Consumer Awareness of Microbial Contamination and Identification of Key Pathogenic Bacteria in Lip Cosmetic Testers
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
2.2. Survey
2.3. Collection of Lip Cosmetic Testers
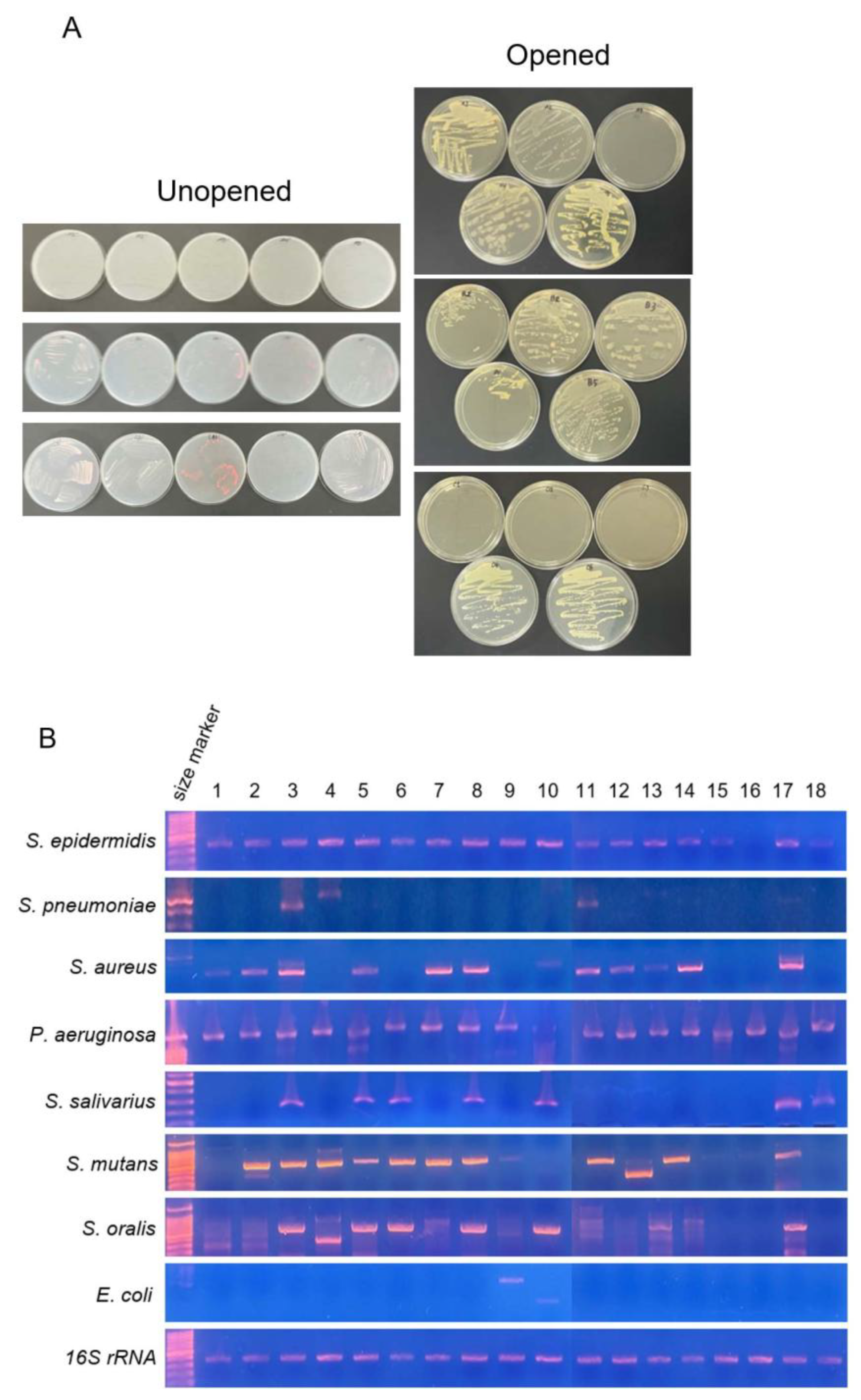
2.4. Bacterial Culture
2.5. Genomic DNA Extraction and Polymerase Chain Reaction (PCR) Analysis
2.6. Statistical Analysis
3. Results
3.1. Characteristics of the Survey Participants
3.2. Usage Patterns and Perceived Contamination of Lip Cosmetic Testers
3.3. Analysis of Microbial Infection Susceptibility and Product Safety Sensitivity Toward Lip Cosmetic Testers by Participant Characteristics
3.4. Detection of Bacterial Contamination in Lip Cosmetic Testers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, Y.; Kwon, K.H. Changes in purchasing patterns in the beauty market due to Post-COVID-19: Literature review. J. Cosmet. Dermatol. 2021, 20, 3074–3079. [Google Scholar] [CrossRef]
- K-Beauty Products Market Size, Share, Trends, and Forecast by Product Type, Distribution Channel, End User, and Region, 2025–2033. Imarc Transforming Ideas into Impact. Available online: https://www.imarcgroup.com/k-beauty-products-market?utm_source=chatgpt.com (accessed on 12 May 2025).
- Al Jbour, N.D. An Overview of New Trends in the Cosmetics Industry. Int. J. Appl. Pharm. 2025, 17, 136–147. [Google Scholar] [CrossRef]
- Korea Consumer Agency. Safety Status of Tester Cosmetics—Press Release. 9 January 2018. Available online: https://www.kca.go.kr/home/sub.do?menukey=4002&mode=view&no=1002596650 (accessed on 22 May 2025).
- Capetta, A. A Woman Claims She Got Herpes From Sephora Lipstick Samples. 3 November 2017. Available online: https://www.self.com/story/woman-claims-she-got-herpes-from-sephora-lipstick-samples?utm_source=chatgpt.com (accessed on 23 May 2025).
- Turner, R.; Shehab, Z.; Osborne, K.; Hendley, J.O. Shedding and survival of herpes simplex virus from “fever blisters”. Pediatrics 1982, 70, 547–549. [Google Scholar] [CrossRef]
- Mawazi, S.M.; Redzal, N.A.B.A.; Othman, N.; Alolayan, S.O. Lipsticks History, Formulations, and Production: A Narrative Review. Cosmetics 2022, 9, 25. [Google Scholar] [CrossRef]
- Halla, N.; Fernandes, I.P.; Heleno, S.A.; Costa, P.; Boucherit-Otmani, Z.; Boucherit, K.; Rodrigues, A.E.; Ferreira, I.C.F.R.; Barreiro, M.F. Cosmetics Preservation: A Review on Present Strategies. Molecules 2018, 23, 1571. [Google Scholar] [CrossRef]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of general 16S rRNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef]
- Bej, A.K.; McCarty, S.C.; Atlas, R.M. Detection of Escherichia coli and Shigella spp. in water by PCR amplification of the uidA gene. Appl. Environ. Microbiol. 1991, 57, 1013–1017. [Google Scholar] [CrossRef]
- De Vos, D.; Lim, A., Jr.; Pirnay, J.P.; Struelens, M.; Vandenvelde, C.; Duinslaeger, L.; Vanderkelen, A.; Cornelis, P. Direct detection and identification of Pseudomonas aeruginosa in clinical samples such as skin biopsy specimens and expectorations by multiplex PCR based on two outer membrane lipoprotein genes, oprI and oprL. J. Clin. Microbiol. 1997, 35, 1295–1299. [Google Scholar] [CrossRef]
- Banas, J.A.; Zhu, M.; Dawson, D.V.; Drake, D.R.; Progulske-Fox, A. PCR-Based Identification of Oral Streptococcal Species. Int. J. Microbiol. 2016, 2016, 3465163. [Google Scholar] [CrossRef]
- Carvalho, M.G.; Tondella, M.L.C.; McCaustland, K.; Weidlich, L.; McGee, L.; Mayer, L.W.; Steigerwalt, A.; Whaley, M.; Facklam, R.R.; Fields, B.; et al. Evaluation and improvement of real-time PCR assays targeting lytA, ply, and psaA genes for detection of pneumococcal DNA. J. Clin. Microbiol. 2007, 45, 2460–2466. [Google Scholar] [CrossRef]
- Brakstad, O.G.; Aasbakk, K.; Maeland, J.A. Detection of Staphylococcus aureus by polymerase chain reaction amplification of the nuc gene. J. Clin. Microbiol. 1992, 30, 1654–1660. [Google Scholar] [CrossRef]
- Martineau, F.; Picard, F.J.; Ke, D.; Paradis, S.; Roy, P.H.; Ouellette, M.; Bergeron, M.G. Development of a PCR assay for identification of staphylococci at genus and species levels. J. Clin. Microbiol. 2001, 39, 2541–2547. [Google Scholar] [CrossRef]
- Oho, T.; Yamashita, Y.; Shimazaki, Y.; Kushiyama, M.; Koga, T. Simple and rapid detection of Streptococcus mutans and Streptococcus sobrinus in human saliva by polymerase chain reaction. Oral Microbiol. Immunol. 2000, 15, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, H.F. The Application of Electronic Computers to Factor Analysis. Educ. Psychol. Meas. 1960, 20, 141–151. [Google Scholar] [CrossRef]
- Cattell, R.B. The screen test for the number of factors. Multivar. Behav. Res. 1966, 1, 245–276. [Google Scholar] [CrossRef] [PubMed]
- Kalender, G.I. The Symbol of Cosmetic Products as Social Distinction and the False Needs of Shopping for Cosmetics at Department Stores Aroused by Women’s Magazines. Adv. J. Commun. 2021, 9, 1–11. [Google Scholar] [CrossRef]
- Park, S.M. Hygiene Management in Cosmetic Store: A Resonsibility Shared by Both Companies and Consumers. Hyundai Economic Daily. 23 January 2019. Available online: https://www.finomy.com/news/articleView.html?idxno=62341&utm_source=chatgpt.com (accessed on 28 May 2025).
- Article 8 of the Cosmetics Safety Standards, Law No. 20901. Ministry of Food and Drug Safety (Cosmetics Policy Division). 1 April 2025. Available online: https://www.law.go.kr/LSW//lsSideInfoP.do?lsiSeq=270323&joNo=0008&joBrNo=00&docCls=jo&urlMode=lsScJoRltInfoR (accessed on 29 May 2025).
- Al-Rifaai, J.M.; Al Haddad, M.A.; Alrefaei, Y.S.N. Types of bacteria found in cosmetics used by female college students in Kuwait. Eur. J. Biol. Med. Sci. Res. 2021, 9, 20–34. [Google Scholar] [CrossRef]
- Muhammed, H.J. Bacterial and Fungal Contamination in Three Brands of Cosmetic Marketed in Iraq. Iraqi J. Pharm. Sci. 2011, 20, 38–42. [Google Scholar] [CrossRef]
- Otto, M. Staphylococcus epidermidis—The ‘accidental’ pathogen. Nat. Rev. Microbiol. 2009, 7, 555–567. [Google Scholar] [CrossRef]
- Lucas, V.S.; Beighton, D.; Roberts, G.J. Composition of the oral streptococcal flora in healthy children. J. Dent. 2000, 28, 45–50. [Google Scholar] [CrossRef]
- Zhang, Y.; Fang, J.; Yang, J.; Gao, X.; Dong, L.; Zheng, X.; Sun, L.; Xia, B.; Zhao, N.; Ma, Z.; et al. Streptococcus mutans-associated bacteria in dental plaque of severe early childhood caries. J. Oral. Microbiol. 2022, 14, 2046309. [Google Scholar] [CrossRef]
- Qin, S.; Xiao, W.; Zhou, C.; Pu, Q.; Deng, X.; Lan, L.; Liang, H.; Song, X.; Wu, M. Pseudomonas aeruginosa: Pathogenesis, virulence factors, antibiotic resistance, interaction with host, technology advances and emerging therapeutics. Signal Transduct. Target Ther. 2022, 7, 199. [Google Scholar] [CrossRef]
- Del Giudice, P. Skin Infections Caused by Staphylococcus aureus. Acta Derm. Venereol. 2020, 100, 208–215. [Google Scholar] [CrossRef]
- Morimura, A.; Hamaguchi, S.; Akeda, Y.; Tomono, K. Mechanisms Underlying Pneumococcal Transmission and Factors Influencing Host-Pneumococcus Interaction: A Review. Front. Cell Infect. Microbiol. 2021, 11, 639450. [Google Scholar] [CrossRef]
- Ministry of Food and Drug Safety (Cosmetics Policy Division). Regulations on Cosmetics Safety Standards. [Enforced on 7 February 2024] [Ministry of Food and Drug Safety Notification No. 2024-9, Partial Amendment, 7 February 2024]. Available online: https://law.go.kr/행정규칙/화장품%20안전기준%20등에%20관한%20규정 (accessed on 6 June 2025).
- 17516:2014; Cosmetics—Microbiology—Microbiological Limits. ISO: Geneva, Switzerland, 2014. Available online: https://www.iso.org/obp/ui/ (accessed on 20 September 2025).
- U.S. Food and Drug Administration (FDA). Microbiological Quality Considerations in Non-Sterile Drug Products. Guidance for Industry. 2021. Available online: https://www.fda.gov/media/152527/download (accessed on 20 September 2025).
| Question | N | % |
|---|---|---|
| Age group (years) | ||
| 20–29 | 78 | 58.21 |
| 30–49 | 21 | 15.67 |
| >50 | 35 | 26.12 |
| Marital status | ||
| Single | 86 | 64.18 |
| Married | 47 | 35.07 |
| Other | 1 | 0.75 |
| Education level | ||
| High school graduate or less | 26 | 19.4 |
| Currently attending college | 56 | 41.79 |
| Bachelor’s degree or higher | 52 | 38.81 |
| Employment status | ||
| Yes (employed or on temporary leave) | 71 | 52.99 |
| No (not economically active or seeking employment) | 63 | 47.01 |
| Question | N | % |
|---|---|---|
| I have used lip cosmetic testers (n = 134) | ||
| Yes | 127 | 94.78 |
| No | 7 | 5.22 |
| What is your usual method of using lip cosmetic testers? (n = 127) | ||
| Direct application to lips | 19 | 14.96 |
| Application to lips using a disposable tool | 9 | 7.09 |
| Application to lips using fingers | 16 | 12.6 |
| Swatching on the back of the hand | 81 | 63.78 |
| Other | 2 | 1.57 |
| What is your purpose for using lip cosmetic testers? (Multiple responses allowed, n = 127) | ||
| To check the color and pigmentation | 120 | 94.49 |
| To assess texture and usability | 69 | 54.33 |
| To check for allergic reactions | 2 | 1.57 |
| To evaluate the taste and scent | 6 | 4.72 |
| To assess overall quality | 4 | 3.15 |
| Other | - | - |
| Have you ever experienced adverse effects after using a lip cosmetic tester? (n = 127) | ||
| Yes | 5 | 3.94 |
| No | 122 | 96.06 |
| Do you think lip cosmetic testers can be contaminated with microorganisms? (n = 134) | ||
| Yes | 120 | 89.55 |
| No | 3 | 2.24 |
| I don’t know | 11 | 8.21 |
| Specify the reason for your perception of microbial contamination (Multiple responses allowed, n = 120) | ||
| Experienced an expired or contaminated product | 15 | 12.5 |
| Because many people use it | 106 | 88.33 |
| Contamination due to saliva | 69 | 57.5 |
| Contamination from contact with hands or skin | 47 | 39.17 |
| Contamination due to exposure to dust or other particles | 37 | 30.83 |
| Question | Factor 1 | Factor 2 | Cronbach’s α | |
|---|---|---|---|---|
| Susceptibility to microorganism | ||||
| I am aware of the effects of microorganisms on oral health. | 0.853 | −0.038 | 0.751 | |
| I generally have an interest in microorganisms. | 0.765 | −0.058 | ||
| I tend to be concerned about microbial infection when using lip cosmetic testers | 0.713 | −0.204 | ||
| I tend to be concerned about side effects when using lip cosmetic testers | 0.649 | −0.199 | ||
| Consumer sensitivity regarding product safety | ||||
| I usually pay attention to the ingredients and contents listed on lip cosmetics. | 0.181 | 0.929 | 0.729 | |
| I usually pay attention to the preservatives and allergy-related information indicated on lip cosmetics. | 0.007 | 0.918 | ||
| I usually pay attention to the expiration date on lip cosmetics. | 0.309 | 0.569 | ||
| Eigen value | 2.115 | 2.370 | ||
| Kaiser–Meyer–Olkin (KMO) = 0.586, Bartlett’ test of sphericity = 401.206 (p < 0.001) | ||||
| Susceptibility to Microorganism | Consumer Sensitivity Regarding Product Safety | ||||||
|---|---|---|---|---|---|---|---|
| Mean | SD | p-Value | Mean | SD | p-Value | ||
| Age group | |||||||
| 20–29 years | 3.52 | 0.78 | 0.004 | 1.97 | 0.79 | 0.128 | |
| 30–49 years | 3.69 | 0.72 | 2.3 | 0.89 | |||
| 50 years and older | 3.09 | 0.7 | 2.3 | 0.88 | |||
| Marital status | |||||||
| Single | 3.45 | 0.782 | 0.79 | 2.18 | 0.88 | 0.241 | |
| Married | 3.4 | 0.761 | 1.97 | 0.77 | |||
| Education level | |||||||
| High school graduate or less | 2.96 | 0.74 | 0.001 | 2.31 | 0.85 | 0.306 | |
| Currently attending college | 3.47 | 0.69 | 2.07 | 0.83 | |||
| Bachelor’s degree or higher | 3.63 | 0.79 | 2.04 | 0.86 | |||
| Employment status | |||||||
| Yes (employed or on temporary leave) | 3.37 | 0.803 | 0.268 | 2.14 | 0.86 | 0.82 | |
| No (not economically active or seeking employment) | 3.5 | 0.736 | 2.07 | 0.82 | |||
| Target Bacterium | Primer (5′→3′) | Target Gene | PCR Product Size (bp) | Annealing Temp.(°C) |
|---|---|---|---|---|
| 16S rRNA [9] | F-5′-CCT ACG GGN GGC WGC AG-3′ R-5′-GAC TAC HVG GGT ATC TAA TCC-3′ | 16S V3–V4 | 460 | 55 |
| E. coli [10] | F-5′-TGG TAA TTA CCG ACG AAA ACG GC-3′ R-5′-ACG CGT GGT TAC AGT CTT GCG-3′ | uidA | 147 | 60 |
| P. aeruginosa [11] | F-5′-ATG GAA ATG CTG AAA TTC GGC-3′ R-5′-CTT CTT CAG CTC GAC GCG ACG-3′ | oprL | 504 | 60 |
| S. salivarius [12] | F-5′-ACA ACT GAA ACC TTT GCA TCT GG-3′ R-5′-CGG TCG CAT CTG TAC GGT AA-3′ | gdh | 278 | 60 |
| S. oralis [12] | F-5′-CCT TGG GAG CAA GGA ATA TTT TGA ATC TG-3′ R-5′-AGA GCG ATA TTG ACC ACG AAT AAA C-3′ | SO3 | 732 | 60 |
| S. pneumoniae [13] | F-5′-ACG CAA TCT AGC AGA TGA AGC A-3′ R-5′-TCG TGC GTT TTA ATT CCA GCT-3′ | lytA | 101 | 60 |
| S. aureus [14] | F-5′-GCG ATT GAT GGT GAT ACG GTT-3′ R-5′-AGC CAA GCC TTG ACG AAC TAA AGC-3′ | nuc | 279 | 60 |
| S. epidermidis [15] | F-5′-TAT GGT GGT GTG ACG GTG AC-3′ R-5′-CGT TGA TGG TGT TGT TGA AC-3′ | tuf | 370 | 60 |
| S. mutans [16] | F-5′-CGG AGT GCT TTT TAC AAG TGC TGG-3′ R-5′-AAC CAC GGC CAG CAA ACC CTT TAT-3′ | gtfB | 750 | 60 |
| Bacterial Strain | No. Detection (n = 18) | Detection Rate (%) |
|---|---|---|
| S. epidermidis | 17 | 94.4 |
| S. pneumoniae | 4 | 22.2 |
| S. aureus | 12 | 66.7 |
| P. aeruginosa | 17 | 94.4 |
| S. salivarius | 7 | 38.9 |
| S. mutans | 12 | 66.7 |
| S. oralis | 8 | 44.5 |
| E. coli | 1 | 5.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.-H.; Jeong, H.-J.; Hwang, Y.S. Consumer Awareness of Microbial Contamination and Identification of Key Pathogenic Bacteria in Lip Cosmetic Testers. Hygiene 2025, 5, 47. https://doi.org/10.3390/hygiene5040047
Kim M-H, Jeong H-J, Hwang YS. Consumer Awareness of Microbial Contamination and Identification of Key Pathogenic Bacteria in Lip Cosmetic Testers. Hygiene. 2025; 5(4):47. https://doi.org/10.3390/hygiene5040047
Chicago/Turabian StyleKim, Myoung-Hee, Ho-Jin Jeong, and Young Sun Hwang. 2025. "Consumer Awareness of Microbial Contamination and Identification of Key Pathogenic Bacteria in Lip Cosmetic Testers" Hygiene 5, no. 4: 47. https://doi.org/10.3390/hygiene5040047
APA StyleKim, M.-H., Jeong, H.-J., & Hwang, Y. S. (2025). Consumer Awareness of Microbial Contamination and Identification of Key Pathogenic Bacteria in Lip Cosmetic Testers. Hygiene, 5(4), 47. https://doi.org/10.3390/hygiene5040047






