [Google Scholar] [CrossRef] [PubMed]
- Giske, L.A.L.; Lindstad, L.H.; Løvdal, T.; Mork, O.J. Design of fish processing equipment: Exploring cleaning brush performance and material properties to minimize biofilm deposits. Procedia CIRP 2020, 91, 140–145. [Google Scholar] [CrossRef]
- Gerba, C.P.; Sifuentes, L.Y.; Tamimi, A.H. A comparison of urethane and cellulose sponges as cleaning tools in household kitchens. Food Prot. Trends 2017, 37, 170–175. [Google Scholar]
- Jacksch, S.; Thota, J.; Shetty, S.; Smidt, H.; Schnell, S.; Egert, M. Metagenomic analysis of regularly microwave-treated and untreated domestic kitchen sponges. Microorganisms 2020, 8, 736. [Google Scholar] [CrossRef]
- Sharma, M.; Eastridge, J.; Mudd, C. Effective household disinfection method of kitchen sponges. Food Control 2009, 20, 310–313. [Google Scholar] [CrossRef]
- Her, E.S.; Seo, S.; Choi, J.; Pool, V.; Ilic, S. Assessment of food safety at university food courts using surveys, observations, and microbial testing. Food Control 2019, 103, 167–174. [Google Scholar] [CrossRef]
- Sharp, K.; Walker, H. A microbiological survey of communal kitchens used by undergraduate students. Int. J. Consum. Stud. 2003, 1, 11–16. [Google Scholar] [CrossRef]
- Kasza, G.; Csenki, E.Z.; Izsó, T.; Scholderer, J. Paradoxical risk mitigation behavior in private households. Food Control 2022, 138, 109032. [Google Scholar] [CrossRef]
- Medeiros, L.; Hillers, V.; Kendall, P.; Mason, A. Evaluation of food safety education for consumers. J. Nutr. Educ. 2001, 33, S27–S34. [Google Scholar] [CrossRef] [PubMed]
- Mullan, B.; Allom, V.; Fayn, K.; Johnston, I. Building habit strength; A pilot intervention designed to improve food-safety behavior. Food Res. Int. 2014, 66, 274–278. [Google Scholar] [CrossRef]
- Bonanno, L.; Bergis, H.; Gnanou-Besse, N.; Asséré, A.; Danan, C. Which domestic refrigerator temperatures in Europe?—Focus on shelf-life studies regarding Listeria monocytogenes (Lm) in ready-to-eat (RTE) foods. Food Microbiol. 2024, 123, 104595. [Google Scholar] [CrossRef] [PubMed]
- Evans, E.W.; Redmond, E.C. Time-temperature profiling of United Kingdom consumers’ domestic refrigerators. J. Food Prot. 2016, 79, 2119–2127. [Google Scholar] [CrossRef] [PubMed]
- James, C.; Onarinde, B.A.; James, S.J. The use and performance of household refrigerators: A review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 160–179. [Google Scholar] [CrossRef] [PubMed]
- Van der Vossen-Wijmenga, W.P.; den Beste, H.M.W.; Zwietering, M.H. Temperature status of domestic refrigerators and its effect on the risk of listeriosis from ready-to-eat (RTE) cooked meat products. Int. J. Food Microvbiol. 2024, 413, 110516. [Google Scholar] [CrossRef] [PubMed]
- Norwood, D.E.; Gilmour, A. Adherence of Listeria monocytogenes strains to stainless steel coupons. J. Appl. Microbiol. 1999, 86, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Breidt, F., Jr.; Kathariou, S. Resistance of Listeria monocytogenes biofilms to sanitizing agents in a simulated food processing environment. Appl. Environ. Microbiol. 2006, 72, 7711–7717. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.; Autio, W.R.; McLandsborough, L.A. Effect of surface roughness and stainless steel finish on Listeria monocytogenes attachment and biofilm formation. J. Food Prot. 2008, 71, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Borucki, M.K.; Peppin, J.D.; White, D.; Loge, F.; Call, D.R. Variation in biofilm formation among strains of Listeria monocytogenes. Appl. Environ. Microbiol. 2003, 69, 7336–7342. [Google Scholar] [CrossRef]
- Lianou, A.; Koutsoumanis, K.P. Strain variability of the biofilm-forming ability of Salmonella enterica under various environmental conditions. Int. J. Food Microbiol. 2012, 160, 171–178. [Google Scholar] [CrossRef]
- Shi, X.; Zhu, X. Biofilm formation and food safety in food industries. Trends Food Sci. Technol. 2009, 20, 407–413. [Google Scholar] [CrossRef]
- Dahlsten, E.; Lindström, M.; Korkeala, H. Mechanisms of food processing and storage-related stress tolerance in Clostridium botulinum. Res. Microbiol. 2015, 166, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; McClane, B. Further comparison of temperature effects on growth and survival of Clostridium perfringens Type A isolates carrying a chromosomal or plasmid-borne enterotoxin gene. Appl. Environ. Microbiol. 2006, 72, 4561–4568. [Google Scholar] [CrossRef]
- Li, J.; McClane, B. Comparative effects of osmotic, sodium nitrite-induced, and pH-induced stress on growth and survival of Clostridium perfringens Type A isolates carrying chromosomal or plasmid-borne enterotoxin genes. Appl. Environ. Microbiol. 2006, 72, 7620–7625. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Hung, Y.-C.; Qi, H. Efficacy of peracetic acid in inactivating foodborne pathogens on fresh produce surface. J. Food Sci. 2018, 83, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Cuggino, S.G.; Posada-Izquierdo, G.; Villegas, I.B.; Theumer, M.G.; Pérez-Rodríguez, F.P. Effects of chlorine and peroxyacetic acid wash treatments on growth kinetics of Salmonella in fresh-cut lettuce. Food Res. Int. 2023, 167, 112451. [Google Scholar] [CrossRef]
- Soltanzadeh, E.; Hosseini, H.; Boroumand, M.; Motallebi, A. Analysis of the frequency, antibiotic susceptibility, and related genes among foodborne pathogenic bacteria isolated from hospital refrigerators in Tehran, Iran. Iran J. Public Health 2024, 53, 680–690. [Google Scholar] [CrossRef]
- Duc, H.M.; Hoa, T.T.K.; Ha, C.T.T.; Hung, L.V.; Thang, N.V.; Son, H.M.; Flory, G.A. Prevalence and antibiotic resistance profile of Clostridium perfringens isolated from pork and chicken meat in Vietnam. Pathogens 2024, 13, 400. [Google Scholar] [CrossRef]
- Bergšpica, I.; Ozola, A.; Miltiņa, E.; Alksne, L.; Meistere, I.; Cibrovska, A.; Grantiņa-Ieviņa, L. Occurrence of pathogenic and potentially pathogenic bacteria in microgreens, sprouts, and sprouted seeds on retail market in Riga, Latvia. Foodborne Pathog. Dis. 2020, 17, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Vojkovská, H.; Myšková, P.; Gelbíčová, T.; Skočková, A.; Koláčková, I.; Karpíšková, R. Occurrence and characterization of food-borne pathogens isolated from fruit, vegetables and sprouts retailed in the Czech Republic. Food Microbiol. 2017, 63, 147–152. [Google Scholar] [CrossRef]
- De Jong, A.E.I.; Verhoeff-Bakkenes, L.; Nauta, M.J.; de Jonge, R. Cross-contamination in the kitchen: Effect of hygiene measures. J. Appl. Microbiol. 2008, 105, 615–624. [Google Scholar] [CrossRef]
- Menini, A.; Mascarello, G.; Giaretta, M.; Brombin, A.; Marcolin, S.; Personeni, F.; Pinto, A.; Crovato, S. The critical role of consumers in the prevention of foodborne diseases: An ethnographic study of Italian families. Foods 2022, 11, 1006. [Google Scholar] [CrossRef]
- Mihalache, O.A.; Møretrø, T.; Borda, D.; Dumitraşcu, L.; Neagu, C.; Nguyen-The, C.; Maître, I.; Didier, P.; Teixeira, P.; Junqueira, L.O.L.; et al. Kitchen layouts and consumers’ food hygiene practices: Ergonomics vesrus safety. Food Control 2022, 131, 108433. [Google Scholar] [CrossRef]
- Duff, S.B.; Scott, E.A.; Mafilios, M.S.; Todd, E.C.; Krilov, L.R.; Geddes, A.M.; Ackerman, S.J. Cost-effectiveness of a targeted disinfection program in household kitchens to prevent foodborne illnesses in the United States, Canada, and the United Kingdom. J. Food Prot. 2003, 66, 2013–2015. [Google Scholar] [CrossRef] [PubMed]
- Mihalache, O.A.; Møretrø, T.; Borda, D.; Dumitraşcu, L.; Neagu, C.; Nguyen-The, C.; Maître, I.; Didier, P.; Teixeira, P.; Junqueira, L.O.L.; et al. Data on European kitchen layouts belonging to vulnerable consumers (elderly people and young families with children or pregnant women) and risk-takers (young single men). Data Brief 2021, 38, 107362. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, J.; Djekic, I.; Smigic, N.; Tomic, N.; Rajkovic, A. Temperature profile and hygiene in household refrigerators in Belgrade, Serbia and their relation to consumer food safety knowledge and characteristics of the refrigerators. Food Control 2022, 136, 108813. [Google Scholar] [CrossRef]
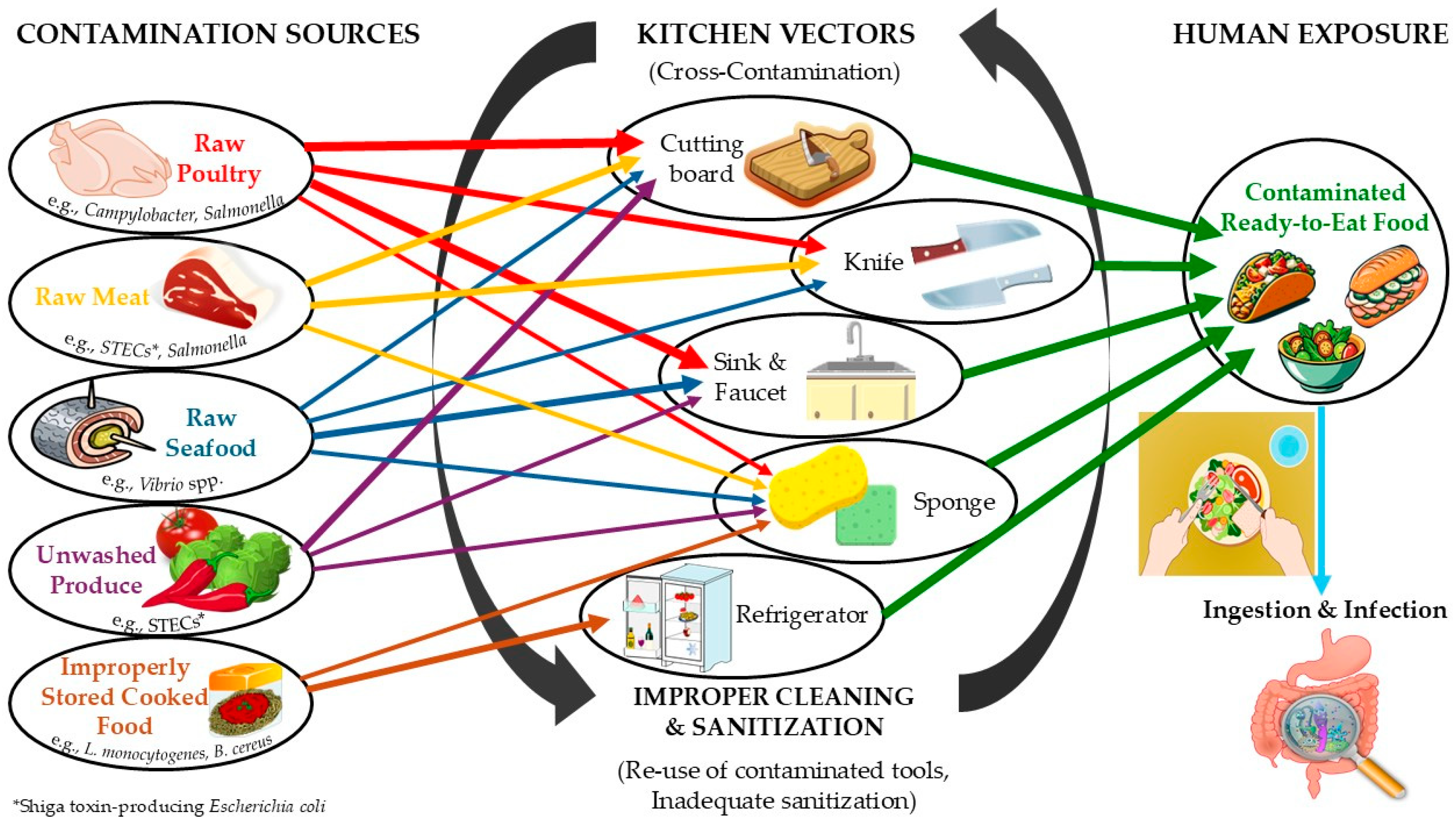
| Pathogen | Domestic Reservoirs | Transmission Routes | Main Consumer Behaviors | References |
|---|---|---|---|---|
| Campylobacter spp. | Raw poultry and meat products | Cutting boards, countertops, sinks, knives, cooking salt | Washing poultry in sinks, cross-use of utensils, poor hand hygiene | [17,18,57,58,59] |
| C. perfringens | Cooked meat, poultry, gravy | Kitchen surfaces, pots, storage containers, utensils | Improper cooling of cooked foods, inadequate refrigeration temperatures | [5,60,61,62] |
| E. coli pathotypes | Raw meat, fresh produce, salads irrigated with sewage water | Sponges, dishcloths, cutting boards, countertops | Inadequate washing of vegetables, reuse of sponges/dishcloths, poor surface sanitation | [16,34,40,63] |
| L. monocytogenes | RTE 1 foods, dairy, raw vegetables | Refrigerators, cutting boards, countertops, sinks | Improper refrigeration temperatures, prolonged food storage, poor refrigerator cleaning | [42,64,65,66,67] |
| Salmonella spp. | Poultry, eggs, raw meat | Cutting boards, dishcloths, sponges, refrigerators | Insufficient cooking, poor disinfection of utensils, inadequate refrigerator cleaning, cross-contamination between raw and RTE foods | [16,26,43,68,69] |
| S. aureus | Human carriers, contaminated foods | Sponges, dishcloths, refrigerators, countertops | Direct contamination from handlers, reuse of cloths, poor refrigerator hygiene | [39,53,70,71,72] |
| Kitchen Equipment | Associated Pathogens | Reservoir Characteristics | Main Consumer Behaviors | References |
|---|---|---|---|---|
| Countertops & kitchen surfaces | Salmonella spp., L. monocytogenes, S. aureus | Support biofilm formation, resistant to desiccation | Wiped with contaminated cloths/sponges, reliance on visual cleanliness instead of microbial hygiene | [18,31,57,59] |
| Cutting boards | Campylobacter spp., Salmonella spp., E. coli pathotypes, L. monocytogenes | Cracks and knife scars, porous materials allow bacterial attachment and persistence | Inadequate cleaning between raw and RTE 1 foods, cross-use of the same board for meat and vegetables | [18,26,40,113] |
| Dishcloths & hand towels | S. aureus, E. coli pathotypes, Salmonella spp. | High bacterial load from repeated use, slow drying promotes survival | Used for drying hands, wiping surfaces, and cleaning utensils without adequate laundering | [39,41,114,115,116] |
| Knives & small utensils | Campylobacter spp., Salmonella spp., E. coli pathotypes | Direct contact with raw meat and produce | Inadequate cleaning between uses, sharing knives for raw and RTE foods | [75,116] |
| Refrigerators | L. monocytogenes, Salmonella spp., S. aureus, C. perfringens | Poor temperature control, long-term storage, biofilm formation on shelves | Operating above recommended temperatures, infrequent cleaning, raw–RTE food contact, stored cooked foods that are improperly cooled | [42,44,53,61,64,65] |
| Sponges | E. coli pathotypes, Salmonella spp., S. aureus, diverse microbiota | Moist, porous structure supports microbial growth and biofilm formation | Frequent reuse, lack of disinfection, multiple-task usage (dishes, counters, hands) | [33,34,117,118] |
| Sinks & faucets | Campylobacter spp., Salmonella spp., L. monocytogenes | Drain areas and faucets harbor biofilms; splash dispersal spreads contamination | Washing raw poultry in sink, poor faucet hygiene, infrequent disinfection | [42,44,53,64,65] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mataragka, A.; Anthi, R.; Christodouli, Z.-E.; Malisova, O.; Andritsos, N.D. Presence of Major Bacterial Foodborne Pathogens in the Domestic Environment and Hygienic Status of Food Cleaning Utensils: A Narrative Review. Hygiene 2025, 5, 60. https://doi.org/10.3390/hygiene5040060
Mataragka A, Anthi R, Christodouli Z-E, Malisova O, Andritsos ND. Presence of Major Bacterial Foodborne Pathogens in the Domestic Environment and Hygienic Status of Food Cleaning Utensils: A Narrative Review. Hygiene. 2025; 5(4):60. https://doi.org/10.3390/hygiene5040060
Chicago/Turabian StyleMataragka, Antonia, Rafaila Anthi, Zoi-Eleni Christodouli, Olga Malisova, and Nikolaos D. Andritsos. 2025. "Presence of Major Bacterial Foodborne Pathogens in the Domestic Environment and Hygienic Status of Food Cleaning Utensils: A Narrative Review" Hygiene 5, no. 4: 60. https://doi.org/10.3390/hygiene5040060
APA StyleMataragka, A., Anthi, R., Christodouli, Z.-E., Malisova, O., & Andritsos, N. D. (2025). Presence of Major Bacterial Foodborne Pathogens in the Domestic Environment and Hygienic Status of Food Cleaning Utensils: A Narrative Review. Hygiene, 5(4), 60. https://doi.org/10.3390/hygiene5040060








