In Vitro Analysis of Cross-Contamination and Disinfection Methods of Prosthetic Components Coming from Laboratories
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Microbial Collection and Seeding Process
2.3. Statistical Analysis
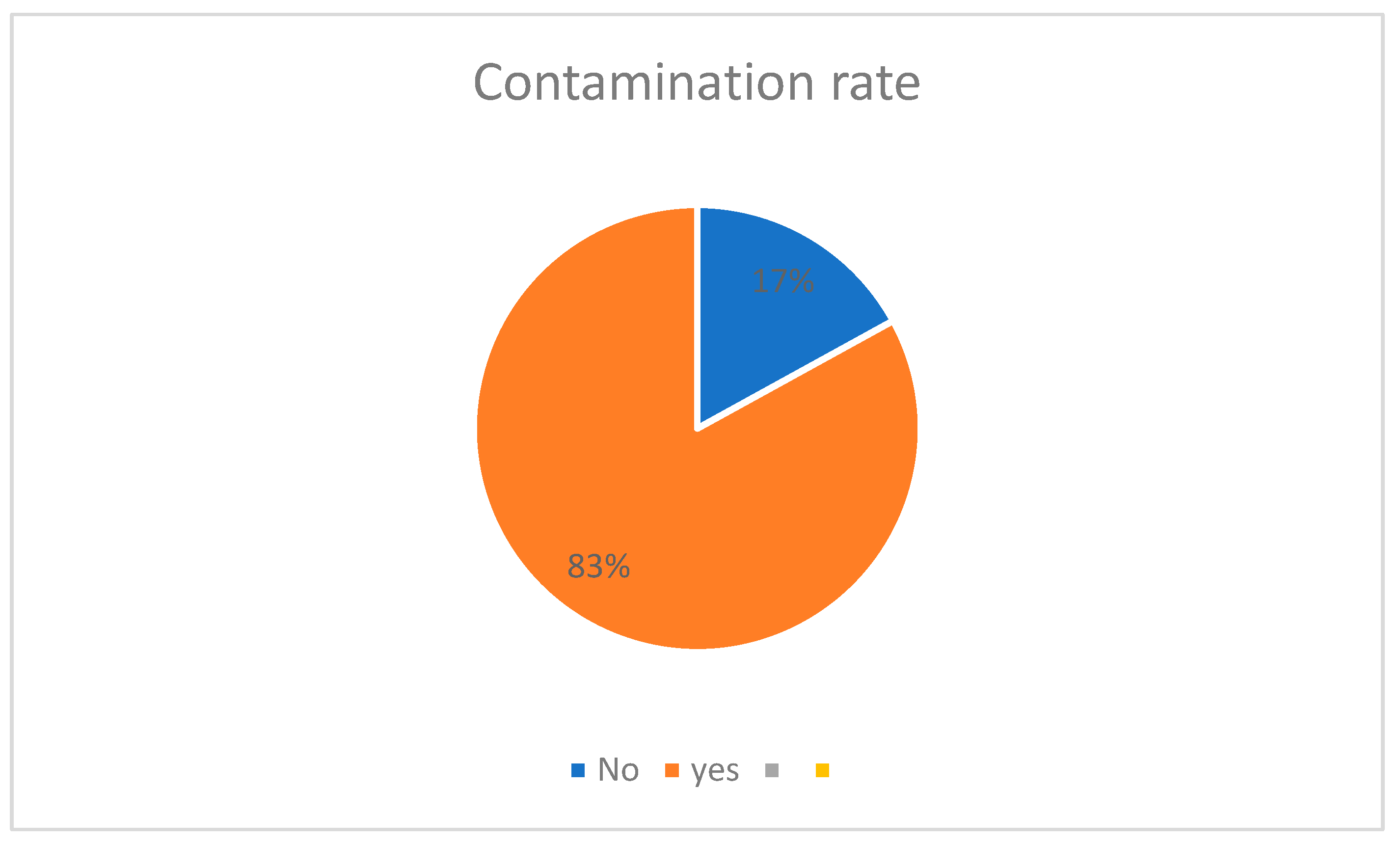
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, S.; Choi, C.; Cha, Y.; Chang, J. The Efficacy of Convenient Cleaning Methods Applicable for Customized Abutments: An In Vitro Study. BMC Oral Health 2021, 21, 78. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Rani, S.; Garg, S. Infection Control Knowledge and Practice: A Cross-Sectional Survey on Dental Laboratories in Dental Institutes of North India. J. Indian Prosthodont. Soc. 2017, 17, 348–354. [Google Scholar]
- Demajo, J.K.; Cassar, V.; Farrugia, C.; Millan-Sango, D.; Sammut, C.; Valdramidis, V.; Camilleri, J. Effectiveness of Disinfectants on Antimicrobial and Physical Properties of Dental Impression Materials. Int. J. Prosthodont. 2016, 29, 63–67. [Google Scholar] [CrossRef]
- Canullo, L.; Micarelli, C.; Lembo-Fazio, L.; Iannello, G.; Clementini, M. Microscopical and Microbiologic Characterization of Customized Titanium Abutments after Different Cleaning Procedures. Clin. Oral Implants Res. 2014, 25, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Barroso, M.G.; Cortela, D.C.B.; Mota, W.P. Endocardite Bacteriana: Da Boca ao Coração. Rev. Ciênc. Estud. Acad. Med. 2014, 2, 47–57. [Google Scholar]
- Silva, M.C.V.S.; Cartaxo, J.U.Q.; Arioli Filho, J.N.; Batista, A.U.D. Avaliação das Condutas de Biossegurança em Laboratórios de Prótese Dentária de João Pessoa, PB, Brasil. Pesqui. Bras. Odontopediatria Clín. Integr. 2010, 10, 101–106. [Google Scholar] [CrossRef]
- Cotrim, L.E.F.; Santos, E.M.; Jorge, A.O.C. Procedimentos de Biossegurança Realizados por Cirurgiões-Dentistas e Laboratórios Durante a Confecção de Próteses Dentárias. Rev. Odontol. 2001, 30, 233–244. [Google Scholar]
- Leung, R.L.; Schonfeld, S.E. Gypsum Casts as a Potential Source of Microbial Cross-Contamination. J. Prosthet. Dent. 1983, 49, 210–211. [Google Scholar] [CrossRef]
- Vilas Bôas, M.V.; Quirino, M.R.S. Controle de Infecção Cruzada: Laboratório de Prótese versus Consultório Odontológico. Rev. Biociênc. 2002, 8, 103–108. [Google Scholar]
- Chidambaranathan, A.S.; Balasubramanium, M. Comprehensive Review and Comparison of the Disinfection Techniques Currently Available in the Literature. J. Prosthodont. 2017, 28, e849–e856. [Google Scholar] [CrossRef]
- Nasser, K.R.P.; Querido, S.M.R.; Brito, G.N.B.; Palhari, L.O. Avaliação in vitro da Microinfiltração Bacteriana na Interface Implante/Pilar Protético. Rev. Ciênc. Saúde 2020, 5, 11–18. [Google Scholar]
- Berglundh, T.; Lindhe, J.; Jonsson, K.; Ericsson, I. The Topography of the Vascular Systems in the Periodontal and Peri-Implant Tissues in the Dog. J. Clin. Periodontol. 1994, 21, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Alresheedi, B.; Alazmi, S. Disinfection of Implant Abutment Connection Using Antimicrobial Photodynamic Therapy and 0.2% Chlorhexidine Gel Applications Immediately Before Prosthesis Delivery: Clinical and Radiographic Status at 1-Year of Follow-Up. Photodiagn. Photodyn. Ther. 2022, 38, 102790. [Google Scholar] [CrossRef] [PubMed]
- Bitton, G. Wastewater Microbiology; Wiley-Liss: Boca Raton, FL, USA, 2005; p. 765. [Google Scholar]
- Purohit, H.; Pisulkar, S.K.; Kambala, S.S.; Rajpurohit, G.S.; Godbole, S.D.; Dahihandekar, C.; Mistry, R.A. Comparative Evaluation of Disinfection on Elastomeric Impression Material Using 2% Glutaraldehyde, Ultraviolet Radiation, and Gaseous Ozone Using Customized Disinfection Unit—An In Vitro Study. Indian J. Forensic Med. Toxicol. 2021, 15, 192–199. [Google Scholar] [CrossRef]
- Prakash, N.; Parmar, A.; Pandey, P.; Mishra, N.; Mukhopadhyay, G.; Bais, K.; Acharya, J. Disinfection of Dental Impressions with the Use of Clinical UV Chamber: A Comparative Study. J. Adv. Med. Dent. Sci. Res. 2019, 7, 177–183. [Google Scholar]
- Abdullatif, F.A.; Al-Askar, M. Does Ultraviolet Radiation Exhibit Antimicrobial Effect against Oral Pathogens Attached on Various Dental Implant Surfaces? A Systematic Review. Dent. J. 2022, 10, 93. [Google Scholar] [CrossRef]
- Alsahhaf, A.; Alrabiah, M.; Ali, K.; Vohra, F.; Abduljabbar, T. Implant Abutment Disinfection Using Plasma of Argon and 0.2% Chlorhexidine Gel Applications Immediately Before Prosthesis Delivery: Clinical and Radiographic Status at 5-Years of Follow-Up. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 116–121. [Google Scholar]
- Sinjari, B.; D’Addazio, G.; De Tullio, I.; Traini, T.; Caputi, S. Peri-Implant Bone Resorption during Healing Abutment Placement: The Effect of a 0.20% Chlorhexidine Gel vs. Placebo—A Randomized Double-Blind Controlled Human Study. Biomed. Res. Int. 2018, 2018, 5326340. [Google Scholar] [CrossRef]
- Hartman, D. Perfecting Your Spread Plate Technique. J. Microbiol. Biol. Educ. 2011, 12, 204–205. [Google Scholar] [CrossRef]
- Machado, A.; Gonçalves, R.; Pinheiro, A.R.; Cordeiro Filho, C.; Rangel, B.C.; Alto, R.M. Immediate Implant and Customized Abutments: Esthetic Peri-Implant Preservation Alternative Without Immediate Loading Esthetic Customized Abutment. Int. J. Growth Factors Stem Cells Dent. 2023, 17, 8–12. [Google Scholar]
- Macedo, A.P.; Fedeli Jr, A.; Fukushigue, C.Y.; Voss, N.R.; Voss, N.R. Análise da Contaminação Bacteriana das Próteses Dentárias Enviadas dos Laboratórios. Prótese News 2020, 7, 46–52. [Google Scholar]
- Agência Nacional de Vigilância Sanitária. Manual de Microbiologia Clínica para Controle de Infecção em Serviços de Saúde; ANVISA, 2014. [Google Scholar]
- Pasternak, J. New Methods of Microbiological Identification Using MALDI-TOF. Einstein 2012, 10, 118. [Google Scholar] [CrossRef] [PubMed]
- Badillo, B.M.; Morales, G.J.; Martínez, C.M.Á.; Carachure, A.A.; Chávez, G.M.G.; García, R.V. Presencia de Bacterias en Prótesis Dentales Durante el Proceso de Elaboración. Rev. ADM 2021, 78, 13–21. [Google Scholar] [CrossRef]
- Marya, C.M.; Shukla, P.; Dahiya, V.; Jnaneswar, A. Current Status of Disinfection of Dental Impressions in Indian Dental Colleges: A Cause of Concern. J. Infect. Dev. Ctries. 2011, 5, 776–780. [Google Scholar] [CrossRef]
- Barenghi, L.; Barenghi, A.; Cadeo, C.; Di Blasio, A. Innovation by Computer-Aided Design/Computer-Aided Manufacturing Technology: A Look at Infection Prevention in Dental Settings. Biomed. Res. Int. 2019, 2019, 6092018. [Google Scholar] [CrossRef]
- ISO 17664; Processing of Health Care Products—Information to Be Provided by the Medical Device Manufacturer for the Processing of Medical Devices. International Organization for Standardization: Geneva, Switzerland, 2017.
- Cleveland, J.L.; Foster, M.; Barker, L.; Brown, G.G.; Lenfestey, N.; Lux, L.; Corley, T.J.; Bonito, A.J. Advancing Infection Control in Dental Care Settings. J. Am. Dent. Assoc. 2012, 143, 1127–1138. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, P.; Riebe, O.; Fischer, C.; Weinhold, C.; Dhom, G.; Sader, R.; Weigl, P. Microbiological Cleaning and Disinfection Efficacy of a Three-Stage Ultrasonic Processing Protocol for CAD-CAM Implant Abutments. J. Adv. Prosthodont. 2022, 14, 273–284. [Google Scholar] [CrossRef]
- Wang, T.; Costa, V.; Jenkins, S.G.; Hartman, B.J.; Westblade, L.F. Acinetobacter radioresistens Infection with Bacteremia and Pneumonia. IDCases 2019, 16, e00495. [Google Scholar] [CrossRef]
- Ross, K.M.; Mehr, J.S.; Greeley, R.D.; Montoya, L.A.; Kulkarni, P.A.; Frontin, S.; Weigle, T.J.; Giles, H.; Montana, B.E. Outbreak of Bacterial Endocarditis Associated with an Oral Surgery Practice: New Jersey Public Health Surveillance, 2013 to 2014. J. Am. Dent. Assoc. 2018, 149, 191–201. [Google Scholar] [CrossRef]
- Kan, J.Y.K.; Rungcharassaeng, K.; Lozada, J.L.; Zimmerman, G. Facial Gingival Tissue Stability Following Immediate Placement and Provisionalization of Maxillary Anterior Single Implants: A 2- to 8-Year Follow-Up. Int. J. Oral Maxillofac. Implants 2011, 26, 179–187. [Google Scholar]
- American Dental Association. Infection Control Recommendations for the Dental Office and the Dental Laboratory. J. Am. Dent. Assoc. 1996, 127, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Doundoulakis, J.H. Surface Analysis of Titanium after Sterilization: Role in Implant-Tissue Interface and Bioadhesion. J. Prosthet. Dent. 1987, 58, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Li, K.C.; Waddell, J.N.; Prior, D.J.; Ting, S.; Girvan, L.; van Vuuren, L.J.; Swain, M.V. Effect of Autoclave-Induced Low-Temperature Degradation on the Adhesion Energy between Yttria-Stabilized Zirconia Veneered with Porcelain. Dent. Mater. 2013, 29, e263–e270. [Google Scholar] [CrossRef]
- Hugo, W.B.; Longworth, A.R. Some Aspects of the Mode of Action of Chlorhexidine. J. Pharm. Pharmacol. 1964, 16, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, T.Q.; Blanco, K.C.; Inada, N.M.; Hortenci, M.F.; Costa, A.A.; Silva, E.D.S.; Gimenes, P.P.d.C.; Pompeu, S.; Silva, R.L.d.H.e.; Figueiredo, W.M.; et al. Manual Operated Ultraviolet Surface Decontamination for Healthcare Environments. Photomed. Laser Surg. 2017, 35, 666–671. [Google Scholar] [CrossRef]
- Alqarni, H.; Jamleh, A.; Chamber, M.S. Chlorhexidine as a Disinfectant in the Prosthodontic Practice: A Comprehensive Review. Cureus 2022, 14, e30566. [Google Scholar] [CrossRef]
- Azeredo, F.; Menezes, L.M.; Silva, R.M.; Rizzato, S.M.D.; Garcia, G.G.; Revers, K. Análises Microbiológicas de Alicates Ortodônticos. Dent. Press J. Orthod. 2011, 16, 102–112. [Google Scholar] [CrossRef]


| ID | Contaminated G2 | Decontaminated G2 | Efficacy Rate G2 | Contaminated G1 | Decontaminated G1 | Reduction Rate G1 |
|---|---|---|---|---|---|---|
| cp1 | 300 | 0 | 100.0 | 700 | 0 | 100 |
| cp2 | 340 | 80 | 76.5 | 530 | 0 | 100 |
| cp3 | 980 | 70 | 92.9 | 240 | 0 | 100 |
| cp4 | 360 | 120 | 66.7 | 570 | 0 | 100 |
| cp5 | 0 | 0 | 670 | 0 | 100 | |
| cp6 | 1120 | 0 | 100.0 | 970 | 0 | 100 |
| cp7 | 120 | 0 | 100.0 | 280 | 0 | 100 |
| cp8 | 660 | 0 | 100.0 | 0 | 0 | 0 |
| cp9 | 160 | 0 | 100.0 | 140 | 0 | 100 |
| cp10 | 950 | 0 | 100.0 | 100 | 0 | 100 |
| cp11 | 110 | 0 | 100.0 | 1700 | 250 | 85.3 |
| cp12 | 900 | 0 | 100.0 | 0 | 0 | 0 |
| cp13 | 410 | 0 | 100.0 | 100 | 0 | 100 |
| cp14 | 0 | 0 | 0 | 0 | 0 | |
| cp15 | 120 | 0 | 100.0 | 1200 | 150 | 87.5 |
| cp16 | 1060 | 130 | 87.7 | 1400 | 0 | 100 |
| cp17 | 870 | 0 | 100.0 | 7600 | 0 | 100 |
| cp18 | 0 | 0 | 1110 | 0 | 100 | |
| cp19 | 0 | 0 | 1300 | 340 | 73.8 | |
| cp20 | 1830 | 390 | 78.7 | 950 | 0 | 100 |
| cp21 | 620 | 0 | 100.0 | 2509 | 0 | 100 |
| cp22 | 980 | 0 | 100.0 | 420 | 0 | 100 |
| cp23 | 240 | 0 | 100.0 | 1350 | 140 | 89.6 |
| cp24 | 0 | 0 | 0 | 0 | 0 | |
| cp25 | 230 | 0 | 100.0 | 1010 | 0 | 100 |
| cp26 | 890 | 380 | 57.3 | 2200 | 900 | 59.1 |
| cp27 | 1500 | 130 | 91.3 | 0 | 0 | 0 |
| cp28 | 530 | 0 | 100.0 | 370 | 0 | 100 |
| cp29 | 310 | 40 | 87.1 | 1210 | 220 | 81.8 |
| cp30 | 430 | 0 | 100.0 | 350 | 0 | 100 |
| TOTAL | 16,020 | 1340 | 91.6 | 28,979 | 2000 | 93.0984506 |
| Microrganisms | Frequency | Percentage |
|---|---|---|
| Acinetobacter iwoffii | 4 | 5.5 |
| Acinetobacter radioresistens | 4 | 5.5 |
| Aerococus viridans | 2 | 2.7 |
| Bacillus licheniformis | 1 | 1.4 |
| Candida albicans | 2 | 2.7 |
| Enterobacter cloacae | 4 | 5.5 |
| Enterococcus faecalis | 25 | 34.2 |
| Enterococcus sp. | 1 | 1.4 |
| Kocuria marina | 1 | 1.4 |
| Paenibacillus lactis | 2 | 2.7 |
| Pichia kudriavzerli | 5 | 6.8 |
| Pseudomonas sp. | 2 | 2.8 |
| Rhodotorula mucilaginosa | 1 | 1.4 |
| Staphylococcus aureus | 4 | 5.5 |
| Staphylococcus epidermidis | 13 | 17.8 |
| Staphylococcus succinus | 1 | 1.4 |
| Staphylococcus warneri | 1 | 1.4 |
| Total | 73 | 100.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braga, C.; Rivaldo, E.G.; Paula, A.S.d.; Bourgi, R.; Hardan, L.; Kharouf, N.; Qaddomi, M.; Haikel, Y.; Klein-Junior, C.A. In Vitro Analysis of Cross-Contamination and Disinfection Methods of Prosthetic Components Coming from Laboratories. Hygiene 2025, 5, 9. https://doi.org/10.3390/hygiene5010009
Braga C, Rivaldo EG, Paula ASd, Bourgi R, Hardan L, Kharouf N, Qaddomi M, Haikel Y, Klein-Junior CA. In Vitro Analysis of Cross-Contamination and Disinfection Methods of Prosthetic Components Coming from Laboratories. Hygiene. 2025; 5(1):9. https://doi.org/10.3390/hygiene5010009
Chicago/Turabian StyleBraga, Carlos, Elken Gomes Rivaldo, Arthur Saavedra de Paula, Rim Bourgi, Louis Hardan, Naji Kharouf, Mohammad Qaddomi, Youssef Haikel, and Celso Afonso Klein-Junior. 2025. "In Vitro Analysis of Cross-Contamination and Disinfection Methods of Prosthetic Components Coming from Laboratories" Hygiene 5, no. 1: 9. https://doi.org/10.3390/hygiene5010009
APA StyleBraga, C., Rivaldo, E. G., Paula, A. S. d., Bourgi, R., Hardan, L., Kharouf, N., Qaddomi, M., Haikel, Y., & Klein-Junior, C. A. (2025). In Vitro Analysis of Cross-Contamination and Disinfection Methods of Prosthetic Components Coming from Laboratories. Hygiene, 5(1), 9. https://doi.org/10.3390/hygiene5010009










