Differential Antimicrobial Effects of Endodontic Irrigant Endocyn on Oral Bacteria
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Bacterial Culture
2.3. DNA Isolation and Analysis
2.4. Experimental Reagents
2.5. Experimental Assays
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HOCl | Hypochlorous acid |
| pH | Potential of hydrogen |
| ATCC | American Type Culture Collection |
| BSL | Biosafety Level |
| PBS | Phosphate-buffered saline |
| TSB | Trypticase Soy Broth |
| MRS | DeMan, Rogosa, and Sharpe |
| BHI | Brain–Heart Infusion |
| qPCR | Quantitative polymerase chain reaction |
| CFR | Colony-forming unit |
References
- Boutsiouki, C.; Frankenberger, R.; Krämer, N. Clinical and radiographic success of (partial) pulpotomy and pulpectomy in primary teeth: A systematic review. Eur. J. Paediatr. Dent. 2021, 22, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.J.; Seremidi, K.; Stratigaki, E.; Kloukos, D.; Duggal, M.; Gizani, S. Deep dentine caries management of immature permanent posterior teeth with vital pulp: A systematic review and meta-analysis. J. Dent. 2022, 124, 104214. [Google Scholar] [CrossRef] [PubMed]
- Matoug-Elwerfelli, M.; ElSheshtawy, A.S.; Duggal, M.; Tong, H.J.; Nazzal, H. Vital pulp treatment for traumatized permanent teeth: A systematic review. Int. Endod. J. 2022, 55, 613–629. [Google Scholar] [CrossRef] [PubMed]
- Coll, J.A.; Dhar, V.; Chen, C.Y.; Crystal, Y.O.; Guelmann, M.; Marghalani, A.A.; AlShamali, S.; Xu, Z.; Glickman, G.N.; Wedeward, R. Use of Vital Pulp Therapies in Primary Teeth 2024. Pediatr. Dent. 2024, 46, 13–26. [Google Scholar]
- Urquhart, O.; Tampi, M.P.; Pilcher, L.; Slayton, R.L.; Araujo, M.W.B.; Fontana, M.; Guzmán-Armstrong, S.; Nascimento, M.M.; Nový, B.B.; Tinanoff, N.; et al. Nonrestorative Treatments for Caries: Systematic Review and Network Meta-analysis. J. Dent. Res. 2019, 98, 14–26. [Google Scholar] [CrossRef]
- Coll, J.A.; Seale, N.S.; Vargas, K.; Marghalani, A.A.; Al Shamali, S.; Graham, L. Primary Tooth Vital Pulp Therapy: A Systematic Review and Meta-analysis. Pediatr. Dent. 2017, 39, 16–123. [Google Scholar]
- Schwendicke, F.; Walsh, T.; Lamont, T.; Al-Yaseen, W.; Bjørndal, L.; Clarkson, J.E.; Fontana, M.; Gomez Rossi, J.; Göstemeyer, G.; Levey, C.; et al. Interventions for treating cavitated or dentine carious lesions. Cochrane Database Syst. Rev. 2021, 7, CD013039. [Google Scholar] [CrossRef]
- Coll, J.A.; Dhar, V.; Chen, C.Y.; Crystal, Y.O.; Guelmann, M.; Marghalani, A.A.; AlShamali, S.; Xu, Z.; Glickman, G.; Wedeward, R. Primary Tooth Vital Pulp Treatment Interventions: Systematic Review and Meta-Analyses. Pediatr. Dent. 2023, 45, 474–546. [Google Scholar]
- Chouchene, F.; Oueslati, A.; Masmoudi, F.; Baaziz, A.; Maatouk, F.; Ghedira, H. Efficacy of non-instrumental Endodontic treatment in primary teeth: A systematic review of clinical randomized trials. Syst. Rev. 2024, 13, 112. [Google Scholar] [CrossRef]
- Elhamouly, Y.; Adham, M.M.; Dowidar, K.M.L.; El Backly, R.M. Outcome assessment methods of bioactive and biodegradable materials as pulpotomy agents in primary and permanent teeth: A scoping review. BMC Oral Health 2024, 24, 496. [Google Scholar] [CrossRef]
- Stringhini Junior, E.; Vitcel, M.E.; Oliveira, L.B. Evidence of pulpotomy in primary teeth comparing MTA, calcium hydroxide, ferric sulphate, and electrosurgery with formocresol. Eur. Arch. Paediatr. Dent. 2015, 16, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Tzanetakis, G.N.; Tsiouma, O.; Mougiou, E.; Koletsi, D. Factors Related to Pulp Survival After Complicated Crown Fracture Following Vital Pulp Therapy: A Systematic Review and Meta-analysis. J. Endod. 2022, 48, 457–478.e4. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, W.; Zeng, Q.; Tang, M.; Massey, J.; Bergeron, B.E.; Gu, L.; Tay, F.R. Efficacy of pulpotomy in managing irreversible pulpitis in mature permanent teeth: A systematic review and meta-analysis. J. Dent. 2024, 144, 104923. [Google Scholar] [CrossRef]
- Afrashtehfar, K.I.; Jurado, C.A.; Al-Hadi, D.; Shetty, K.P. Pulpotomy versus root canal treatment in permanent teeth with spontaneous pain: Comparable clinical and patient outcomes, but insufficient evidence. Evid. Based Dent. 2023, 24, 54–56. [Google Scholar] [CrossRef]
- Taylor, G.D.; Vernazza, C.R.; Abdulmohsen, B. Success of endodontic management of compromised first permanent molars in children: A systematic review. Int. J. Paediatr. Dent. 2020, 30, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Lopes, L.B.; Neves, J.A.; Botelho, J.; Machado, V.; Mendes, J.J. Regenerative Endodontic Procedures: An Umbrella Review. Int. J. Environ. Res. Public Health 2021, 18, 754. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, Y.; Chen, X.; Zhang, Y.; Zhou, F.; Deng, J.; Zou, J.; Wang, Y. Materials for pulpotomy in immature permanent teeth: A systematic review and meta-analysis. BMC Oral Health 2019, 19, 227. [Google Scholar] [CrossRef] [PubMed]
- Snigdha, N.T.; Karobari, M.I.; Kamarudin, A.; Samsudin, N.A.; Baharin, F.; Dziaruddin, N.; Assiry, A.A.; Luke, A.M.; Scardina, G.A. A Systematic Review and Meta-analysis of Regenerative Pulpotomy in the Treatment of Vital Primary Teeth. Eur. J. Paediatr. Dent. 2024, 1, 1–16. [Google Scholar] [CrossRef]
- Saoud, T.M.A.; Ricucci, D.; Lin, L.M.; Gaengler, P. Regeneration and Repair in Endodontics-A Special Issue of the Regenerative Endodontics-A New Era in Clinical Endodontics. Dent. J. 2016, 4, 3. [Google Scholar] [CrossRef]
- Kim, S.G.; Malek, M.; Sigurdsson, A.; Lin, L.M.; Kahler, B. Regenerative endodontics: A comprehensive review. Int. Endod. J. 2018, 51, 1367–1388. [Google Scholar] [CrossRef]
- Galler, K.M. Clinical procedures for revitalization: Current knowledge and considerations. Int. Endod. J. 2016, 49, 926–936. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; De Deus, G.; Kristoffersen, I.M.; Wiig, E.; Reseland, J.E.; Johnsen, G.F.; Silva, E.J.N.L.; Haugen, H.J. Regenerative Endodontics by Cell Homing: A Review of Recent Clinical trials. J. Endod. 2023, 49, 4–17. [Google Scholar] [CrossRef] [PubMed]
- Duarte, M.L.; Pires, P.M.; Ferreira, D.M.; Pintor, A.V.B.; de Almeida Neves, A.; Maia, L.C.; Primo, L.G. Is there evidence for the use of lesion sterilization and tissue repair therapy in the endodontic treatment of primary teeth? A systematic review and meta-analyses. Clin. Oral Investig. 2020, 24, 2959–2972. [Google Scholar] [CrossRef]
- Pozos-Guillen, A.; Garcia-Flores, A.; Esparza-Villalpando, V.; Garrocho-Rangel, A. Intracanal irrigants for pulpectomy in primary teeth: A systematic review and meta-analysis. Int. J. Paediatr. Dent. 2016, 26, 412–425. [Google Scholar] [CrossRef]
- Sreenivasan, P.K.; Haraszthy, V.I. Chlorhexidine Improves Hygiene Reducing Oral Polymorphonuclear Leukocytes with Antimicrobial Effects at Distinct Microenvironments amongst Subjects Stratified by Health Status. Antibiotics 2022, 11, 603. [Google Scholar] [CrossRef]
- Ruksakiet, K.; Hanák, L.; Farkas, N.; Hegyi, P.; Sadaeng, W.; Czumbel, L.M.; Sang-Ngoen, T.; Garami, A.; Mikó, A.; Varga, G.; et al. Antimicrobial Efficacy of Chlorhexidine and Sodium Hypochlorite in Root Canal Disinfection: A Systematic Review and Meta-analysis of Randomized Controlled Trials. J. Endod. 2020, 46, 1032–1041.e7. [Google Scholar] [CrossRef] [PubMed]
- Wikström, A.; Romani Vestman, N.; Rakhimova, O.; Lazaro Gimeno, D.; Tsilingaridis, G.; Brundin, M. Microbiological assessment of success and failure in pulp revitalization: A randomized clinical trial using calcium hydroxide and chlorhexidine gluconate in traumatized immature necrotic teeth. J. Oral Microbiol. 2024, 16, 2343518. [Google Scholar] [CrossRef]
- Nogales, C.G.; Cazares, R.X.R.; Nardello, L.C.L.; Mayer, M.P.A.; Gavini, G.; Zehnder, M.; Pinheiro, E.T. Evaluating the Impact of Ultrasonic Irrigation on Bacterial Levels and Activity Following Chemomechanical Procedures. J. Endod. 2025, 51, 118–123. [Google Scholar] [CrossRef]
- Tirali, R.E.; Bodur, H.; Ece, G. In vitro antimicrobial activity of sodium hypochlorite, chlorhexidine gluconate and octenidine dihydrochloride in elimination of microorganisms within dentinal tubules of primary and permanent teeth. Med. Oral Patol. Oral Cir. Bucal. 2012, 17, e517–e522. [Google Scholar] [CrossRef]
- Daood, U.; Parolia, A.; Matinlinna, J.; Yiu, C.; Ahmed, H.M.A.; Fawzy, A. Properties of a modified quaternary ammonium silane formulation as a potential root canal irrigant in endodontics. Dent. Mater. 2020, 36, e386–e402. [Google Scholar] [CrossRef]
- Daood, U.; Parolia, A.; Elkezza, A.; Yiu, C.K.; Abbott, P.; Matinlinna, J.P.; Fawzy, A.S. An in vitro study of a novel quaternary ammonium silane endodontic irrigant. Dent. Mater. 2019, 35, 1264–1278. [Google Scholar] [CrossRef] [PubMed]
- Scott MB 2nd Zilinski, G.S.; Kirkpatrick, T.C.; Himel, V.T.; Sabey, K.A.; Lallier, T.E. The Effects of Irrigants on the Survival of Human Stem Cells of the Apical Papilla, Including Endocyn. J. Endod. 2018, 44, 263–268. [Google Scholar] [CrossRef]
- Lin, Y.C.; Tsai, C.F.; Huang, H.L. Effects of hypochlorous acid mouthwash on salivary bacteria including Staphylococcus aureus in patients with periodontal disease: A randomized controlled trial. BMC Oral Health 2023, 23, 698. [Google Scholar] [CrossRef]
- Lineback, C.B.; Nkemngong, C.A.; Wu, S.T.; Li, X.; Teska, P.J.; Oliver, H.F. Hydrogen peroxide and sodium hypochlorite disinfectants are more effective against Staphylococcus aureus and Pseudomonas aeruginosa biofilms than quaternary ammonium compounds. Antimicrob. Resist. Infect. Control 2018, 7, 154. [Google Scholar] [CrossRef]
- Tyski, S.; Bocian, E.; Laudy, A.E. Application of normative documents for determination of biocidal activity of disinfectants and antiseptics dedicated to the medical area: A narrative review. J. Hosp. Infect. 2022, 125, 75–91. [Google Scholar] [CrossRef] [PubMed]
- Rôças, I.N.; Provenzano, J.C.; Neves, M.A.; Siqueira, J.F., Jr. Disinfecting Effects of Rotary Instrumentation with Either 2.5% Sodium Hypochlorite or 2% Chlorhexidine as the Main Irrigant: A Randomized Clinical Study. J. Endod. 2016, 42, 943–947. [Google Scholar] [CrossRef]
- Gonçalves, L.S.; Rodrigues, R.C.; Andrade Junior, C.V.; Soares, R.G.; Vettore, M.V. The Effect of Sodium Hypochlorite and Chlorhexidine as Irrigant Solutions for Root Canal Disinfection: A Systematic Review of Clinical Trials. J. Endod. 2016, 42, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Lafaurie, G.I.; Zaror, C.; Díaz-Báez, D.; Castillo, D.M.; De Ávila, J.; Trujillo, T.G.; Calderón-Mendoza, J. Evaluation of substantivity of hypochlorous acid as an antiplaque agent: A randomized controlled trial. Int. J. Dent. Hyg. 2018, 16, 527–534. [Google Scholar] [CrossRef]
- Wilson, J.; Swanbeck, S.; Banning, G.; Alhwayek, T.; Sullivan, V.; Howard, K.M.; Kingsley, K. Assessment of Sodium Diamine Fluoride (SDF) with Light Curing Technique: A Pilot Study of Antimicrobial Effects. Methods Protoc. 2022, 5, 31. [Google Scholar] [CrossRef]
- Emett, J.; David, R.; McDaniel, J.; McDaniel, S.; Kingsley, K. Comparison of DNA Extracted from Pediatric Saliva, Gingival Crevicular Fluid and Site-Specific Biofilm Samples. Methods Protoc. 2020, 3, 48. [Google Scholar] [CrossRef]
- Tsou, S.H.; Hu, S.W.; Yang, J.J.; Yan, M.; Lin, Y.Y. Potential Oral Health Care Agent from Coffee Against Virulence Factor of Periodontitis. Nutrients 2019, 11, 2235. [Google Scholar] [CrossRef]
- Gulati, R.K.; Jain, N.; Singh, A.; Jain, A.; Jindal, A.; Kumawat, M.K.; Paiwal, K. Comparative Evaluation of the Antimicrobial Efficacy of Chemical and Phytomedicinal Agents When Used As Intracanal Irrigants: An In Vitro Study. Cureus 2023, 15, e48754. [Google Scholar] [CrossRef]
- Botu, R.K.; Rachuri, P.; Martha, S.; Raparla, M.; Matilda, S.; Yemparla, S. Comparative Evaluation of the Efficacy of Chlorhexidine, Diode Laser, and Saline in Reducing the Microbial Count in Primary Teeth Root Canals: An In Vivo Study. Int. J. Clin. Pediatr. Dent. 2023, 16, 459–463. [Google Scholar] [CrossRef]
- Chauhan, N.; Kumar, G.; Goswami, M.; Johar, S.; Sharma, A. Antimicrobial efficacy of sodium hypochlorite, saline, and Er, Cr:YSGG laser-assisted disinfection in root canal treatment of primary molars: An in vivo study. J. Indian. Soc. Pedod. Prev. Dent. 2023, 41, 70–75. [Google Scholar] [CrossRef]
- Kumar, R.S.; Ankola, A.; Peerzade, M.; Sankeshwari, R.; Hampiholi, V.; Pai Khot, A.; Shah, M.A. Comparative Efficacy of Different Irrigant Activation Techniques for Irrigant Delivery Up to the Working Length of Mature Permanent Teeth: A Systematic Review and Meta-Analysis. Eur. Endod. J. 2023, 8, 1–19. [Google Scholar] [CrossRef]
- Teja, K.V.; Janani, K.; Srivastava, K.C.; Shrivastava, D.; Jose, J.; Marya, A.; Karobari, M.I. Comparison of Herbal Agents with Sodium Hypochlorite as Root Canal Irrigant: A Systematic Review of In Vitro Studies. Evid. Based Complement. Altern. Med. 2021, 2021, 8967219. [Google Scholar] [CrossRef]
- Nascimento, G.G.; Rabello, D.G.D.; Corazza, B.J.M.; Gomes, A.P.M.; Silva, E.G.; Martinho, F.C. Comparison of the effectiveness of single- and multiple-sessions disinfection protocols against endotoxins in root canal infections: Systematic review and meta-analysis. Sci. Rep. 2021, 11, 1226. [Google Scholar] [CrossRef]
- Zandi, H.; Rodrigues, R.C.; Kristoffersen, A.K.; Enersen, M.; Mdala, I.; Ørstavik, D.; Rôças, I.N.; Siqueira, J.F., Jr. Antibacterial Effectiveness of 2 Root Canal Irrigants in Root-filled Teeth with Infection: A Randomized Clinical Trial. J. Endod. 2016, 42, 1307–1313. [Google Scholar] [CrossRef]
- Tonini, R.; Salvadori, M.; Audino, E.; Sauro, S.; Garo, M.L.; Salgarello, S. Irrigating Solutions and Activation Methods Used in Clinical Endodontics: A Systematic Review. Front. Oral Health 2022, 3, 838043, Erratum in Front. Oral Health 2022, 3, 876265. https://doi.org/10.3389/froh.2022.876265. [Google Scholar] [CrossRef]
- Kharchi, A.S.; Tagiyeva-Milne, N.; Kanagasingam, S. Regenerative Endodontic Procedures, Disinfectants and Outcomes: A Systematic Review. Prim. Dent. J. 2020, 9, 65–84. [Google Scholar] [CrossRef]
- Prada, I.; Micó-Muñoz, P.; Giner-Lluesma, T.; Micó-Martínez, P.; Collado-Castellano, N.; Manzano-Saiz, A. Influence of microbiology on endodontic failure. Literature review. Med. Oral Patol. Oral Cir. Bucal 2019, 24, e364–e372. [Google Scholar] [CrossRef]
- Swimberghe, R.C.D.; Coenye, T.; De Moor, R.J.G.; Meire, M.A. Biofilm model systems for root canal disinfection: A literature review. Int. Endod. J. 2019, 52, 604–628. [Google Scholar] [CrossRef]
- Gasparelli, C.H.C.; Marceliano-Alves, M.F.V.; Bastos, L.F.; Lopes, R.T.; Limoeiro, A.G.D.S.; Nascimento, W.M.; Dos Santos, L.M.H.; Boukpessi, T.; Soares, A.J.; Frozoni, M. Analysis of the penetration of NaOCl 5.25% into dentinal tubules using different irrigation protocols: An ex vivo study. Aust. Endod. J. 2024, 50, 140–147. [Google Scholar] [CrossRef]
- Sabino-Silva, R.; Cardoso, I.V.; Vitali, F.C.; Alves, A.M.H.; Souza, B.D.M.; Bortoluzzi, E.A.; da Fonseca Roberti Garcia, L.; da Silveira Teixeira, C. Prevalence of postoperative pain after endodontic treatment using low and high concentrations of sodium hypochlorite: A systematic review and meta-analysis. Clin. Oral Investig. 2023, 27, 4157–4171. [Google Scholar] [CrossRef]
- Briggs, E.A.; Toner, R.; Kilgariff, J.K. Evidence-based Standard Operating Procedures FoR the Prevention and Management of Sodium Hypochlorite Accidents in Dentistry. Prim. Dent. J. 2023, 12, 97–109. [Google Scholar] [CrossRef]
- Baruwa, A.O.; Martins, J.N.R.; Maravic, T.; Mazzitelli, C.; Mazzoni, A.; Ginjeira, A. Effect of Endodontic Irrigating Solutions on Radicular Dentine Structure and Matrix Metalloproteinases-A Comprehensive Review. Dent. J. 2022, 10, 219. [Google Scholar] [CrossRef]
- da Cruz Nizer, W.S.; Inkovskiy, V.; Overhage, J. Surviving Reactive Chlorine Stress: Responses of Gram-Negative Bacteria to Hypochlorous Acid. Microorganisms 2020, 8, 1220. [Google Scholar] [CrossRef]
- Kampf, G. Biocidal Agents Used for Disinfection Can Enhance Antibiotic Resistance in Gram-Negative Species. Antibiotics 2018, 7, 110. [Google Scholar] [CrossRef] [PubMed]
- Septimus, E.J.; Schweizer, M.L. Decolonization in Prevention of Health Care-Associated Infections. Clin. Microbiol. Rev. 2016, 29, 201–222. [Google Scholar] [CrossRef]
- Boyce, J.M. Hand and environmental hygiene: Respective roles for MRSA, multi-resistant gram negatives, Clostridioides difficile, and Candida spp. Antimicrob. Resist. Infect. Control 2024, 13, 110. [Google Scholar] [CrossRef]
- Kampf, G. Antibiotic ResistanceCan Be Enhanced in Gram-Positive Species by Some Biocidal Agents Used for Disinfection. Antibiotics 2019, 8, 13. [Google Scholar] [CrossRef]
- Tsai, C.F.; Chung, J.J.; Ding, S.J.; Chen, C.C. In vitro cytotoxicity and antibacterial activity of hypochlorous acid antimicrobial agent. J. Dent. Sci. 2024, 19, 345–356. [Google Scholar] [CrossRef]
- Anari, R.K.; Nikkhahi, F.; Javadi, A.; Bakht, M.; Rostamani, M.; Kelishomi, F.Z.; Alizadeh, S.A. Evaluation of antibacterial activity of five biocides and the synergistic effect of biocide/EDTA combinations on biofilm-producing and non-producing Stenotrophomonas maltophilia strains isolated from clinical specimens in Iran. BMC Microbiol. 2022, 22, 257. [Google Scholar] [CrossRef]
- Alhadainy, H.A.; Abdel-Karim, A.H.; Fouad, A.F. Prevalence of Fusobacterium Species in Endodontic Infections Detected With Molecular Methods: Systematic Review and Meta-Analysis. J. Endod. 2023, 49, 1249–1261. [Google Scholar] [CrossRef]
- Cuevas-Gonzalez, M.V.; Mungarro-Cornejo, G.A.; Espinosa-Cristóbal, L.F.; Donohue-Cornejo, A.; Tovar Carrillo, K.L.; Saucedo Acuña, R.A.; García Calderón, A.G.; Guzmán Gastelum, D.A.; Zambrano-Galván, G.; Cuevas-Gonzalez, J.C. Antimicrobial resistance in odontogenic infections: A protocol for systematic review. Medicine 2022, 101, e31345. [Google Scholar] [CrossRef]
- Ardila, C.M.; Bedoya-García, J.A.; González-Arroyave, D. Antimicrobial resistance in patients with endodontic infections: A systematic scoping review of observational studies. Aust. Endod. J. 2023, 49, 386–395. [Google Scholar] [CrossRef]
- Bronzato, J.D.; Bomfim, R.A.; Hayasida, G.Z.P.; Cúri, M.; Estrela, C.; Paster, B.J.; Gomes, B.P.F.A. Analysis of microorganisms in periapical lesions: A systematic review and meta-analysis. Arch. Oral Biol. 2021, 124, 105055. [Google Scholar] [CrossRef]
- Al-Ahmad, A.; Elamin, F.; Gärttner, R.; Anderson, A.; Wittmer, A.; Mirghani, Y.; Hellwig, E. New Bacterial Combinations in Secondary Endodontic Infections of Patients with a Recent Systematic Antibiotic Therapy. Monogr. Oral Sci. 2021, 29, 144–154. [Google Scholar] [CrossRef]
- Brave, D.R.; Langaliya, A.K.; Rai, J.S.; Buch, A.; Mahendra, M.; Patel, S. The Impact of Chlorhexidine as an Endodontic Irrigant/Medicament on Post-Obturation Pain in Patients Following Endodontic Therapy: A Preliminary Bayesian Meta-Analysis. J. Pharm. Bioallied Sci. 2024, 16 (Suppl. S3), S2935–S2937. [Google Scholar] [CrossRef]
- Azizi Mazreah, S.; Shirvani, A.; Azizi Mazreah, H.; Dianat, O. Evaluation of irrigant extrusion following the use of different root canal irrigation techniques: A systematic review and meta-analysis. Aust. Endod. J. 2023, 49, 396–417. [Google Scholar] [CrossRef]
- Kendell-Wall, R.; Nguyen, J.T.; Salleras, F.; Kamboj, A.S.; Diwen Tan, S.A.; Manish Trivedi, V.; de Mello-Neto, J.M.; Rodrigues Amaral, R. Antimicrobial efficacy of Odontopaste in endodontics: A systematic review. Evid. Based Dent. 2024, 25, 166. [Google Scholar] [CrossRef] [PubMed]
- Duggal, M.; Tong, H.J.; Al-Ansary, M.; Twati, W.; Day, P.F.; Nazzal, H. Interventions for the endodontic management of non-vital traumatised immature permanent anterior teeth in children and adolescents: A systematic review of the evidence and guidelines of the European Academy of Paediatric Dentistry. Eur. Arch. Paediatr. Dent. 2017, 18, 139–151, Erratum in Eur. Arch. Paediatr. Dent. 2017, 18, 153. https://doi.org/10.1007/s40368-017-0292-x. [Google Scholar] [CrossRef]
- Malu, K.; Khubchandani, M. Triple Antibiotic Paste: A Suitable Medicament for Intracanal Disinfection. Cureus. 2022, 14, e29186. [Google Scholar] [CrossRef] [PubMed]
- Panchal, K.G.; Virani, K.; Patel, V.; Ali Khan, A.; Pettiwala, A.; Puranik, S.S.; Joshi, S. Triple Antibiotic Paste: A Game Changer in Endodontics. J. Pharm. Bioallied Sci. 2024, 16 (Suppl. S3), S1913–S1915. [Google Scholar] [CrossRef]
- Boutsioukis, C.; Arias-Moliz, M.T. Present status and future directions—Irrigants and irrigation methods. Int. Endod. J. 2022, 55 (Suppl. S3), 588–612. [Google Scholar] [CrossRef] [PubMed]
- Gomes, B.P.F.A.; Aveiro, E.; Kishen, A. Irrigants and irrigation activation systems in Endodontics. Braz. Dent. J. 2023, 34, 1–33. [Google Scholar] [CrossRef]
- Yadav, Y.; Anil, C.K.; Kumar, N.S.; Batra, D.; Kapur, I.; Chaturvedi, M.; Mustafa, M. Exploring Efficacy and Safety: Comparative Evaluation of Different Irrigation Solutions in Root Canal Therapy at a Tertiary Care Setting. J. Pharm. Bioallied Sci. 2024, 16 (Suppl. S3), S2546–S2548. [Google Scholar] [CrossRef]
- Arul, B.; Suresh, N.; Sivarajan, R.; Natanasabapathy, V. Influence of volume of endodontic irrigants used in different irrigation techniques on root canal dentin microhardness. Indian. J. Dent. Res. 2021, 32, 230–235. [Google Scholar] [CrossRef]
- Paixão, S.; Rodrigues, C.; Grenho, L.; Fernandes, M.H. Efficacy of sonic and ultrasonic activation during endodontic treatment: A Meta-analysis of in vitro studies. Acta Odontol. Scand. 2022, 80, 588–595. [Google Scholar] [CrossRef]
- Smaïl-Faugeron, V.; Glenny, A.M.; Courson, F.; Durieux, P.; Muller-Bolla, M.; Fron Chabouis, H. Pulp treatment for extensive decay in primary teeth. Cochrane Database Syst. Rev. 2018, 5, CD003220. [Google Scholar] [CrossRef]
- Tewari, N.; Goel, S.; Mathur, V.P.; O’Connell, A.C.; Johnson, R.M.; Rahul, M.; Sultan, F.; Goswami, M.; Srivastav, S.; Ritwik, P. Success of medicaments and techniques for pulpotomy of primary teeth: An overview of systematic reviews. Int. J. Paediatr. Dent. 2022, 32, 828–842. [Google Scholar] [CrossRef]
- Colombo, M.; Gallo, S.; Garofoli, A.; Poggio, C.; Arciola, C.R.; Scribante, A. Ozone Gel in Chronic Periodontal Disease: A Randomized Clinical Trial on the Anti-Inflammatory Effects of Ozone Application. Biology 2021, 10, 625. [Google Scholar] [CrossRef]
- Goswami, P.; Sharma, K.; Maiti, N.; Yadav, S.; Verma, V.; Puthenkandathil, R. Ozone: An Adjunct in Dental Treatment. J. Pharm. Bioallied Sci. 2024, 16 (Suppl. S1), S2–S4. [Google Scholar] [CrossRef]
- Silva, E.J.N.L.; Prado, M.C.; Soares, D.N.; Hecksher, F.; Martins, J.N.R.; Fidalgo, T.K.S. The effect of ozone therapy in root canal disinfection: A systematic review. Int. Endod. J. 2020, 53, 317–332. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Ren, C.; Jiang, Y.; Yan, J.; Wu, M. Clinical application of Er:YAG laser and traditional dental turbine in caries removal in children. J. Clin. Pediatr. Dent. 2024, 48, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Valério, R.A.; Borsatto, M.C.; Serra, M.C.; Polizeli, S.A.; Nemezio, M.A.; Galo, R.; Aires, C.P.; Dos Santos, A.C.; Corona, S.A. Caries removal in deciduous teeth using an Er:YAG laser: A randomized split-mouth clinical trial. Clin. Oral Investig. 2016, 20, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Wenyan, H.; Pujue, Z.; Yuhang, H.; Zhenni, L.; Yuejun, W.; Wenbin, W.; Ziling, L.; Pathak, J.L.; Sujuan, Z. The impact of Er:YAG laser combined with fluoride treatment on the supragingival plaque microbiome in children with multiple caries: A dynamic study. BMC Oral Health 2022, 22, 537. [Google Scholar] [CrossRef]
- Susila, A.V.; Sai, S.; Sharma, N.; Balasubramaniam, A.; Veronica, A.K.; Nivedhitha, S. Can natural irrigants replace sodium hypochlorite? A systematic review. Clin. Oral Investig. 2023, 27, 1831–1849. [Google Scholar] [CrossRef]
- Dumitrel, S.I.; Matichescu, A.; Dinu, S.; Buzatu, R.; Popovici, R.; Dinu, D.C.; Bratu, D.C. New Insights Regarding the Use of Relevant Synthetic Compounds in Dentistry. Molecules 2024, 29, 3802. [Google Scholar] [CrossRef]
- Coaguila-Llerena, H.; Raphael da Silva, L.; Faria, G. Research methods assessing sodium hypochlorite cytotoxicity: A scoping review. Heliyon 2023, 10, e23060. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Truman, B.; Ma, L.; Stewart, S.; Kingsley, K.; Sullivan, V. Assessment of Endocyn on Dental Pulp Stem Cells (DPSCs): A Pilot Study of Endodontic Irrigant Effects. Methods Protoc. 2025, 8, 18. [Google Scholar] [CrossRef] [PubMed]
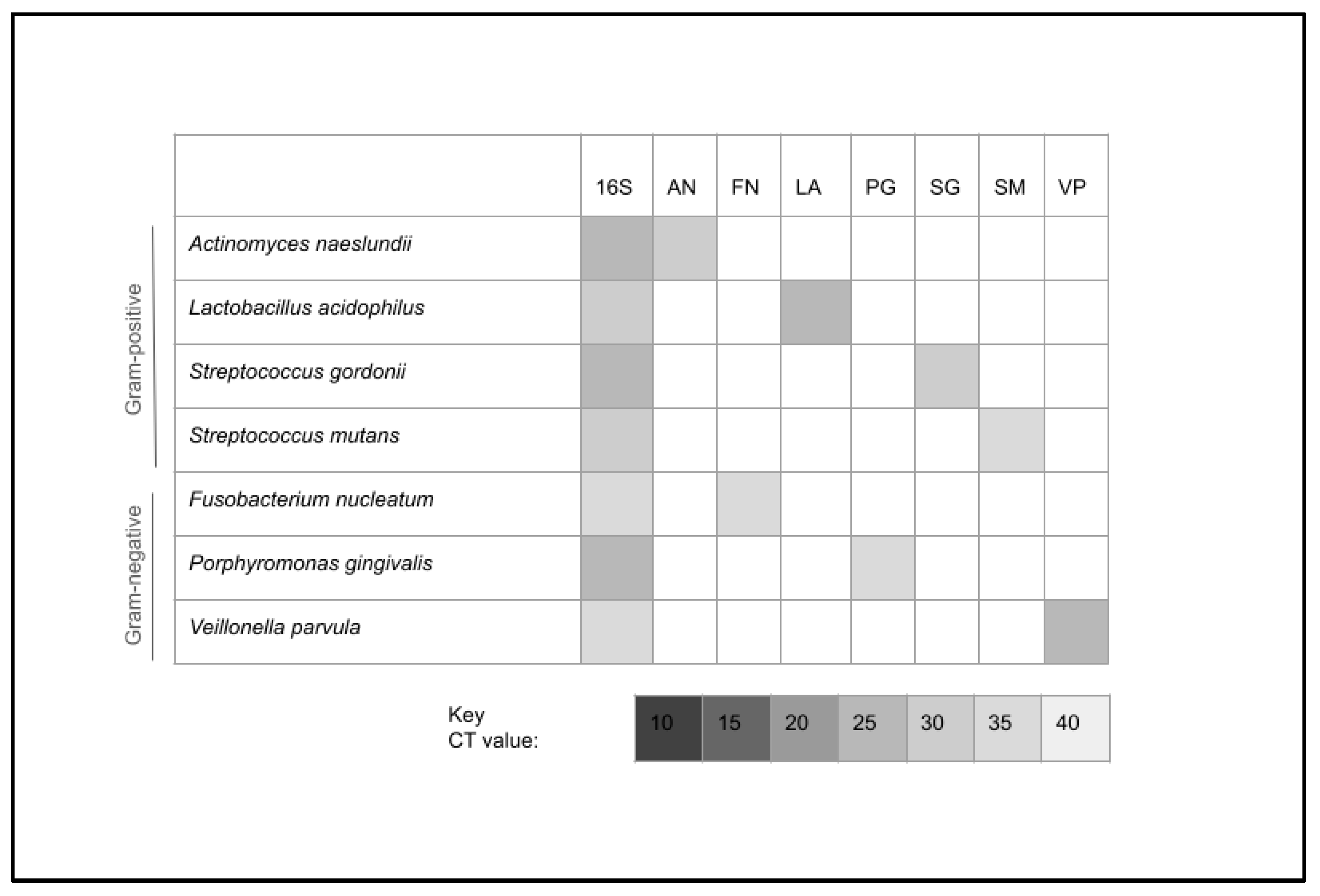
| Organism | Culture Medium | Requirements |
|---|---|---|
| Actinomyces naeslundii | Trypticase Soy Broth (TSB) | Anaerobic |
| Fusobacterium nucleatum | TSB with defibrinated sheep blood | Anaerobic |
| Lactobacillus acidophilus | DeMan, Rogosa, and Sharp broth | Anaerobic |
| Porphyromonas gingivalis | TSB with defibrinated sheep blood | Anaerobic |
| Streptococcus mutans | Brain–Heart Infusion (BHI) broth | Facultative |
| Streptococcus gordonii | TSB with defibrinated sheep blood | Anaerobic |
| Veillonella parvula | TSB with defibrinated sheep blood | Anaerobic |
| 16S rRNA forward primer | 5′-ACGCGTCGACAGAGTTTGATCCTGG-3′; 25 nt |
| 16S rRNA reverse primer | 5′-GGGACTACCAGGGTATCTAAT-3′; 21 nt |
| A. naeslundii forward primer | 5′-GTCTCAGTTCGGATCGGTGT-3′; 20 nt |
| A. naeslundii reverse primer | 5′-CCGGTACGGCTACCTTGTTA-3′; 20 nt |
| F. nucleatum forward primer | 5′-CGCAGAAGGTGAAAGTCCTGTAT-3′; 23 nt |
| F. nucleatum reverse primer | 5′-TGGTCCTCACTGATTCACACAGA-3′; 23 nt |
| L. acidophilus forward primer | 5′- AATTCTCTTCTCGGTCGCTCTA-3′; 22 nt |
| L. acidophilus reverse primer | 5′-CCTTTCTAAGGAAGCGAAGGAT-3′; 22 nt |
| P. gingivalis forward primer | 5′-TACCCATCGTCGCCTTGGT-3′; 19 nt |
| P. gingivalis reverse primer | 5′-CGGACTAAAACCGCATACACTTG-3′; 23 nt |
| S. mutans forward primer | 5′-GCCTACAGCTCAGAGATGCTATTCT-3′; 25 nt |
| S. mutans reverse primer | 5′-GCCATACACCACTCATGAATTGA-3′; 23 nt |
| S. gordonii forward primer | 5′-TGTACCCCGTATCGTTCCTGTG-3′; 22 nt |
| S. gordonii reverse primer | 5′-AAAGACTGGAGTTGCAATGTGAATA-3′; 25 nt |
| V. parvula forward primer | 5′-GGACAACGCTTGCCACCTA-3′; 19 nt |
| V. parvula reverse primer | 5′-GGTTACCTTGTTACGACTT-3′; 19 nt |
| Bacteria | Treatment Condition Response | Average |
|---|---|---|
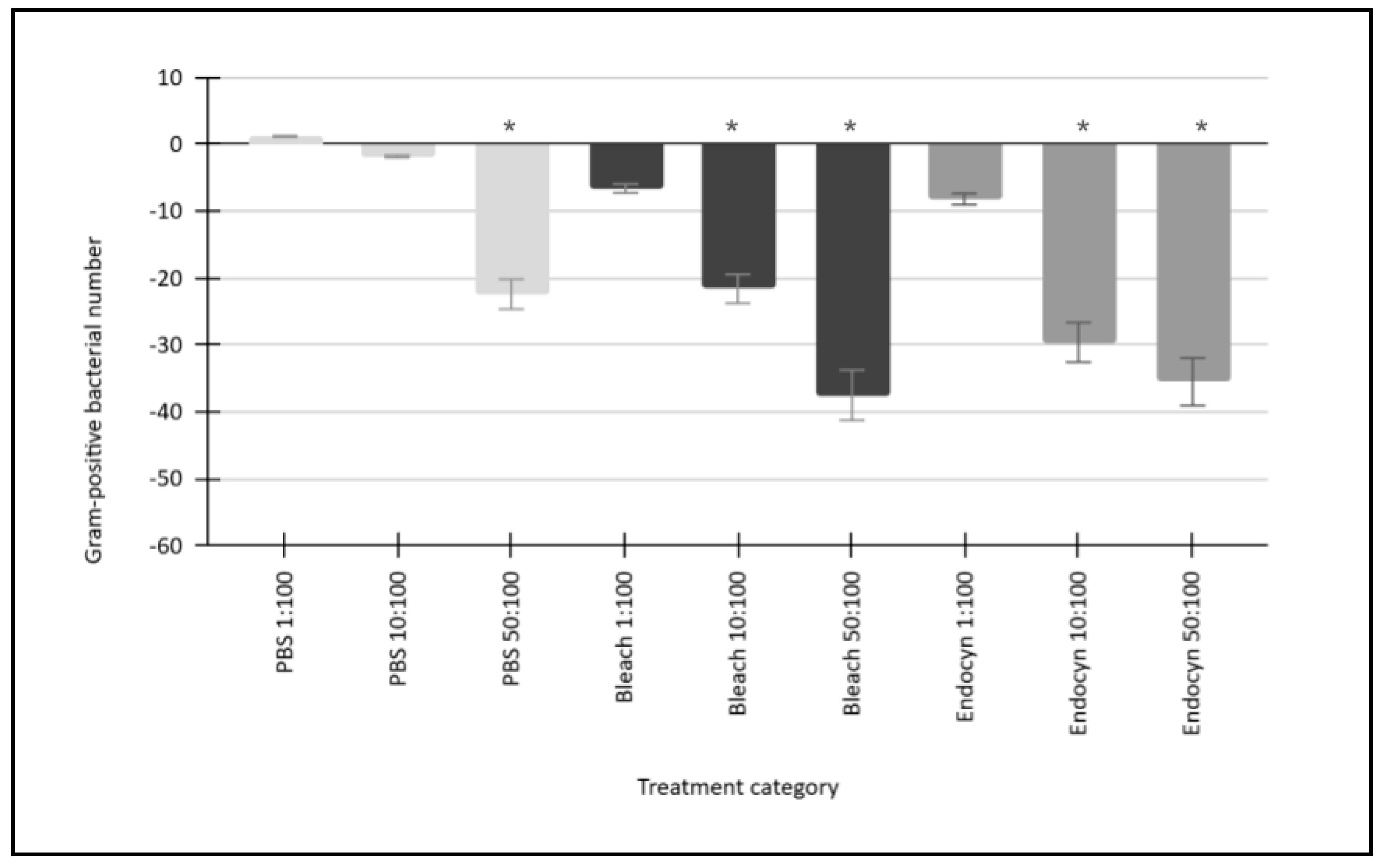
| Gram-positive bacteria: Actinomyces naeslundii Lactobacillus acidophilus Streptococcus mutans Streptococcus gordonii | [PBS 1:100] Growth 1.1% 1.3% 1.2% 1.1% | [PBS 1:100] Growth Gram-positive mean: 1.2% Standard deviation: 0.09 Median: 1.15% Minimum: 1.1% Maximum: 1.3% |
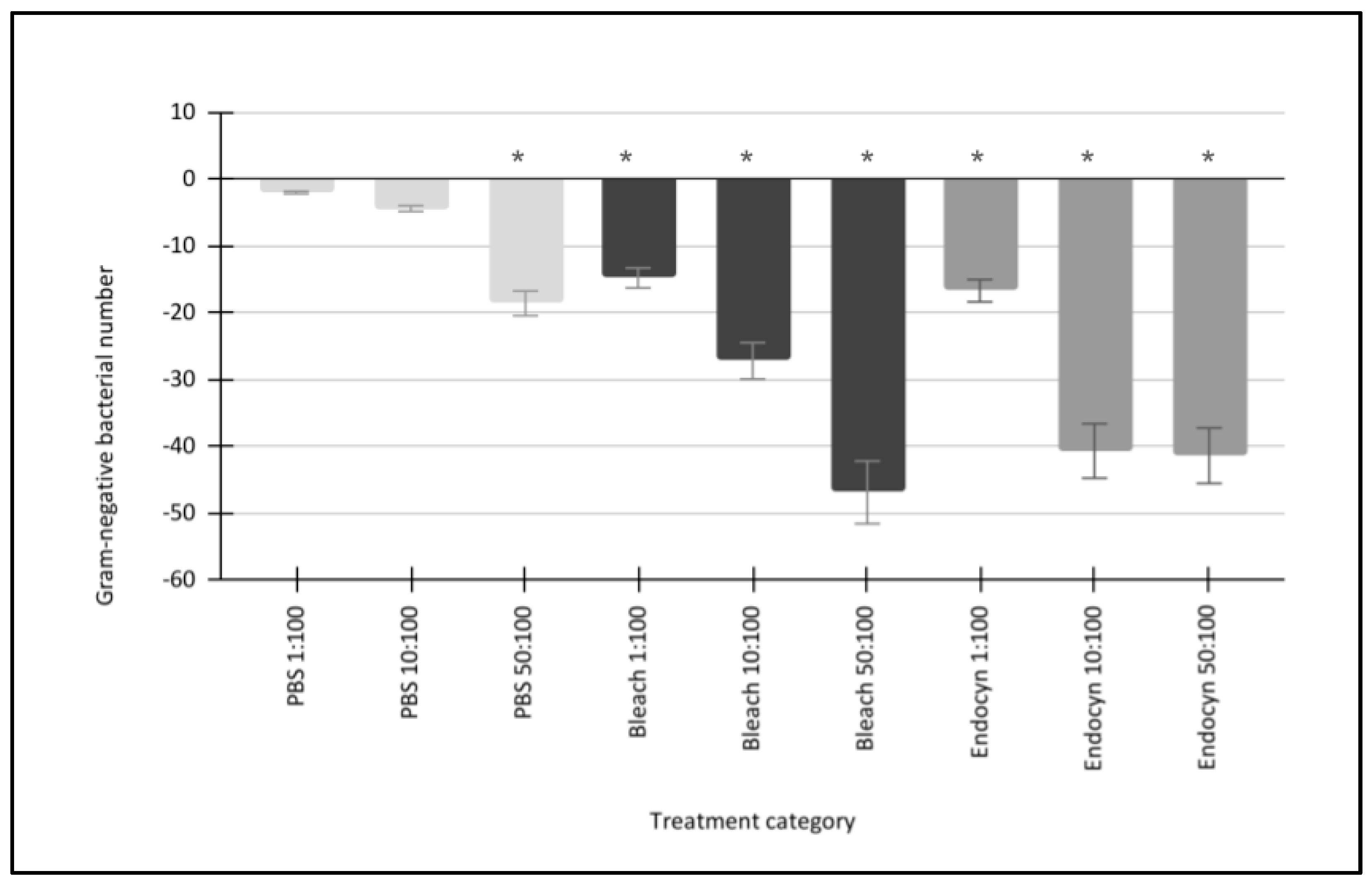
| Gram-negative bacteria: Fusobacterium nucleatum Porphyromonas gingivalis Veillonella parvula | [PBS 1:100] Growth −1.9% −2.0% −2.1% | [PBS 1:100] Growth Gram-negative mean: −2.0% Standard deviation: 0.1 Median: −2.0% Minimum: −1.9% Maximum: −2.1% |
| Gram-positive bacteria: Actinomyces naeslundii Lactobacillus acidophilus Streptococcus mutans Streptococcus gordonii | [PBS 10:100] Growth −1.9% −1.7% −1.9% −1.8% | [PBS 10:100] Growth Gram-positive mean: −1.8% Standard deviation: 0.09 Median: −1.85% Minimum: −1.7% Maximum: 1.9% |
| Gram-negative bacteria: Fusobacterium nucleatum Porphyromonas gingivalis Veillonella parvula | [PBS 10:100] Growth −4.4% −4.3% −4.5% | [PBS 10:100] Growth Gram-negative mean: −4.4% Standard deviation: 0.1 Median: −4.4% Minimum: −4.3% Maximum: −4.5% |
| Gram-positive bacteria: Actinomyces naeslundii Lactobacillus acidophilus Streptococcus mutans Streptococcus gordonii | [PBS 50:100] Growth −22.4% −22.6% −23.1% −21.3% | [PBS 50:100] Growth Gram-positive mean: −22.4% Standard deviation: 0.75 Median: −22.5% Minimum: −21.3% Maximum: −23.1% |
| Gram-negative bacteria: Fusobacterium nucleatum Porphyromonas gingivalis Veillonella parvula | [PBS 50:100] Growth −18.0% −19.1% −18.6% | [PBS 50:100] Growth Gram-negative mean: −18.6% Standard deviation: 0.55 Median: −18.6% Minimum: −18.0% Maximum −19.1% |
| Gram-positive bacteria: Actinomyces naeslundii Lactobacillus acidophilus Streptococcus mutans Streptococcus gordonii | [NaOCl 1:100] Growth −5.9% −6.8% −6.9% −6.7% | [NaOCl 1:100] Growth Gram-positive mean: −6.6% Standard deviation: 0.45 Median: −6.75% Minimum: −5.9% Maximum −6.9% |
| Gram-negative bacteria: Fusobacterium nucleatum Porphyromonas gingivalis Veillonella parvula | [NaOCl 1:100] Growth −14.1% −15.3% −14.9% | [NaOCl 1:100] Growth Gram-negative mean: −14.8% Standard deviation: 0.61 Median: −14.9% Minimum: −14.1% Maximum: −15.3% |
| Gram-positive bacteria: Actinomyces naeslundii Lactobacillus acidophilus Streptococcus mutans Streptococcus gordonii | [NaOCl 10:100] Growth −21.1% −22.2% −23.5% −19.6% | [NaOCl 10:100] Growth Gram-positive mean: −21.6% Standard deviation: 1.65 Median: −21.65% Minimum: −21.1% Maximum: −23.5% |
| Gram-negative bacteria: Fusobacterium nucleatum Porphyromonas gingivalis Veillonella parvula | [NaOCl 10:100] Growth −28.5% −26.2% −27.0% | [NaOCl 10:100] Growth Gram-negative mean: −27.2% Standard deviation: 1.17 Median: −27.0% Minimum: −26.2% Maximum: −28.5% |
| Gram-positive bacteria: Actinomyces naeslundii Lactobacillus acidophilus Streptococcus mutans Streptococcus gordonii | [NaOCl 50:100] Growth −38.2% −36.6% −37.6% −37.5% | [NaOCl 50:100] Growth Gram-positive mean: −37.5% Standard deviation: 0.66 Median: −37.55% Minimum: −36.6% Maximum: −38.2% |
| Gram-negative bacteria: Fusobacterium nucleatum Porphyromonas gingivalis Veillonella parvula | [NaOCl 50:100] Growth −47.2% −46.1% −47.3% | [NaOCl 50:100] Growth Gram-negative mean: −46.9% Standard deviation: 0.67 Median: −47.2% Minimum: −46.1% Maximum: −47.3% |
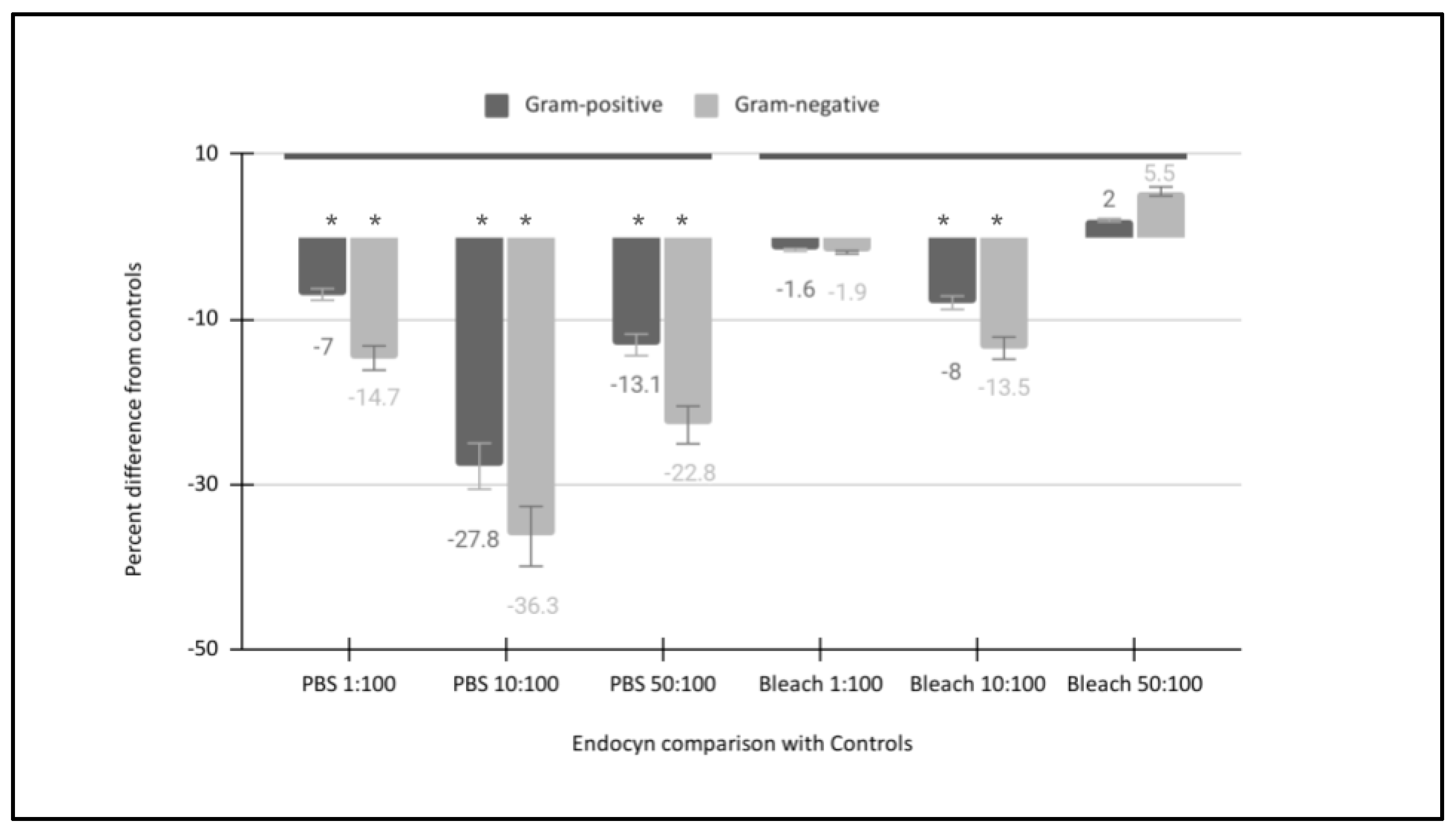
| Gram-positive bacteria: Actinomyces naeslundii Lactobacillus acidophilus Streptococcus mutans Streptococcus gordonii | [Endocyn 1:100] Growth −8.1% −8.3% −8.5% −7.9% | [Endocyn 1:100] Growth Gram-positive mean: −8.2% Standard deviation: 0.26 Median: −8.2% Minimum: −7.9% Maximum: −8.5% |
| Gram-negative bacteria: Fusobacterium nucleatum Porphyromonas gingivalis Veillonella parvula | [Endocyn 1:100] Growth −18.3% −15.5% −16.2% | [Endocyn 1:100] Growth Gram-negative mean: −16.7% Standard deviation: 1.46 Median: −16.2% Minimum: −15.5% Maximum: −18.3% |
| Gram-positive bacteria: Actinomyces naeslundii Lactobacillus acidophilus Streptococcus mutans Streptococcus gordonii | [Endocyn 10:100] Growth −31.4% −28.8% −28.5% −29.6% | [Endocyn 10:100] Growth Gram-positive mean: −29.6% Standard deviation: 1.30 Median: −29.2% Minimum: −28.8% Maximum: −31.4% |
| Gram-negative bacteria: Fusobacterium nucleatum Porphyromonas gingivalis Veillonella parvula | [Endocyn 10:100] Growth −41.2% −39.9% −40.9% | [Endocyn 10:100] Growth Gram-negative mean: −40.7% Standard deviation: 0.68 Median: −40.9% Minimum: −39.9% Maximum: −41.2% |
| Gram-positive bacteria: Actinomyces naeslundii Lactobacillus acidophilus Streptococcus mutans Streptococcus gordonii | [Endocyn 50:100] Growth −37.4% −34.8% −35.1% −34.8% | [Endocyn 50:100] Growth Gram-positive mean: −35.5% Standard deviation: 1.26 Median: −34.95% Minimum: −34.8% Maximum:−37.4% |
| Gram-negative bacteria: Fusobacterium nucleatum Porphyromonas gingivalis Veillonella parvula | [Endocyn 50:100] Growth −41.5% −40.9% −41.7% | [Endocyn 50:100] Growth Gram-negative mean: −41.4% Standard deviation: 0.42 Median: −41.5% Minimum: −40.9% Maximum: −41.7% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pearson, M.; Stewart, S.; Ma, L.; Kingsley, K.; Sullivan, V. Differential Antimicrobial Effects of Endodontic Irrigant Endocyn on Oral Bacteria. Hygiene 2025, 5, 11. https://doi.org/10.3390/hygiene5010011
Pearson M, Stewart S, Ma L, Kingsley K, Sullivan V. Differential Antimicrobial Effects of Endodontic Irrigant Endocyn on Oral Bacteria. Hygiene. 2025; 5(1):11. https://doi.org/10.3390/hygiene5010011
Chicago/Turabian StylePearson, Michael, Samuel Stewart, Linda Ma, Karl Kingsley, and Victoria Sullivan. 2025. "Differential Antimicrobial Effects of Endodontic Irrigant Endocyn on Oral Bacteria" Hygiene 5, no. 1: 11. https://doi.org/10.3390/hygiene5010011
APA StylePearson, M., Stewart, S., Ma, L., Kingsley, K., & Sullivan, V. (2025). Differential Antimicrobial Effects of Endodontic Irrigant Endocyn on Oral Bacteria. Hygiene, 5(1), 11. https://doi.org/10.3390/hygiene5010011







