The Effectiveness of Phosphate-Based Bioactive Glass on Candida albicans Adherence in Dental Soft Lining Material (In Vitro Study)
Abstract
1. Introduction
2. Materials and Methods
2.1. Bioactive Glass Preparation
2.2. Fabrication of Soft Liner Specimens
2.3. Adherence Test
2.4. Characterization Procedures
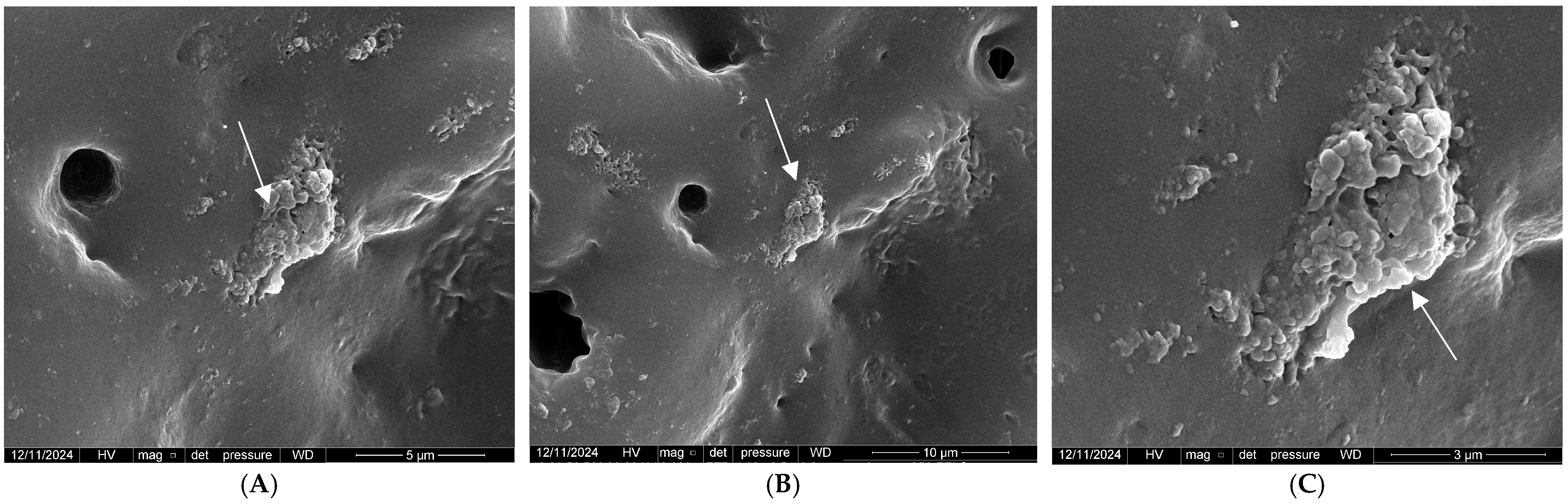
2.4.1. Field Emission Scanning Electron Microscope (FE-SEM)
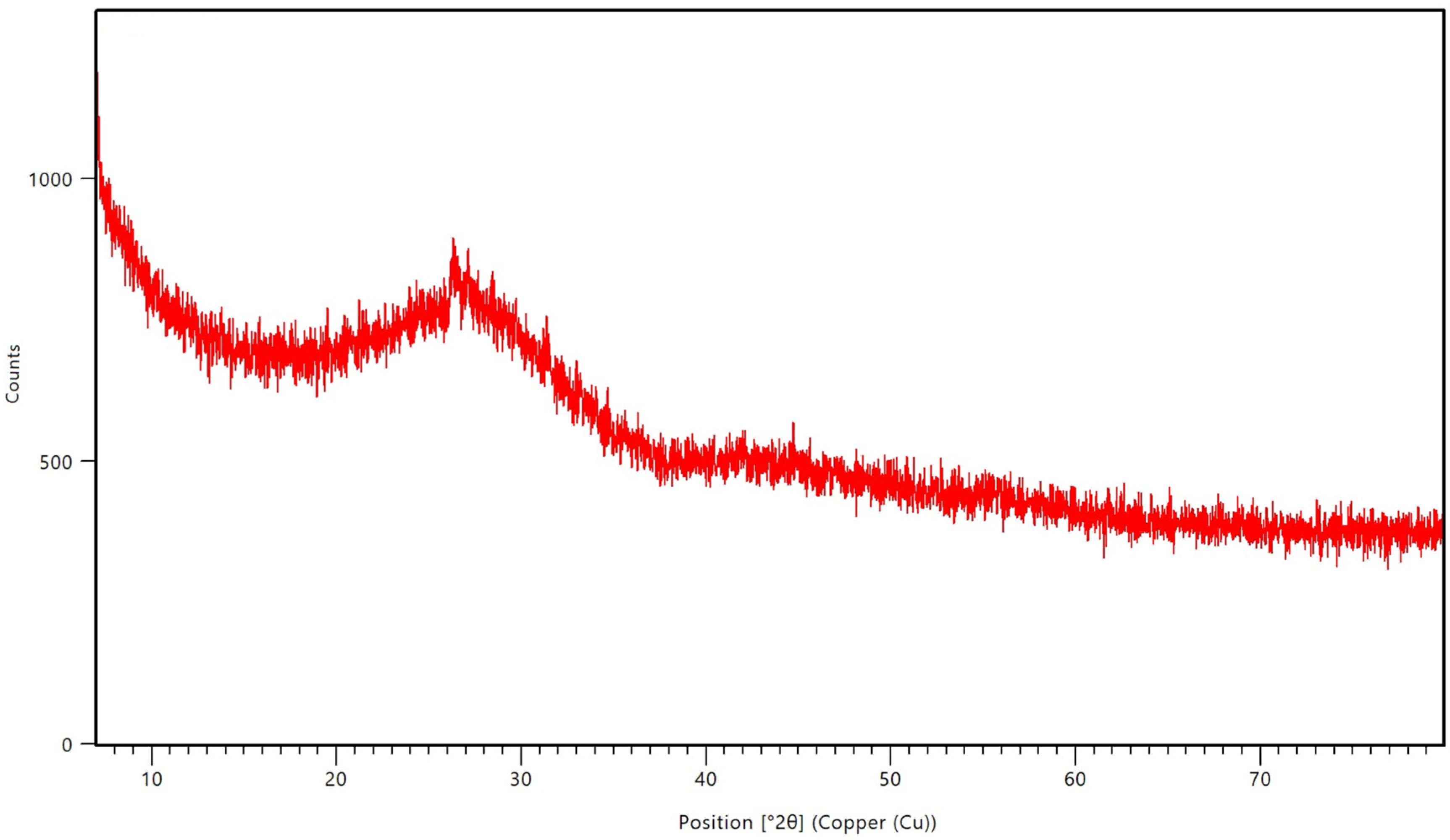
2.4.2. X-Ray Diffractometer (XRD)
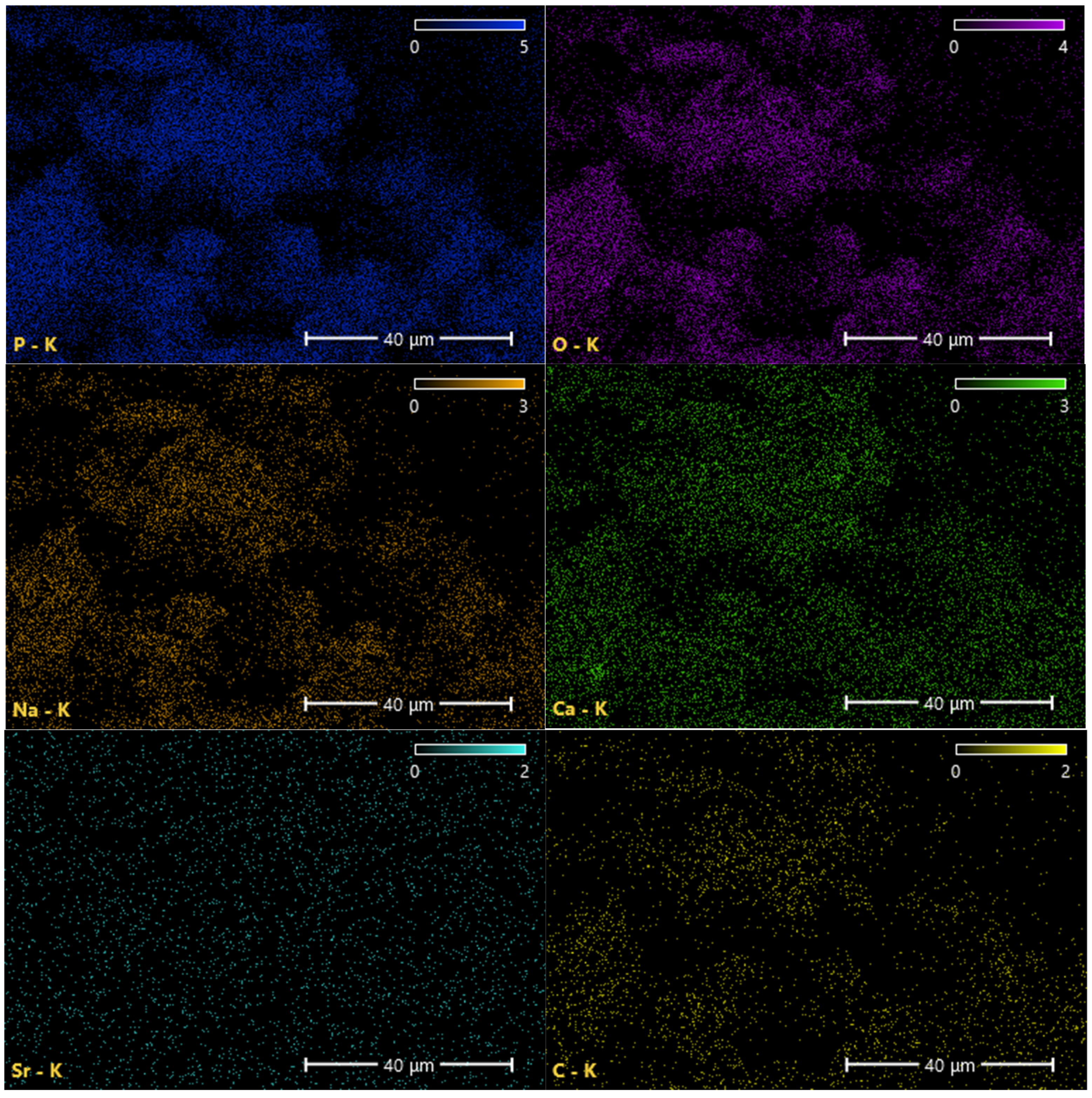
2.4.3. Energy Dispersive X-Ray Analyser (EDX)
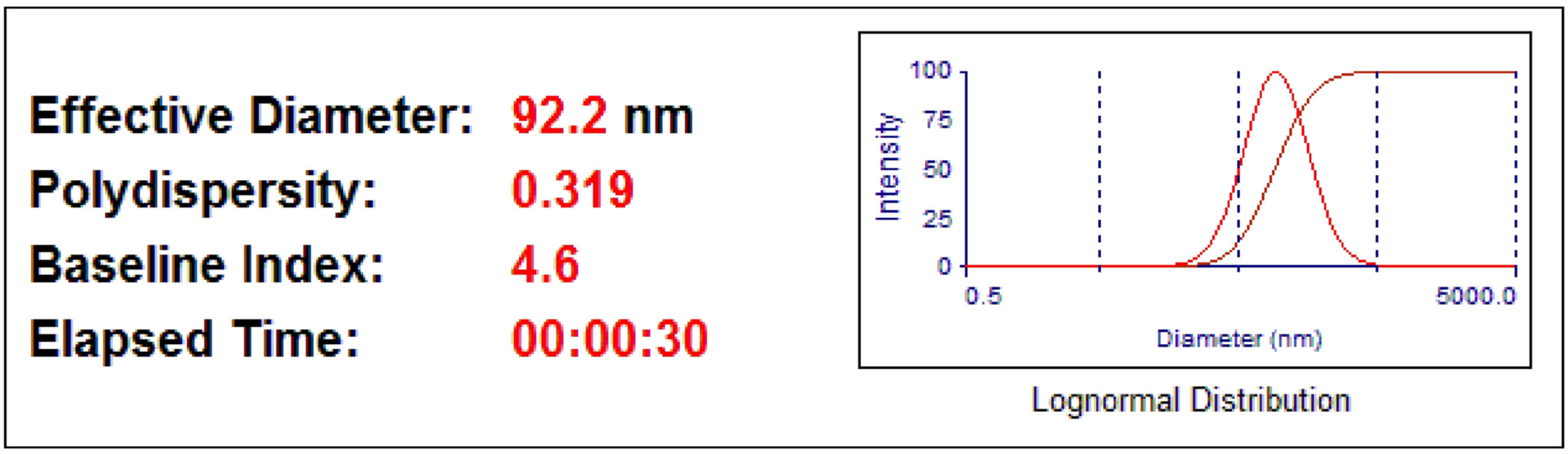
2.4.4. Particle Size Analyser (PSA)
3. Results
3.1. Characterizations of the PBG-Sr
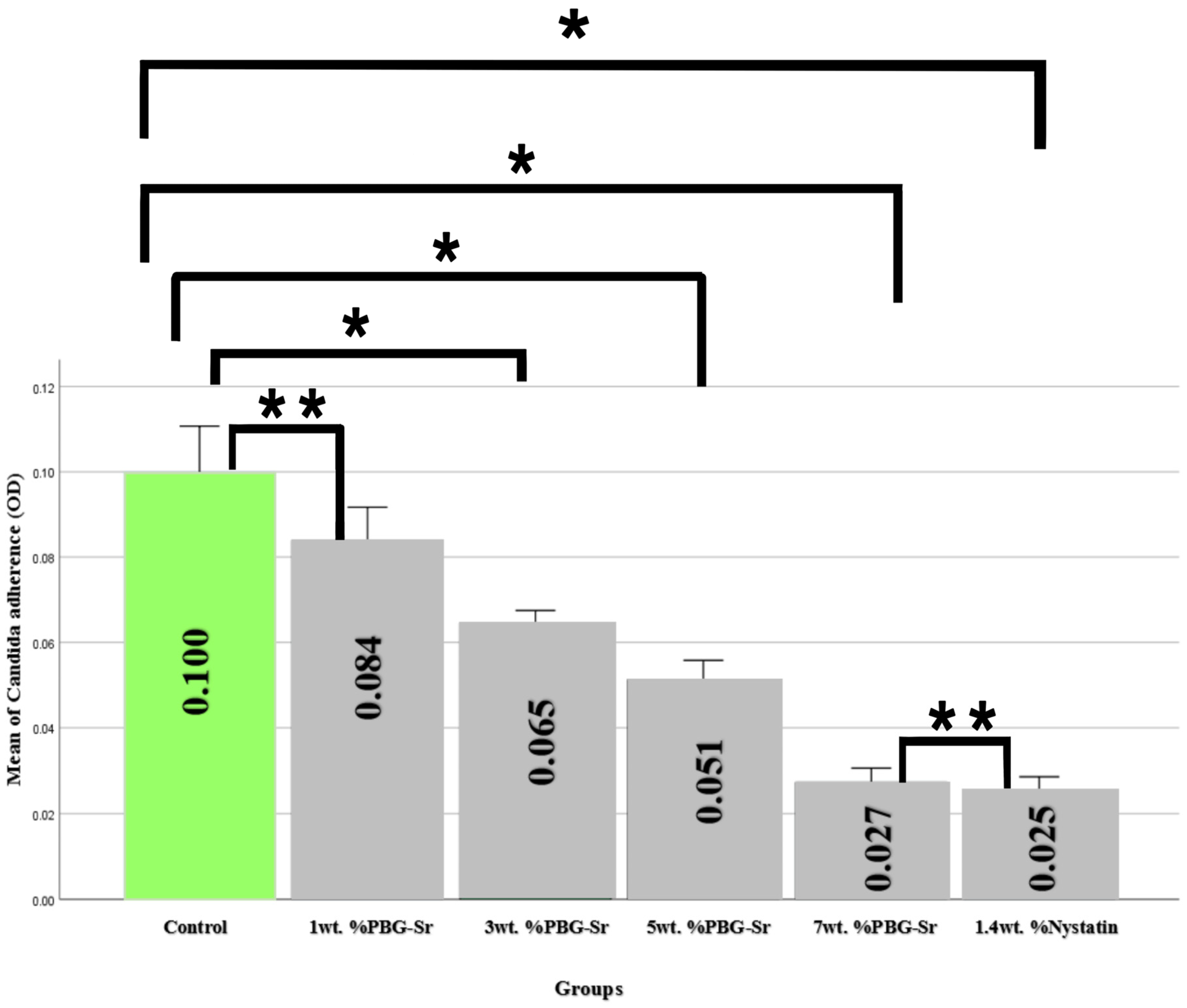
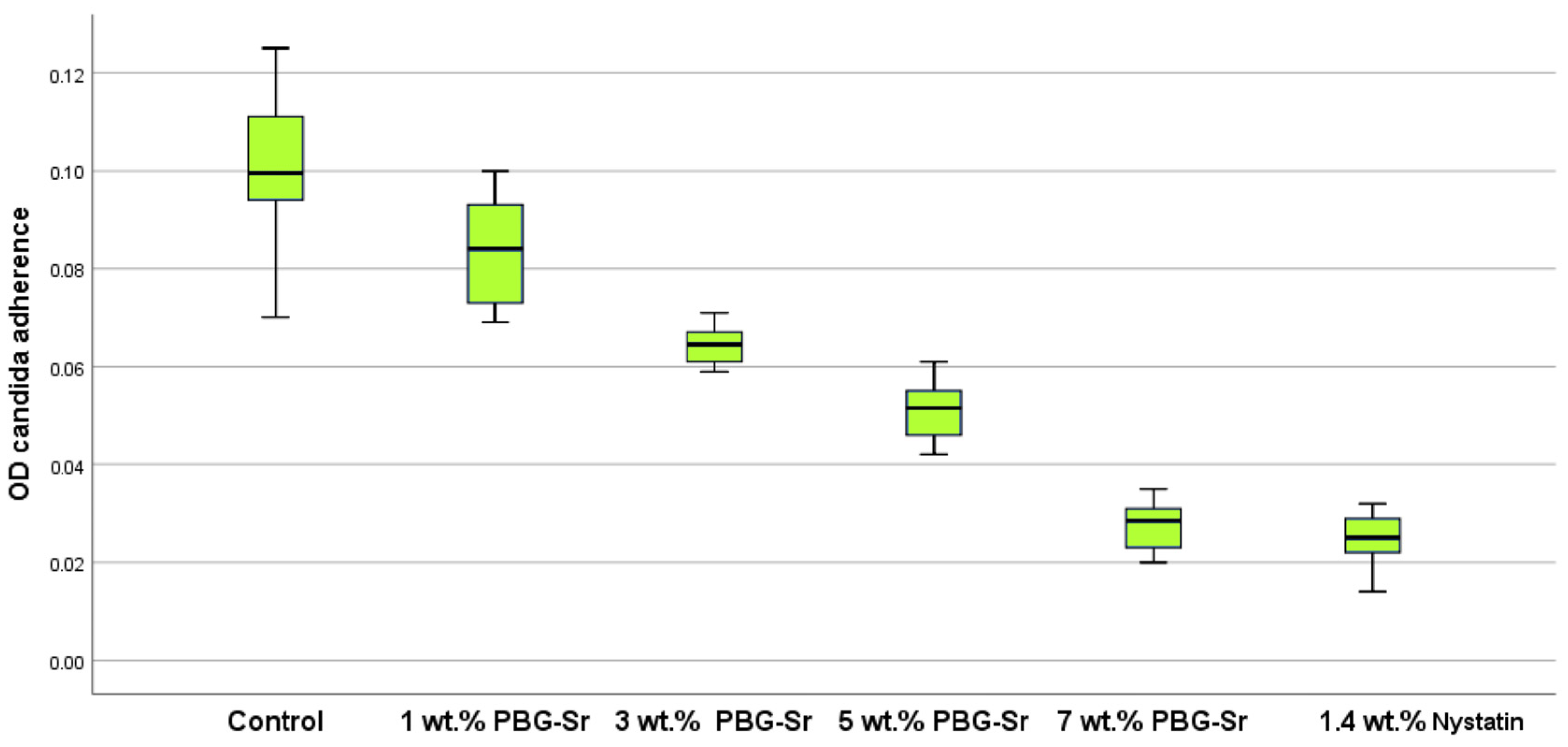
3.2. Candidal Adherence Results and Statistics
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Waltenberger, L.; Reissmann, B.S.; Fritzer, E.; Foerster, Y.; Schmitter, M.; Heydecke, G. Impact of loading protocol of a mandibular single implant-supported complete denture on oral health-related quality of life over a period of 5 years: A randomized controlled trial. J. Dent. 2024, 142, 104769. [Google Scholar]
- Chu, M.; Ibrahim, A.M.B.R.; Moores, C.J.; Moynihan, P. The impact of wearing complete denture in one or both arches, on eating-related quality of life and patients’ perceived need for advice to support eating well: Results from a qualitative study. J. Oral Rehabil. 2024, 51, 1956–1965. [Google Scholar] [PubMed]
- AlHamdan, E.M. Soft denture liner and microbial disinfection with contemporary and conventional agents. Photodiagnosis Photodyn. Ther. 2022, 38, 102768. [Google Scholar] [CrossRef] [PubMed]
- Naser, H.J.; Abdul-Ameer, F.M. Evaluating the effect of lemongrass essential oil addition on some properties of heat cure acrylic soft-lining material. Med. J. Babylon 2022, 19, 646–652. [Google Scholar]
- Mohad, A.; Fatalla, A.A. The Effectiveness of Aluminum Potassium Sulfate Micro-Particles Addition into Soft Denture Lining Material on Tensile strength and Peel bond Strength of Soft Denture Lining Material. J. Baghdad Coll. Dent. 2019, 31, 29–35. [Google Scholar] [CrossRef]
- Gibson, B.J.; Baker, S.R.; Broomhead, T.; El-Dhuwaib, B.; Martin, N.; McKenna, G.; Alavi, A. ‘It’s like being in a tunnel’: Understanding the patient journey from tooth loss to life with removable dentures. J. Dent. 2024, 145, 104964. [Google Scholar] [CrossRef]
- Rakasevic, D.; Marinkovic, J.; Rakonjac, B.; Arce, M.; Joksimovic, E.; Markovic, J.; Kulic, M.; Hadzi-Mihailovic, M.; Markovic, A. Evaluation of photodynamic therapy efficacy vs. conventional antifungal therapy in patients with poor-fitting dentures suffering from denture stomatitis. a prospective clinical study. Photodiagnosis Photodyn. Ther. 2024, 45, 103913. [Google Scholar]
- Ribeiro, A.B.; Pizziolo, P.G.; Clemente, L.M.; Aguiar, H.C.; Poker, B.d.C.; Silva, A.A.M.e.; Makrakis, L.R.; Fifolato, M.A.; Souza, G.C.; Oliveira, V.d.C.; et al. Strategies for preventing and treating oral mucosal infections associated with removable dentures: A scoping review. Antibiotics 2024, 13, 273. [Google Scholar] [CrossRef]
- Mostafa, B.; Elboraey, A.N.; El-Masry, H.M.; Dehis, W.M. Evaluation of the effect of ozonated water in treatment of denture stomatitis associated with removable prosthetics. Bull. Natl. Res. Cent. 2024, 48, 1. [Google Scholar] [CrossRef]
- Procópio, A.L.F.; Lara, V.S.; Porto, V.C.; Soares, S.; Fernandes, M.H.; Urban, V.M.; Neppelenbroek, K.H. Resilient liner modified by antimicrobials for denture stomatitis treatment: A randomized controlled trial. J. Dent. 2022, 126, 104297. [Google Scholar] [CrossRef]
- Damian-Buda, A.I.; Alipanah, N.; Bider, F.; Sisman, O.; Neščáková, Z.; Boccaccini, A.R. Metal–organic-framework (MOF)-bioactive glass (BG) systems for biomedical applications—A review. Mater. Today Bio 2024, 30, 101413. [Google Scholar] [CrossRef] [PubMed]
- Bromet, B.A.; Blackwell, N.P.; Abokefa, N.; Freudenberger, P.; Blatt, R.L.; Brow, R.K.; Semon, J.A. The angiogenic potential of pH-neutral borophosphate bioactive glasses. J. Biomed. Mater. Res. A 2023, 111, 1554–1564. [Google Scholar] [CrossRef] [PubMed]
- Intawin, P.; Kraipok, A.; Barnthip, N.; Kantha, P.; Potong, R.; Panyata, S.; Eitssayeam, S.; Pengpat, K. Optimization of Mn-Zn ferrite doping in phosphate-based glass ceramics for enhanced hyperthermia efficiency and bioactivity. Ceram. Int. 2024, 50, 52957–52966. [Google Scholar] [CrossRef]
- Gobbo, V.A.; Parihar, V.S.; Prato, M.; Kellomäki, M.; Verne, E.; Spriano, S.; Massera, J. Surface modification of silicate, borosilicate and phosphate bioactive glasses to improve/control protein adsorption: Part I. Ceram. Int. 2023, 49, 1261–1275. [Google Scholar] [CrossRef]
- Mukherjee, S.; Mishra, M. Application of strontium-based nanoparticles in medicine and environmental sciences. Nanotechnol. Environ. Eng. 2021, 24, 6. [Google Scholar] [CrossRef]
- Liu, J.; Rawlinson, S.C.F.; Hill, R.G.; Fortune, F. Strontium-substituted bioactive glasses in vitro osteogenic and antibacterial effects. Dent. Mater. 2016, 32, 412–422. [Google Scholar] [CrossRef]
- Elangovan, U.; Anbarasu, K.; Shanmugam, R.; Veeramuthu, D.; John, J.G. Biological Properties of Mycosynthesized Novel Strontium Oxide Nanoparticles from Biomass Free Filtrate of Endophytic Fungus Aspergillus sp. LCJ315. BioNanoScience 2024, 14, 2145–2158. [Google Scholar] [CrossRef]
- Deng, J.; Ren, L.; Pan, Y.; Gao, H.; Meng, X. Antifungal property of acrylic denture soft liner containing silver nanoparticles synthesized in situ. J. Dent. 2021, 106, 103589. [Google Scholar] [CrossRef]
- Go, H.B.; Lee, M.J.; Seo, J.Y.; Byun, S.Y.; Kwon, J.S. Mechanical properties and sustainable bacterial resistance effect of strontium-modified phosphate-based glass microfiller in dental composite resins. Sci. Rep. 2023, 13, 17763. [Google Scholar] [CrossRef]
- Gobbo, V.A.; Parihar, V.S.; Prato, M.; Kellomäki, M.; Verne, E.; Spriano, S.; Massera, J. Surface modification of silicate, borosilicate, and phosphate bioactive glasses to improve/control protein adsorption: Part II. Ceram. Int. 2023, 49, 12856–12865. [Google Scholar] [CrossRef]
- Ryu, J.H.; Mangal, U.; Lee, M.J.; Seo, J.Y.; Jeong, I.J.; Park, J.Y.; Na, J.Y.; Lee, K.J.; Yu, H.S.; Cha, J.K.; et al. Effect of strontium substitution on functional activity of phosphate-based glass. Biomater. Sci. 2023, 11, 6299–6310. [Google Scholar] [CrossRef] [PubMed]
- Al-Shammari, S.S.; Abdul-Ameer, F.M.; Bairam, L.R.; Al-Salihi, Z. The influence of lemongrass essential oil addition into heat cured acrylic resin against Candida albicans adhesion. J. Baghdad Coll. Dent. 2023, 35, 67–75. [Google Scholar] [CrossRef]
- Isam, M.A.; Mahmood, W.S. Impact of cerium oxide nanoparticles incorporation on shear bond strength and surface hardness of acrylic-based soft lining material. J. Emerg. Med. Trauma Acute Care 2024, 2024, 4. [Google Scholar] [CrossRef]
- Memon, M.R.; Memon, H.; Shoro, M.; Bhurgri, H.; Issrani, R.; Iqbal, A.; Khattak, O.; Altassan, M.; Almabadi, A.A.; Sultan, S.; et al. Effectiveness of chitosan versus natural aloe vera on candida adherence in denture soft lining material. Scientifica 2024, 2024, 9918914. [Google Scholar] [CrossRef]
- Fleischmann, J.; Sripuntanagoon, E.M. Pellicle associated adherence film above incubation broth surface-an inexpensive adjunct to recognizing Candida krusei in the laboratory. BMC Res. Notes 2011, 4, 74. [Google Scholar] [CrossRef]
- Bakhurji, E.A.; AlEraky, D.M.; Alshammary, H.; Alamoudi, M.; Alrayes, N.; Hassan, M. of Candida albicans on preformed crowns used to restore primary molars: An in vitro study. Saudi Dent. J. 2024, 36, 1539–1543. [Google Scholar] [CrossRef]
- Naser, H.J.; Abdul-Ameer, F.M. Evaluation of the effect of lemongrass essential oil on candida albicans adhesion on heat cured acrylic based soft lining material. J. Med. Chem. Sci. 2022, 6, 1685–1695. [Google Scholar] [CrossRef]
- Avukat, E.N.; Akay, C.; Ersöz, M.B.T.; Mumcu, E.; Pat, S.; Erdönmez, D. Could Helium Plasma Treatment be a Novel Approach to Prevent the Biofilm Formation of Candida albicans? Mycopathologia 2023, 188, 361–369. [Google Scholar] [CrossRef]
- Lazarin, A.A.; Zamperini, C.A.; Vergani, C.E.; Wady, A.F.; Giampaolo, E.T.; Machado, A.L. Candida albicans adherence to an acrylic resin modified by experimental photopolymerised coatings: An in vitro study. Gerodontology 2012, 31, 25–33. [Google Scholar] [CrossRef]
- Gulati, M.; Lohse, M.B.; Ennis, C.L.; Gonzalez, R.E.; Perry, A.M.; Bapat, P.; Arevalo, A.V.; Rodriguez, D.L.; Nobile, C.J. In Vitro Culturing and Screening of Candida albicans Biofilms. Curr. Protoc. Microbiol. 2018, 50, e60. [Google Scholar] [CrossRef]
- Noori, Z.S.; Al-Khafaji, A.M.; Dabaghi, F. Effect of tea tree oil on candida adherence and surface roughness of heat cure acrylic resin. J. Baghdad Coll. Dent. 2023, 35, 46–54. [Google Scholar] [CrossRef]
- Al-Azzawi, M.F.J. The effect of five disinfection methods on candida albicans-Infected Chair-Side soft liners: A comparative in vitro study. Osol J. Med. Sci. 2024, 2, 8–18. [Google Scholar] [CrossRef]
- Hammami, I.; Gavinho, S.R.; Pádua, A.S.; Lança, M.d.C.; Borges, J.P.; Silva, J.C.; Sá-Nogueira, I.; Jakka, S.K.; Graça, M.P.F. Extensive investigation on the effect of niobium insertion on the physical and biological properties of 45S5 bioactive glass for dental implant. Int. J. Mol. Sci. 2023, 24, 5244. [Google Scholar] [CrossRef] [PubMed]
- Krupińska, A.M.; Bogucki, Z. Lactoferrin as a potential therapeutic for the treatment of Candida-associated denture stomatitis. J. Oral Biosci. 2024, 66, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Al-Qaysi, S.A.S.; Al-Rubaie, S.R.M.A.R. Detection of some virulence factors among Candida albicans isolated from patients and prevalence of candidalysin gene ECE1. Baghdad Sci. J. 2024, 21, 1173. [Google Scholar] [CrossRef]
- Chladek, G.; Nowak, M.; Pakieła, W.; Barszczewska-Rybarek, I.; Żmudzki, J.; Mertas, A. The effect of exposure to Candida albicans suspension on the properties of silicone dental soft lining material. Materials 2024, 17, 723. [Google Scholar] [CrossRef]
- Bertolini, M.M.; Portela, M.B.; Curvelo, J.A.R.; Soares, R.M.; Lourenço, E.J.; Telles, D.M. Resins-based denture soft lining materials modified by chlorhexidine salt incorporation: An in vitro analysis of antifungal activity, drug release and hardness. Dent. Mater. 2014, 30, 793–798. [Google Scholar] [CrossRef]
- Chladek, G.; Kalamarz, I.; Pakieła, W.; Barszczewska-Rybarek, I.; Czuba, Z.; Mertas, A. A temporary acrylic soft denture lining material enriched with silver-releasing filler-cytotoxicity, mechanical and antifungal properties. Materials 2024, 17, 902. [Google Scholar] [CrossRef]
- Tite, T.; Popa, A.C.; Stuart, B.W.; Fernandes, H.R.; Chirica, I.M.; Lungu, G.A.; Macovei, D.; Bartha, C.; Albulescu, L.; Tanase, C.; et al. Independent and complementary bio-functional effects of CuO and Ga2O3 incorporated as therapeutic agents in silica- and phosphate-based bioactive glasses. J. Mater. 2022, 8, 893–905. [Google Scholar] [CrossRef]
- Patel, U.; Macri-Pellizzeri, L.; Zakir Hossain, K.M.; Scammell, B.E.; Grant, D.M.; Scotchford, C.A.; Hannon, A.C.; Kennedy, A.R.; Barney, E.R.; Ahmed, I.; et al. In vitro cellular testing of strontium/calcium substituted phosphate glass discs and microspheres shows potential for bone regeneration. J. Tissue Eng. Regen. Med. 2019, 13, 396–405. [Google Scholar] [CrossRef]
- Azizi, L.; Turkki, P.; Huynh, N.; Massera, J.M.; Hytonen, V.P. Surface modification of bioactive glass promotes cell attachment and spreading. ACS Omega 2021, 6, 22635–22642. [Google Scholar] [CrossRef] [PubMed]
- Mubina, K.; Shailajha, S.; Sankaranarayanan, R.; Saranya, L. In vitro bioactivity, mechanical behavior and antibacterial properties of mesoporous SiO2-CaO-Na2O-P2O5 nano bioactive glass ceramics. J. Mech. Behav. Biomed. Mater. 2019, 100, 103379. [Google Scholar] [CrossRef] [PubMed]
- Moreno, M.G.; Butini, M.E.; Maiolo, E.M.; Sessa, L.; Trampuz, A. Antimicrobial activity of bioactive glass S53P4 against representative microorganisms causing osteomyelitis—Real-time assessment by isothermal microcalorimetry. Colloids Surf. B Biointerfaces 2020, 189, 110853. [Google Scholar] [CrossRef]
- Gad, M.M.; Abu-Rashid, K.; Alkhaldi, A.; Alshehri, O.; Khan, S.Q. Evaluation of the effectiveness of bioactive glass fillers against Candida albicans adhesion to PMMA denture base materials: An in vitro study. Saudi Dent. J. 2022, 34, 730–737. [Google Scholar] [CrossRef]
- Alhotan, A.; Raszewski, Z.; Chojnacka, K.; Mikulewicz, M.; Kulbacka, J.; Alaqeely, R.; Mirdad, A.; Haider, J. Evaluating the translucency, surface roughness, and cytotoxicity of a PMMA acrylic denture base reinforced with bioactive glasses. J. Funct. Biomater. 2023, 15, 16. [Google Scholar] [CrossRef]
- Baheiraei, N.; Eyni, H.; Bakhshi, B.; Najafloo, R.; Rabiee, N. Effects of strontium ions with potential antibacterial activity on in vivo bone regeneration. Sci. Rep. 2021, 11, 8745. [Google Scholar] [CrossRef]
- Skallevold, H.E.; Rokaya, D.; Khurshid, Z.; Zafar, M.S. Bioactive glass applications in dentistry. Int. J. Mol. Sci. 2019, 20, 5960. [Google Scholar] [CrossRef]
- Dai, L.L.; Mei, M.L.; Chu, C.H.; Zhao, I.S.; Lo, E.C.M. Effect of Strontium-Doped bioactive glass on preventing formation of demineralized lesion. Materials 2021, 14, 4645. [Google Scholar] [CrossRef]
- Sohrabi, M.; Hesaraki, S.; Shahrezaee, M.; Shams-Khorasani, A.; Roshanfar, F.; Glasmacher, B.; Heinemann, S.; Xu, Y.; Makvandi, P. Antioxidant flavonoid-loaded nano-bioactive glass bone paste: In vitro apatite formation and flow behavior. Nanoscale Adv. 2024, 6, 1011–1022. [Google Scholar] [CrossRef]
- Khvostenko, D.; Hilton, T.J.; Ferracane, J.L.; Mitchell, J.C.; Kruzic, J.J. Bioactive glass fillers reduce bacterial penetration into marginal gaps for composite restorations. Dent. Mater. 2016, 32, 73–81. [Google Scholar] [CrossRef]
- Ozer, H.; Ozcan, M. Antibacterial effect of bioactive glass incorporated in acrylic resins against Streptococcus Mutans and Lactobacillus Acidophilus activity in biofilm. Braz. Dent. Sci. 2022, 25, e3317. [Google Scholar] [CrossRef]
- Hammami, I.; Gavinho, S.R.; Pádua, A.S.; Sá-Nogueira, I.; Silva, J.C.; Borges, J.P.; Valente, M.A.; Graça, M.P. Bioactive Glass Modified with Zirconium Incorporation for Dental Implant Applications: Fabrication, Structural, Electrical, and Biological Analysis. Int. J. Mol. Sci. 2023, 24, 10571. [Google Scholar] [CrossRef]
- Tonprasong, W.; Inokoshi, M.; Tamura, M.; Hatano, K.; Minakuchi, S. Impact of surface pre-reacted glass ionomer filler eluate on lipase gene expression in Candida albicans: An in vitro study. Dent. Mater. J. 2022, 42, 49–54. [Google Scholar] [CrossRef]
- Jang, E.J.; Hong, Y.J.; Jeong, Y.H.; Kim, K.E.; Jo, E.S.; Lee, M.J.; Yang, S.Y. In vitro antifungal and physicochemical properties of polymerized acrylic resin containing strontium-modified phosphate-based glass. BMC Oral Health 2024, 24, 775. [Google Scholar] [CrossRef] [PubMed]
- Drago, L.; Toscano, M.; Bottagisio, M. Recent evidence on bioactive glass antimicrobial and antibiofilm activity: A mini-review. Materials 2018, 11, 326. [Google Scholar] [CrossRef] [PubMed]
- Correia, B.L.; Gomes, A.T.P.C.; Noites, R.; Ferreira, J.M.F.; Duarte, A.S. New and Efficient Bioactive Glass Compositions for Controlling Endodontic Pathogens. Nanomaterials 2022, 12, 1577. [Google Scholar] [CrossRef] [PubMed]
- Anwar, A.; Kanwal, Q.; Sadiqa, A.; Razaq, T.; Khan, I.H.; Javaid, A.; Khan, S.; Tag-Eldin, E.; Ouladsmane, M. Synthesis and antimicrobial analysis of high surface area Strontium-Substituted calcium phosphate nanostructures for bone regeneration. Int. J. Mol. Sci. 2023, 24, 14527. [Google Scholar] [CrossRef]
- Lee, M.J.; Kim, M.J.; Mangal, U.; Seo, J.Y.; Kwon, J.S.; Choi, S.H. Zinc-modified phosphate-based glass micro-filler improves Candida albicans resistance of auto-polymerized acrylic resin without altering mechanical performance. Sci. Rep. 2022, 12, 19456. [Google Scholar] [CrossRef]
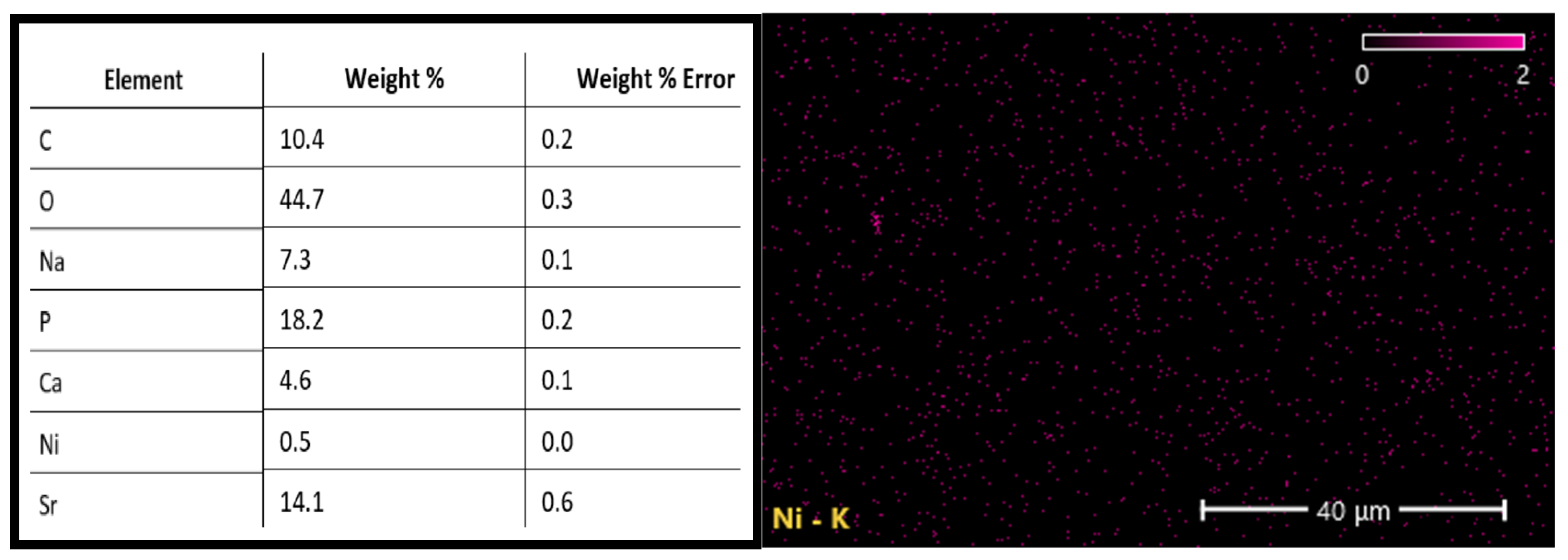
| Properties | Groups | Sum of Squares | df | Mean Square | F | p Value |
|---|---|---|---|---|---|---|
| Candida adherence | Between Groups | 18.765 | 4 | 4.691 | 189.794 | 0.000 |
| Within Groups | 1.112 | 45 | 0.025 | |||
| Total | 19.878 | 49 |
| (I) Groups | (J) Groups | Mean Difference (I-J) | p Value |
|---|---|---|---|
| 0 wt.% PBG-Sr | 1 wt.% PBG-Sr | 0.01610 | 0.163 ** |
| 3 wt.% PBG-Sr | 0.03550 | 0.000 * | |
| 5 wt.% PBG-Sr | 0.04880 | 0.000 * | |
| 7 wt.% PBG-Sr | 0.07270 | 0.000 * | |
| 1.4 wt.% Nystatin | 0.07520 | 0.000 * | |
| 1 wt.% PBG-Sr | 3 wt.% PBG-Sr | 0.01940 | 0.003 * |
| 5 wt.% PBG-Sr | 0.03270 | 0.000 * | |
| 7 wt.% PBG-Sr | 0.05660 | 0.000 * | |
| 1.4 wt.% Nystatin | 0.05910 | 0.000 * | |
| 3 wt.% PBG-Sr | 5 wt.% PBG-Sr | 0.01330 | 0.001 * |
| 7 wt.% PBG-Sr | 0.03720 | 0.000 * | |
| 1.4 wt.% Nystatin | 0.03970 | 0.000 * | |
| 5 wt.% PBG-Sr | 7 wt.% PBG-Sr | 0.02390 | 0.000 * |
| 1.4 wt.% Nystatin | 0.02640 | 0.000 * | |
| 7 wt.% PBG-Sr | 1.4 wt.% Nystatin | 0.00250 | 0.981 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ielewi, N.H.; Abdul-Ameer, F.M. The Effectiveness of Phosphate-Based Bioactive Glass on Candida albicans Adherence in Dental Soft Lining Material (In Vitro Study). Hygiene 2025, 5, 49. https://doi.org/10.3390/hygiene5040049
Ielewi NH, Abdul-Ameer FM. The Effectiveness of Phosphate-Based Bioactive Glass on Candida albicans Adherence in Dental Soft Lining Material (In Vitro Study). Hygiene. 2025; 5(4):49. https://doi.org/10.3390/hygiene5040049
Chicago/Turabian StyleIelewi, Nada Hussien, and Faiza M. Abdul-Ameer. 2025. "The Effectiveness of Phosphate-Based Bioactive Glass on Candida albicans Adherence in Dental Soft Lining Material (In Vitro Study)" Hygiene 5, no. 4: 49. https://doi.org/10.3390/hygiene5040049
APA StyleIelewi, N. H., & Abdul-Ameer, F. M. (2025). The Effectiveness of Phosphate-Based Bioactive Glass on Candida albicans Adherence in Dental Soft Lining Material (In Vitro Study). Hygiene, 5(4), 49. https://doi.org/10.3390/hygiene5040049







