Contamination of High-Touch Surfaces in the Ophthalmic Clinical Environment—A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Phase I—Circuit Simulation
2.2. Phase II—Evaluating Surface Contamination and Transmission
3. Results
4. Discussion
4.1. Contact Sequences and Possible Routes of UV Fluorescent Marker Transmission
4.2. Hand Hygiene and Disinfection of Ophthalmic Equipment
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Breathnach, A.S. Nosocomial infections and infection control. Medicine 2013, 41, 649–653. [Google Scholar] [CrossRef]
- Leung, N.H.L.; Chu, D.K.W.; Shiu, E.Y.C.; Chan, K.-H.; McDevitt, J.J.; Hau, B.J.P.; Yen, H.-L.; Li, Y.; Ip, D.K.M.; Peiris, J.S.M.; et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat. Med. 2020, 26, 676–680. [Google Scholar] [CrossRef] [PubMed]
- Felfeli, T.; Batawi, H.; Aldrees, S.; Hatch, W.; Mandelcorn, E.D. Utility of patient face masks to limit droplet spread from simulated coughs at the slit lamp. Can. J. Ophthalmol. 2020, 55, e163–e165. [Google Scholar] [CrossRef]
- Sobolewska, B.; Buhl, M.; Liese, J.; Ziemssen, F. Slit lamps and lenses: A potential source of nosocomial infections? Eye 2018, 32, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Chin, A.W.H.; Chu, J.T.S.; Perera, M.R.A.; Hui, K.P.Y.; Yen, H.-L.; Chan, M.C.W.; Peiris, M.; Poon, L.L.M. Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe 2020, 1, e10. [Google Scholar] [CrossRef]
- Han, Y.; Wu, N.; Zhu, W.; Li, Y.; Zuo, L.; Ye, J.; Qiu, Z.; Xie, J.; Li, T. Detection of HIV-1 viruses in tears of patients even under long-term HAART. Aids 2011, 25, 1925–1927. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, H.E.; Azcuy, A.M.; Varnell, E.D.; Sloop, G.D.; Thompson, H.W.; Hill, J.M. HSV-1 DNA in Tears and Saliva of Normal Adults. Investig. Ophthalmol. Vis. Sci. 2005, 46, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Lakkis, C.; Lian, K.; Napper, G.; Kiely, P.M. Infection control guidelines for optometrists 2007. Clin. Exp. Optom. 2007, 90, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Moniz, E.; Feldman, F.; Newkirk, M.; Feinman, S.V.; Berris, B. Removal of Hepatitis B Surface Antigen from a Contaminated Applanation Tonometer. Am. J. Ophthalmol. 1981, 91, 522–525. [Google Scholar] [CrossRef]
- Lim, L.W.; Yip, L.W.; Tay, H.W.; Ang, X.L.; Lee, L.K.; Chin, C.F.; Yong, V. Sustainable practice of ophthalmology during COVID-19: Challenges and solutions. Graefe’s Arch. Clin. Exp. Ophthalmol. 2020, 258, 1427–1436. [Google Scholar] [CrossRef]
- Chan, C.X.C.; Lau, B.Y.Y.; Ng, X.L.; Lim, D.K.A.; Lim, B.X.H.; Lim, C.H.L. Beware what lurks on the surface–persistent contamination of high-touch surfaces on slit lamps despite regular cleaning. J. Infect. Prev. 2022, 23, 186–189. [Google Scholar] [CrossRef] [PubMed]
- Reem, R.E.; Van Balen, J.; Hoet, A.E.; Cebulla, C.M. Screening and characterization of Staphylococcus aureus from ophthalmology clinic surfaces: A proposed surveillance tool. Am. J. Ophthalmol. 2014, 157, 781–787.e2. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Otter, J.A.; Yezli, S.; Salkeld, J.A.G.; French, G.L. Evidence that contaminated surfaces contribute to the transmission of hospital pathogens and an overview of strategies to address contaminated surfaces in hospital settings. Am. J. Infect. Control 2013, 41, S6–S11. [Google Scholar] [CrossRef] [PubMed]
- Jinadatha, C.; Villamaria, F.C.; Coppin, J.D.; Dale, C.R.; Williams, M.D.; Whitworth, R.; Stibich, M. Interaction of healthcare worker hands and portable medical equipment: A sequence analysis to show potential transmission opportunities. BMC Infect. Dis. 2017, 17, 800. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Xiao, S.; Cowling, B.J.; Li, Y. Hand hygiene and surface cleaning should be paired for prevention of fomite transmission. Indoor Air 2020, 30, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Kwok, Y.L.A.; Gralton, J.; McLaws, M.-L. Face touching: A frequent habit that has implications for hand hygiene. Am. J. Infect. Control 2015, 43, 112–114. [Google Scholar] [CrossRef]
- Boyce, J.M.; Havill, N.L.; Dumigan, D.G.; Golebiewski, M.; Balogun, O.; Rizvani, R. Monitoring the effectiveness of hospital cleaning practices by use of an adenosine triphosphate bioluminescence assay. Infect. Control Hosp. Epidemiol. 2009, 30, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Randle, J.; Arthur, A.; Vaughan, N. Twenty-four-hour observational study of hospital hand hygiene compliance. J. Hosp. Infect. 2010, 76, 252–255. [Google Scholar] [CrossRef] [PubMed]
- FitzGerald, G.; Moore, G.; Wilson, A.P.R. Hand hygiene after touching a patient’s surroundings: The opportunities most commonly missed. J. Hosp. Infect. 2013, 84, 27–31. [Google Scholar] [CrossRef]
- Gordon, N.C. Infection control for safety and quality. Community Eye Health 2021, 34, 5–7. [Google Scholar]
- Junk, A.K.; Chen, P.P.; Lin, S.C.; Nouri-Mahdavi, K.; Radhakrishnan, S.; Singh, K.; Chen, T.C. Disinfection of Tonometers: A Report by the American Academy of Ophthalmology. Ophthalmology 2017, 124, 1867–1875. [Google Scholar] [CrossRef]
- Chodosh, J.; Holland, G.N.; Yeh, S. Important Coronavirus Updates for Ophthalmologists; American Academy of Ophthalmology: San Francisco, CA, USA, 2020; Available online: https://www.aao.org/headline/d6e1ca3c-0c30-4b20-87e0-7668fa5bf906 (accessed on 24 May 2021).
- Kampf, G.; Todt, D.; Pfaender, S.; Steinmann, E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020, 104, 246–251. [Google Scholar] [CrossRef]
- Kundrapu, S.; Sunkesula, V.; Jury, L.A.; Sitzlar, B.M.; Donskey, C.J. Daily Disinfection of High-Touch Surfaces in Isolation Rooms to Reduce Contamination of Healthcare Workers’ Hands. Infect. Control Hosp. Epidemiol. 2012, 33, 1039–1042. [Google Scholar] [CrossRef]
- Sun, C.; Wang, Y.; Liu, G.; Liu, Z. Role of the Eye in Transmitting Human Coronavirus: What We Know and What We Do Not Know. Front. Public Health 2020, 8, 155. [Google Scholar] [CrossRef]
- Boyce, J.M.; Pittet, D. Guideline for Hand Hygiene in Health-Care Settings. Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. MMWR Recomm. Rep. 2002, 51, 1–45. [Google Scholar] [CrossRef]
- Leslie, R.A.; Zhou, S.S.; Macinga, D.R. Inactivation of SARS-CoV-2 by commercially available alcohol-based hand sanitizers. Am. J. Infect. Control 2021, 49, 401–402. [Google Scholar] [CrossRef]
- McLaughlin, A.C.; Walsh, F. Self-reported reasons for hand hygiene in 3 groups of health care workers. Am. J. Infect. Control 2012, 40, 653–658. [Google Scholar] [CrossRef]
- Marchesi, I.; Sala, A.; Frezza, G.; Paduano, S.; Turchi, S.; Bargellini, A.; Borella, P.; Cermelli, C. In vitro virucidal efficacy of a dry steam disinfection system against Human Coronavirus, Human Influenza Virus, and Echovirus. J. Occup. Environ. Hyg. 2021, 18, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Schneider, P.M. New technologies and trends in sterilization and disinfection. Am. J. Infect. Control 2013, 41, S81–S86. [Google Scholar] [CrossRef]
- Dancer, S.J.; King, M.F. Systematic review on use, cost and clinical efficacy of automated decontamination devices. Antimicrob. Resist. Infect. Control 2021, 10, 34. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.J.; Anderson, D.; Rutala, W.A. The role of the surface environment in healthcare-associated infections. Curr. Opin. Infect. Dis. 2013, 26, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gong, X.; Wang, Z.; Chen, R.; Li, T.; Zeng, D.; Li, M. Clinical features of familial clustering in patients infected with 2019 novel coronavirus in Wuhan, China. Virus Res. 2020, 286, 198043. [Google Scholar] [CrossRef] [PubMed]


| Station | Action Sequence |
|---|---|
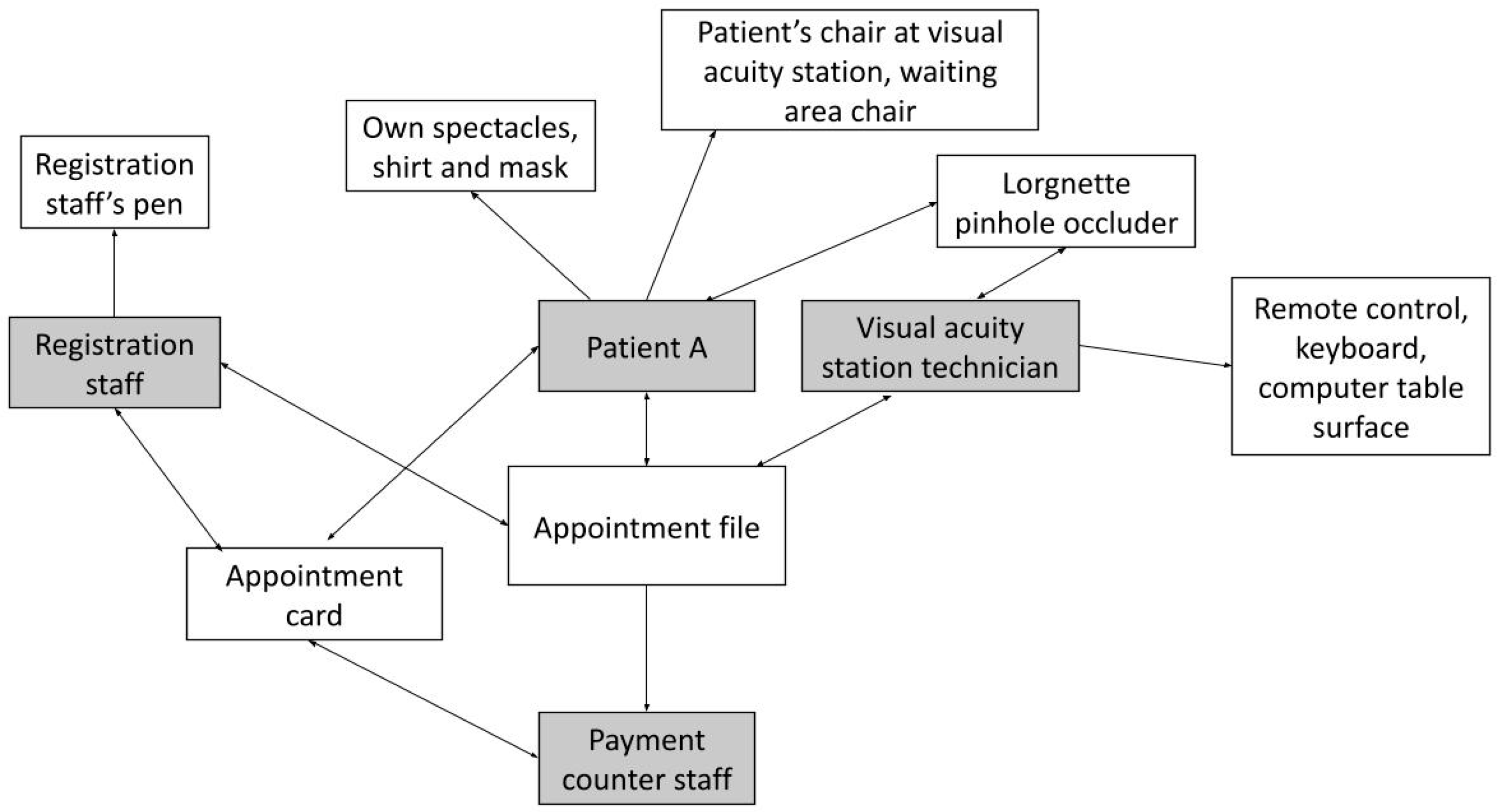
| Registration | 1. Patient A passes the appointment card to the registration staff. 2. Registration staff takes the appointment card, then hands the card, an appointment file and an appointment number slip to Patient A. |
| Visual Acuity Station | 1. Patient A walks to the visual acuity station and passes the appointment file to the visual acuity station technician. 2. The technician hands a Lorgnette pinhole occluder to Patient A. 3. Patient A holds the Lorgnette pinhole occluder to the eyes and is assessed. 4. The technician uses a remote control to switch between different Snellen charts. 5. The technician takes the occluder back from Patient A without cleaning, uses the computer keyboard to type the findings and hands the appointment file back to Patient A. |
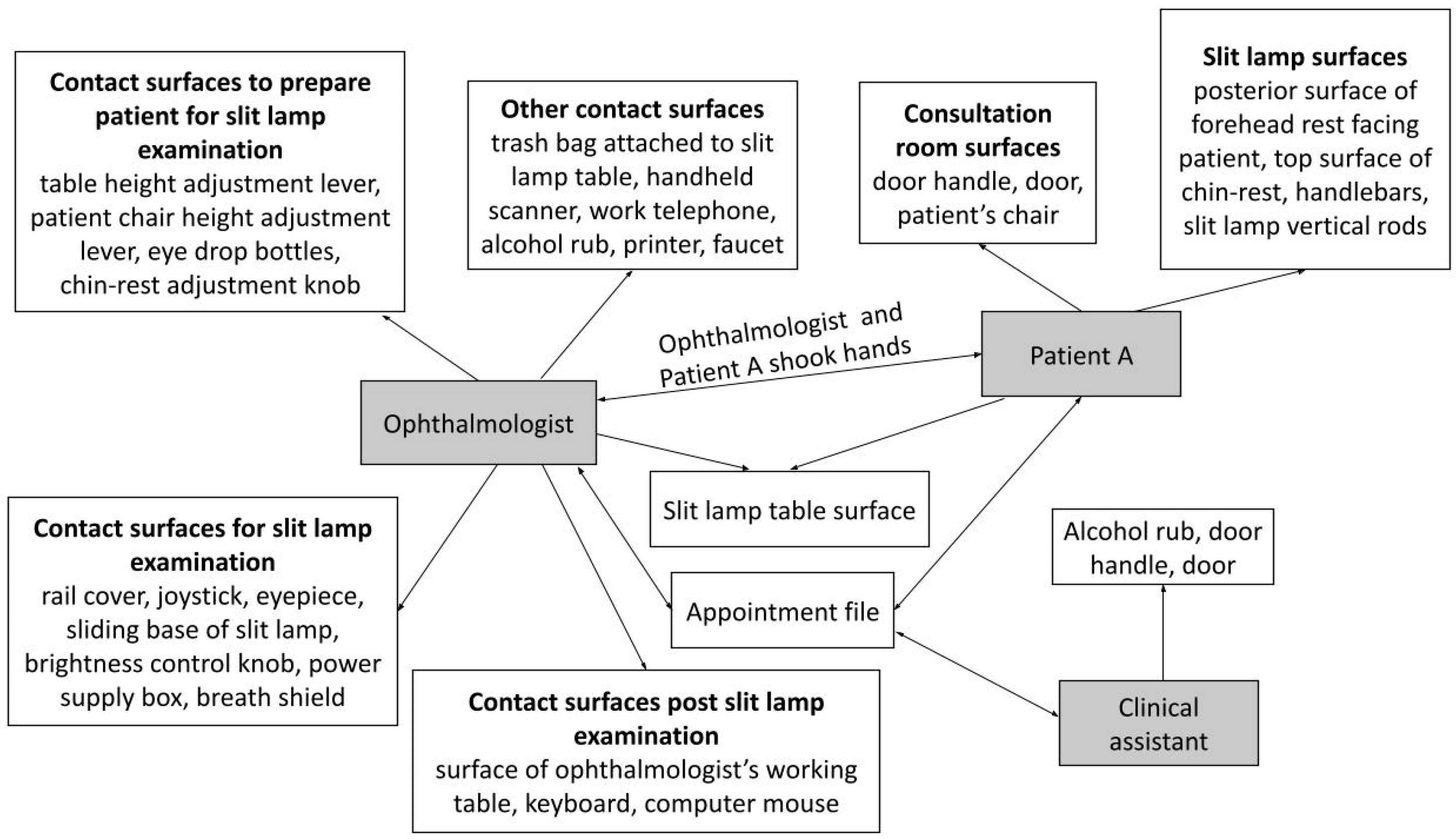
| Consultation Room | 1. Patient A slides the appointment file into a slot outside the consultation room and sits in the waiting area. 2. Consultation room clinical assistant picks up the file and gives the file to the ophthalmologist, before calling for Patient A to enter the consultation room. 3. The ophthalmologist uses a handheld scanner to scan a barcode on the documents in the file. 4. The ophthalmologist positions Patient A for a slit lamp examination—chin on the chin-rest, forehead against the forehead rest and hands holding the handlebars. 5. The ophthalmologist conducts a slit lamp examination on Patient A. 6. The ophthalmologist uses the computer mouse and keyboard to type the relevant notes. 7. The ophthalmologist returns the appointment file and card to the patient. 8. Items such as the printer, work telephone and alcohol rub were used when necessary. |
| Payment | 1. Patient A returns the appointment file to the payment counter staff. 2. Payment counter staff hands the appointment card back to Patient A. |
| Individuals whose hands were contaminated | 1. Patient A 2. Patient B 3. Registration staff 4. Consultation room clinical assistant | 5. Ophthalmologist 6. Visual acuity station technician 7. Payment counter staff |
| Patients’ contaminated surfaces | 1. Patient A’s surgical mask 2. Patient A’s spectacles 3. Patient A’s shirt | 4. Patient B’s surgical mask 5. Patient B’s shirt |
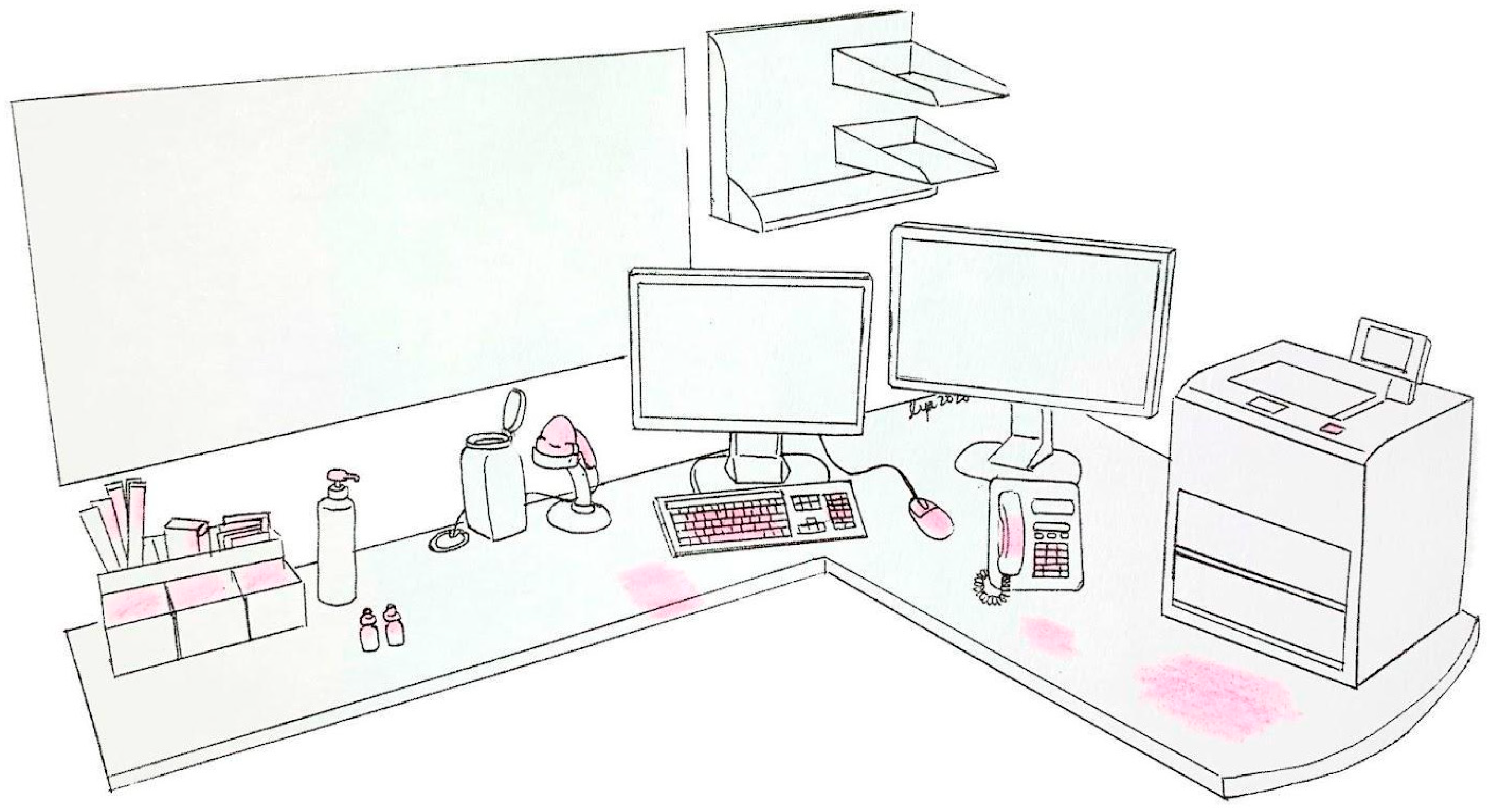
| Contaminated surfaces in the consultation room | 1. Surface of ophthalmologist’s working table 2. Keyboard 3. Computer mouse 4. Consultation room door and door handle 5. Backrest of patient’s chair in consultation room 6. Edge of sink basin | 7. Base of the sink (near the sink drain) 8. Water tap handle 9. Patient chair height adjustment lever 10. Handheld scanner 11. Eye drop bottles 12. Work telephone 13. Alcohol rub 14. Printer |
| Contaminated surfaces at the visual acuity station | 1. Backrest of patient’s chair 2. Lorgnette pinhole occluder: eyepiece and handle | 3. Remote control 4. Keyboard 5. Computer table surface |
| Contaminated surfaces on the slit lamp | 1. Posterior surface of forehead rest contacted by patients 2. Top surface of chin-rest contacted by patients 3. Handlebars 4. Slit lamp rail cover 5. Chin-rest adjustment knob 6. Slit lamp vertical rods 7. Joystick 8. Breath shield | 9. Eyepiece 10. Sliding base of slit lamp 11. Brightness control knob 12. Power supply box 13. Table height adjustment lever 14. Slit lamp table surface (top and side) 15. Inferior surface of slit lamp table (next to table height adjustment lever) 16. Trash bag attached to the slit lamp table |
| Miscellaneous | 1. Appointment card and file 2. Backrest of waiting area chair | 3. Registration staff’s pen |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lau, B.Y.Y.; Chan, C.X.C.; Ng, X.L.; Lim, D.K.A.; Lim, B.X.H.; Lim, C.H.L. Contamination of High-Touch Surfaces in the Ophthalmic Clinical Environment—A Pilot Study. Hygiene 2024, 4, 258-268. https://doi.org/10.3390/hygiene4030021
Lau BYY, Chan CXC, Ng XL, Lim DKA, Lim BXH, Lim CHL. Contamination of High-Touch Surfaces in the Ophthalmic Clinical Environment—A Pilot Study. Hygiene. 2024; 4(3):258-268. https://doi.org/10.3390/hygiene4030021
Chicago/Turabian StyleLau, Berdjette Y. Y., Cassandra X. C. Chan, Xin Le Ng, Dawn K. A. Lim, Blanche X. H. Lim, and Chris H. L. Lim. 2024. "Contamination of High-Touch Surfaces in the Ophthalmic Clinical Environment—A Pilot Study" Hygiene 4, no. 3: 258-268. https://doi.org/10.3390/hygiene4030021
APA StyleLau, B. Y. Y., Chan, C. X. C., Ng, X. L., Lim, D. K. A., Lim, B. X. H., & Lim, C. H. L. (2024). Contamination of High-Touch Surfaces in the Ophthalmic Clinical Environment—A Pilot Study. Hygiene, 4(3), 258-268. https://doi.org/10.3390/hygiene4030021






