Gastric Cancer Epidemiology: Current Trend and Future Direction
Abstract
1. Introduction
2. Materials and Methods
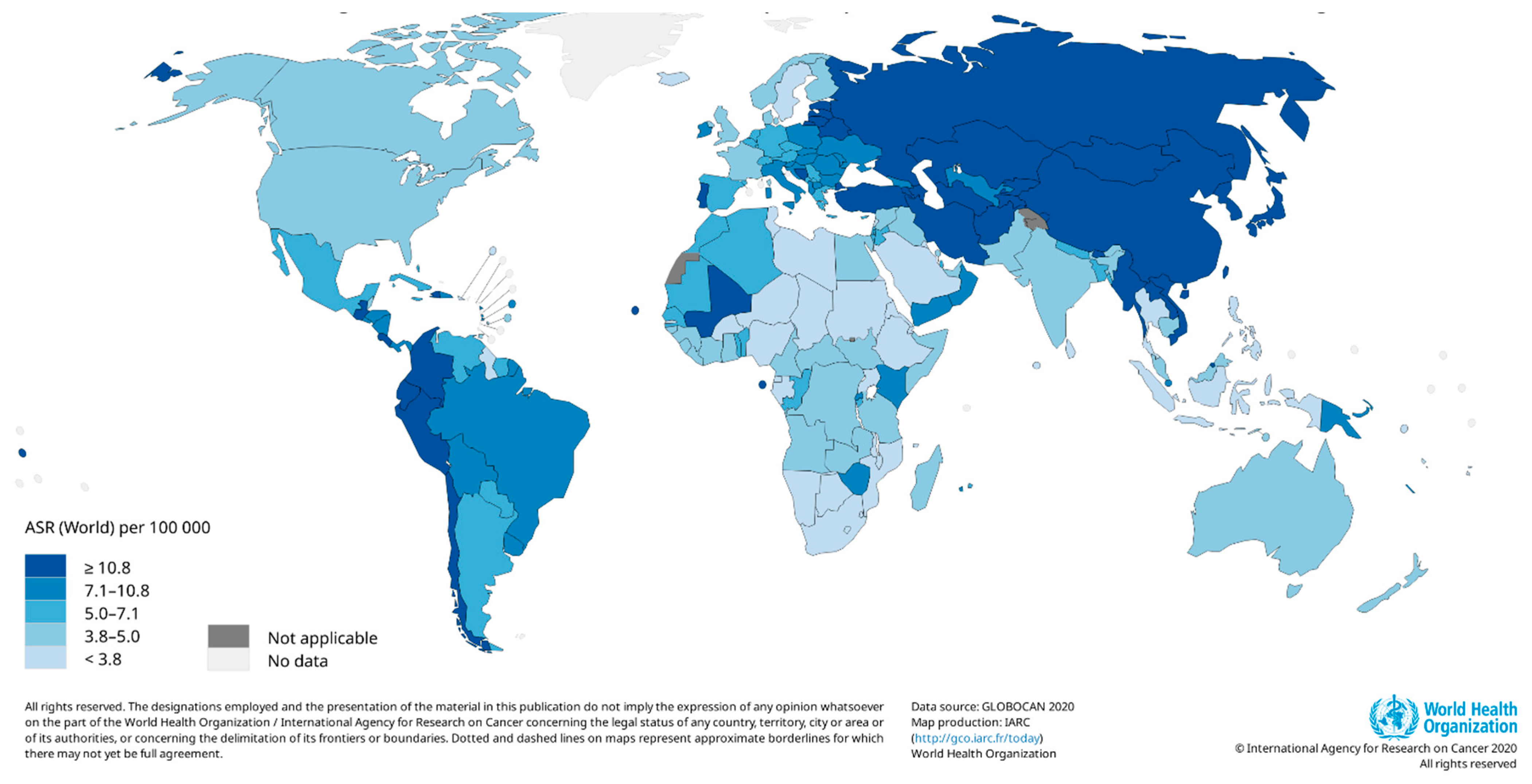
3. Current Global Trends in Measures of Gastric Cancer
4. Etiology and Known Risk Factors of Gastric Cancer
4.1. Helicobacter Pylori
4.2. Other Risk Factors and Susceptible Population
5. Pathogenesis and Hallmarks of Gastric Cancer
6. Treatment and Prevention of Gastric Cancer
7. Study Strengths and Limitations
8. Conclusions and Future Direction
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Arnold, M.; Rutherford, M.J.; Bardot, A.; Ferlay, J.; Andersson, T.M.L.; Myklebust, T.Å.; Tervonen, H.; Thursfield, V.; Ransom, D.; Shack, L.; et al. Progress in Cancer Survival, Mortality, and Incidence in Seven High-Income Countries 1995-2014 (ICBP SURVMARK-2): A Population-Based Study. Lancet Oncol. 2019, 20, 1493–1505. [Google Scholar] [CrossRef]
- Arnold, M.; Park, J.Y.; Camargo, M.C.; Lunet, N.; Forman, D.; Soerjomataram, I. Is Gastric Cancer Becoming a Rare Disease? A Global Assessment of Predicted Incidence Trends to 2035. Gut 2020, 69, 823–829. [Google Scholar] [CrossRef]
- Stock, M.; Otto, F. Gene Deregulation in Gastric Cancer. Gene 2005, 360, 1–19. [Google Scholar] [CrossRef]
- Arnold, M.; Ferlay, J.; Van Berge Henegouwen, M.I.; Soerjomataram, I. Global Burden of Oesophageal and Gastric Cancer by Histology and Subsite in 2018. Gut 2020, 69, 1564–1571. [Google Scholar] [CrossRef]
- Rawla, P.; Barsouk, A. Epidemiology of Gastric Cancer: Global Trends, Risk Factors and Prevention. Gastroenterol. Rev. Gastroenterol. 2018, 14, 26–38. [Google Scholar] [CrossRef]
- Fay, S. A Spotlight on Stomach Cancer Subtypes—WCRF International. Available online: https://www.wcrf.org/a-spotlight-on-stomach-cancer-subtypes/ (accessed on 25 June 2023).
- Plummer, M.; de Martel, C.; Vignat, J.; Ferlay, J.; Bray, F.; Franceschi, S. Global Burden of Cancers Attributable to Infections in 2012: A Synthetic Analysis. Lancet Glob. Health 2016, 4, e609–e616. [Google Scholar] [CrossRef]
- IARC International Agency for Research on Cancer. Global Cancer Observatory. Available online: https://gco.iarc.fr/ (accessed on 5 March 2023).
- Morgan, E.; Arnold, M.; Camargo, M.C.; Gini, A.; Kunzmann, A.T.; Matsuda, T.; Meheus, F.; Verhoeven, R.H.A.; Vignat, J.; Laversanne, M.; et al. The Current and Future Incidence and Mortality of Gastric Cancer in 185 Countries, 2020–2040: A Population-Based Modelling Study. EClinicalMedicine 2022, 47, 101404. [Google Scholar] [CrossRef]
- Yamaoka, Y.; Kato, M.; Asaka, M. Geographic Differences in Gastric Cancer Incidence Can Be Explained by Differences between Helicobacter Pylori Strains. Intern. Med. 2008, 47, 1077. [Google Scholar] [CrossRef]
- Maskarinec, G.; Noh, J.J. The Effect of Migration on Cancer Incidence among Japanese in Hawaii. Ethn. Dis. 2004, 14, 431–439. [Google Scholar]
- IARC International Agency for Research on Cancer. Schistosomes, Liver Flukes and Helicobacter Pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7–14 June 1994. IARC Monogr. Eval. Carcinog. Risks Hum. 1994, 61, 177–240. [Google Scholar]
- Kayali, S.; Manfredi, M.; Gaiani, F.; Bianchi, L.; Bizzarri, B.; Leandro, G.; Di Mario, F.; De’angelis, G.L. Helicobacter Pylori, Transmission Routes and Recurrence of Infection: State of the Art. Acta Bio Medica Atenei Parm. 2018, 89, 72. [Google Scholar] [CrossRef]
- Bravo, D.; Hoare, A.; Soto, C.; Valenzuela, M.A.; Quest, A.F. Helicobacter Pylori in Human Health and Disease: Mechanisms for Local Gastric and Systemic Effects. World J. Gastroenterol. 2018, 24, 3071. [Google Scholar] [CrossRef] [PubMed]
- Fagoonee, S.; Pellicano, R. Helicobacter Pylori: Molecular Basis for Colonization and Survival in Gastric Environment and Resistance to Antibiotics. A Short Review. Infect. Dis. 2019, 51, 399–408. [Google Scholar] [CrossRef]
- Espinoza, J.L.; Matsumoto, A.; Tanaka, H.; Matsumura, I. Gastric Microbiota: An Emerging Player in Helicobacter Pylori-Induced Gastric Malignancies. Cancer Lett. 2018, 414, 147–152. [Google Scholar] [CrossRef]
- Backert, S.; Tegtmeyer, N.; Fischer, W. Composition, Structure and Function of the Helicobacter Pylori Cag Pathogenicity Island Encoded Type IV Secretion System. Future Microbiol. 2015, 10, 955–965. [Google Scholar] [CrossRef]
- Maleki Kakelar, H.; Barzegari, A.; Dehghani, J.; Hanifian, S.; Saeedi, N.; Barar, J.; Omidi, Y. Pathogenicity of Helicobacter Pylori in Cancer Development and Impacts of Vaccination. Gastric Cancer 2018, 22, 23–36. [Google Scholar] [CrossRef]
- Chauhan, N.; Tay, A.C.Y.; Marshall, B.J.; Jain, U. Helicobacter Pylori VacA, a Distinct Toxin Exerts Diverse Functionalities in Numerous Cells: An Overview. Helicobacter 2019, 24, e12544. [Google Scholar] [CrossRef]
- Cover, T.L. Helicobacter Pylori Diversity and Gastric Cancer Risk. MBio 2016, 7, e01869-15. [Google Scholar] [CrossRef]
- Alipour, M. Molecular Mechanism of Helicobacter Pylori-Induced Gastric Cancer. J. Gastrointest. Cancer 2021, 52, 23–30. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Megraud, F.; O’Morain, C.; Gisbert, J.P.; Kuipers, E.J.; Axon, A.; Bazzoli, F.; Gasbarrini, A.; Atherton, J.; Graham, D.Y.; et al. Management of Helicobacter Pylori Infection—The Maastricht V/Florence Consensus Report. Gut 2017, 66, 6–30. [Google Scholar] [CrossRef] [PubMed]
- Chey, W.D.; Leontiadis, G.I.; Howden, C.W.; Moss, S.F. ACG Clinical Guideline: Treatment of Helicobacter Pylori Infection. Am. J. Gastroenterol. 2017, 112, 212–238. [Google Scholar] [CrossRef]
- Iizasa, H.; Nanbo, A.; Nishikawa, J.; Jinushi, M.; Yoshiyama, H. Epstein-Barr Virus (EBV)-Associated Gastric Carcinoma. Viruses 2012, 4, 3420–3439. [Google Scholar] [CrossRef] [PubMed]
- Camargo, M.C.; Murphy, G.; Koriyama, C.; Pfeiffer, R.M.; Kim, W.H.; Herrera-Goepfert, R.; Corvalan, A.H.; Carrascal, E.; Abdirad, A.; Anwar, M.; et al. Determinants of Epstein-Barr Virus-Positive Gastric Cancer: An International Pooled Analysis. Br. J. Cancer 2011, 105, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Jia, K.; Lv, H.; Wang, S.Q.; Wu, Y.; Lei, H.; Chen, X. EBV-Positive Gastric Cancer: Current Knowledge and Future Perspectives. Front. Oncol. 2020, 10, 583463. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shao, L.; Liu, X.; Ji, F.; Mei, Y.; Cheng, Y.; Liu, F.; Yan, C.; Li, L.; Ling, Z. Alterations of Gastric Mucosal Microbiota across Different Stomach Microhabitats in a Cohort of 276 Patients with Gastric Cancer. EBioMedicine 2019, 40, 336–348. [Google Scholar] [CrossRef]
- Hu, Y.L.; Pang, W.; Huang, Y.; Zhang, Y.; Zhang, C.J. The Gastric Microbiome Is Perturbed in Advanced Gastric Adenocarcinoma Identified through Shotgun Metagenomics. Front. Cell. Infect. Microbiol. 2018, 8, 433. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.; Peng, Y.; Tong, L.; Feng, L.; Ma, K. Characterization of the Diethyl Phthalate-Degrading Bacterium Sphingobium Yanoikuyae SHJ. Data Br. 2018, 20, 1758–1763. [Google Scholar] [CrossRef]
- Zhao, Q.; Hu, H.; Wang, W.; Peng, H.; Zhang, X. Genome Sequence of Sphingobium Yanoikuyae B1, a Polycyclic Aromatic Hydrocarbon-Degrading Strain. Genome Announc. 2015, 3, 1522–1536. [Google Scholar] [CrossRef]
- Schulz, C.; Schütte, K.; Mayerle, J.; Malfertheiner, P. The Role of the Gastric Bacterial Microbiome in Gastric Cancer: Helicobacter Pylori and Beyond. Ther. Adv. Gastroenterol. 2019, 12, 1–11. [Google Scholar] [CrossRef]
- Baghban, A.; Gupta, S. Parvimonas Micra: A Rare Cause of Native Joint Septic Arthritis. Anaerobe 2016, 39, 26–27. [Google Scholar] [CrossRef]
- Coker, O.O.; Dai, Z.; Nie, Y.; Zhao, G.; Cao, L.; Nakatsu, G.; Wu, W.K.; Wong, S.H.; Chen, Z.; Sung, J.J.Y.; et al. Mucosal Microbiome Dysbiosis in Gastric Carcinogenesis. Gut 2018, 67, 1024–1032. [Google Scholar] [CrossRef]
- Pinheiro, H.; Oliveira, C.; Seruca, R.; Carneiro, F. Hereditary Diffuse Gastric Cancer—Pathophysiology and Clinical Management. Best Pract. Res. Clin. Gastroenterol. 2014, 28, 1055–1068. [Google Scholar] [CrossRef]
- Corso, G.; Marrelli, D. Frequency of Familial Gastric Cancer. In Spotlight on Familial and Hereditary Gastric Cancer; Springer: Dordrecht, The Netherlands, 2013; pp. 11–18. [Google Scholar]
- Aird, I.; Bentall, H.H.; Roberts, J.A.F. A Relationship between Cancer of Stomach and the ABO Blood Groups. Br. Med. J. 1953, 1, 799–801. [Google Scholar] [CrossRef]
- Yu, H.; Xu, N.; Li, Z.K.; Xia, H.; Ren, H.T.; Li, N.; Wei, J.B.; Bao, H.Z. Association of ABO Blood Groups and Risk of Gastric Cancer. Scand. J. Surg. 2020, 109, 309–313. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, L.; Ji, J.; Zhang, J.; Yan, M.; Zhang, J.; Liu, B.; Zhu, Z.; Yu, Y. ABO Blood Group System and Gastric Cancer: A Case-Control Study and Meta-Analysis. Int. J. Mol. Sci. 2012, 13, 13308–13321. [Google Scholar] [CrossRef]
- Edgren, G.; Hjalgrim, H.; Rostgaard, K.; Norda, R.; Wikman, A.; Melbye, M.; Nyrén, O. Risk of Gastric Cancer and Peptic Ulcers in Relation to ABO Blood Type: A Cohort Study. Am. J. Epidemiol. 2010, 172, 1280–1285. [Google Scholar] [CrossRef] [PubMed]
- Vasan, S.K.; Hwang, J.; Rostgaard, K.; Nyrén, O.; Ullum, H.; Pedersen, O.B.V.; Erikstrup, C.; Melbye, M.; Hjalgrim, H.; Pawitan, Y.; et al. ABO Blood Group and Risk of Cancer: A Register-Based Cohort Study of 1.6 Million Blood Donors. Cancer Epidemiol. 2016, 44, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Paré, G.; Chasman, D.I.; Kellogg, M.; Zee, R.Y.L.; Rifai, N.; Badola, S.; Miletich, J.P.; Ridker, P.M. Novel Association of ABO Histo-Blood Group Antigen with Soluble ICAM-1: Results of a Genome-Wide Association Study of 6578 Women. PLoS Genet. 2008, 4, e1000118. [Google Scholar] [CrossRef]
- Wu, C.C.; Hsu, T.W.; Chang, C.M.; Yu, C.H.; Wang, Y.F.; Lee, C.C. The Effect of Individual and Neighborhood Socioeconomic Status on Gastric Cancer Survival. PLoS ONE 2014, 9, e89655. [Google Scholar] [CrossRef]
- Gupta, S.; Tao, L.; Murphy, J.D.; Camargo, M.C.; Oren, E.; Valasek, M.A.; Gomez, S.L.; Martinez, M.E. Race/Ethnicity-, Socioeconomic Status-, and Anatomic Subsite-Specific Risks for Gastric Cancer. Gastroenterology 2019, 156, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Uthman, O.A.; Jadidi, E.; Moradi, T. Socioeconomic Position and Incidence of Gastric Cancer: A Systematic Review and Meta-Analysis. J. Epidemiol. Commun. Health 2013, 67, 854–860. [Google Scholar] [CrossRef]
- Kim, J.; Cho, Y.A.; Choi, W.J.; Jeong, S.H. Gene-Diet Interactions in Gastric Cancer Risk: A Systematic Review. World J. Gastroenterol. 2014, 20, 9600. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, X. Salt Taste Preference, Sodium Intake and Gastric Cancer in China. Asian Pac. J. Cancer Prev. 2011, 12, 1207–1210. [Google Scholar]
- Keszei, A.P.; Goldbohm, R.A.; Schouten, L.J.; Jakszyn, P.; Van Den Brandt, P.A. Dietary N-Nitroso Compounds, Endogenous Nitrosation, and the Risk of Esophageal and Gastric Cancer Subtypes in the Netherlands Cohort Study. Am. J. Clin. Nutr. 2013, 97, 135–146. [Google Scholar] [CrossRef]
- Yang, L.; Ying, X.; Liu, S.; Lyu, G.; Xu, Z.; Zhang, X.; Li, H.; Li, Q.; Wang, N.; Ji, J. Gastric Cancer: Epidemiology, Risk Factors and Prevention Strategies. Chin. J. Cancer Res. 2020, 32, 695. [Google Scholar] [CrossRef]
- Moy, K.A.; Fan, Y.; Wang, R.; Gao, Y.T.; Yu, M.C.; Yuan, J.M. Alcohol and Tobacco Use in Relation to Gastric Cancer: A Prospective Study of Men in Shanghai, China. Cancer Epidemiol. Biomark. Prev. 2010, 19, 2287–2297. [Google Scholar] [CrossRef]
- Duell, E.J.; Travier, N.; Lujan-Barroso, L.; Clavel-Chapelon, F.; Boutron-Ruault, M.C.; Morois, S.; Palli, D.; Krogh, V.; Panico, S.; Tumino, R.; et al. Alcohol Consumption and Gastric Cancer Risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) Cohort. Am. J. Clin. Nutr. 2011, 94, 1266–1275. [Google Scholar] [CrossRef]
- Forman, D.; Burley, V.J. Gastric Cancer: Global Pattern of the Disease and an Overview of Environmental Risk Factors. Best Pract. Res. Clin. Gastroenterol. 2006, 20, 633–649. [Google Scholar] [CrossRef]
- Cocco, P.; Palli, D.; Buiatti, E.; Cipriani, F.; DeCarli, A.; Manca, P.; Ward, M.H.; Blot, W.J.; Fraumeni, J.F. Occupational Exposures as Risk Factors for Gastric Cancer in Italy. Cancer Causes Control 1994, 5, 241–248. [Google Scholar] [CrossRef]
- Adzersen, K.H.; Becker, N.; Steindorf, K.; Frentzel-Beyme, R. Cancer Mortality in a Cohort of Male German Iron Foundry Workers. Am. J. Ind. Med. 2003, 43, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Sarbia, M.; Becker, K.F.; Höfler, H. Pathology of Upper Gastrointestinal Malignancies. Semin. Oncol. 2004, 31, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Lauren, P. The Two Histological Main Types of Gastric Carcinoma: Diffuse and so-Called Intestinal-Type Carcinoma. An Attempt at a Histo-Clinical Classification. Acta Pathol. Microbiol. Scand. 1965, 64, 31–49. [Google Scholar] [CrossRef] [PubMed]
- Corso, G.; Seruca, R.; Roviello, F. Gastric Cancer Carcinogenesis and Tumor Progression. Ann. Ital. Chir. 2012, 83, 172–176. [Google Scholar]
- Correa, P.; Piazuelo, M.B. The Gastric Precancerous Cascade. J. Dig. Dis. 2012, 13, 2–9. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. The Hallmarks of Cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Parisi, A.; Reim, D.; Borghi, F.; Nguyen, N.T.; Qi, F.; Coratti, A.; Cianchi, F.; Cesari, M.; Bazzocchi, F.; Alimoglu, O.; et al. Minimally Invasive Surgery for Gastric Cancer: A Comparison between Robotic, Laparoscopic and Open Surgery. World J. Gastroenterol. 2017, 23, 2376. [Google Scholar] [CrossRef]
- Bobo, Z.; Xin, W.; Jiang, L.; Quan, W.; Liang, B.; Xiangbing, D.; Ziqiang, W. Robotic Gastrectomy versus Laparoscopic Gastrectomy for Gastric Cancer: Meta-Analysis and Trial Sequential Analysis of Prospective Observational Studies. Surg. Endosc. 2019, 33, 1033–1048. [Google Scholar] [CrossRef]
- Yoshida, K.; Yamaguchi, K.; Okumura, N.; Tanahashi, T.; Kodera, Y. Is Conversion Therapy Possible in Stage IV Gastric Cancer: The Proposal of New Biological Categories of Classification. Gastric Cancer 2016, 19, 329–338. [Google Scholar] [CrossRef]
- Santoro, R.; Ettorre, G.M.; Santoro, E. Subtotal Gastrectomy for Gastric Cancer. World J. Gastroenterol. 2014, 20, 13667. [Google Scholar] [CrossRef]
- Lise, M.; Nitti, D.; Marchet, A.; Sahmoud, T.; Buyse, M.; Duez, N.; Fiorentino, M.; Guimaraes Dos Santos, J.; Labianca, R.; Rougier, P.; et al. Final Results of a Phase III Clinical Trial of Adjuvant Chemotherapy with the Modified Fluorouracil, Doxorubicin, and Mitomycin Regimen in Resectable Gastric Cancer. J. Clin. Oncol. 2016, 13, 2757–2763. [Google Scholar] [CrossRef]
- Nakajima, T.; Nashimoto, A.; Kitamura, M.; Kito, T.; Iwanaga, T.; Okabayashi, K.; Sasaki, M.; Goto, M. Adjuvant Mitomycin and Fluorouracil Followed by Oral Uracil plus Tegafur in Serosa-Negative Gastric Cancer: A Randomised Trial. Lancet 1999, 354, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Paoletti, X.; Oba, K.; Burzykowski, T.; Michiels, S.; Ohashi, Y.; Pignon, J.P.; Rougier, P.; Sakamoto, J.; Sargent, D.; Sasako, M.; et al. Benefit of Adjuvant Chemotherapy for Resectable Gastric Cancer: A Meta-Analysis. JAMA 2010, 303, 1729–1737. [Google Scholar] [CrossRef] [PubMed]
- Van Den Ende, T.; Ter Veer, E.; Machiels, M.; Mali, R.M.A.; Abe Nijenhuis, F.A.; De Waal, L.; Laarman, M.; Gisbertz, S.S.; Hulshof, M.C.C.M.; Van Oijen, M.G.H.; et al. The Efficacy and Safety of (Neo)Adjuvant Therapy for Gastric Cancer: A Network Meta-Analysis. Cancers 2019, 11, 80. [Google Scholar] [CrossRef] [PubMed]
- Hartgrink, H.H.; van de Velde, C.J.H.; Putter, H.; Songun, I.; Tesselaar, M.E.T.; Kranenbarg, E.K.; de Vries, J.E.; Wils, J.A.; van der Bijl, J.; van Krieken, J.H.J.M. Neo-Adjuvant Chemotherapy for Operable Gastric Cancer: Long Term Results of the Dutch Randomised FAMTX Trial. Eur. J. Surg. Oncol. 2004, 30, 643–649. [Google Scholar] [CrossRef]
- Cunningham, D.; Allum, W.H.; Stenning, S.P.; Thompson, J.N.; Van de Velde, C.J.; Nicolson, M.; Howard Scarffe, J.; Lofts, F.J.; Falk, S.J.; Iveson, T.J.; et al. Perioperative Chemotherapy versus Surgery Alone for Resectable Gastroesophageal Cancer. N. Engl. J. Med. 2006, 355, 11–20. [Google Scholar] [CrossRef]
- Tsai, C.; Mueller, A.; Maubach, J.; Warschkow, R.; Nussbaum, D.P.; Schmied, B.M.; Blazer, D.; Gloor, B.; Worni, M. No Difference in Survival between Neo-Adjuvant Chemotherapy and Neo-Adjuvant Chemoradiation Therapy in Gastric Cardia Cancer Patients: A Contemporary View from the National Cancer Database. Dig. Surg. 2020, 37, 249–257. [Google Scholar] [CrossRef]
- De Vita, F.; Borg, C.; Farina, G.; Geva, R.; Carton, I.; Cuku, H.; Wei, R.; Muro, K. Ramucirumab and Paclitaxel in Patients with Gastric Cancer and Prior Trastuzumab: Subgroup Analysis from RAINBOW Study. Future Oncol. 2019, 15, 2723–2731. [Google Scholar] [CrossRef]
- Lordick, F.; Luber, B.; Lorenzen, S.; Hegewisch-Becker, S.; Folprecht, G.; Wöll, E.; Decker, T.; Endlicher, E.; Röthling, N.; Schuster, T.; et al. Cetuximab plus Oxaliplatin/Leucovorin/5-Fluorouracil in First-Line Metastatic Gastric Cancer: A Phase II Study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). Br. J. Cancer 2010, 102, 500–505. [Google Scholar] [CrossRef]
- Inokuchi, M.; Fujimori, Y.; Otsuki, S.; Sato, Y.; Nakagawa, M.; Kojima, K. Therapeutic Targeting of Fibroblast Growth Factor Receptors in Gastric Cancer. Gastroenterol. Res. Pract. 2015, 2015, 796380. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, R.K.; McDonough, S.L.; Kennecke, H.F.; Iqbal, S.; Baranda, J.C.; Seery, T.E.; Lim, H.J.; Hezel, A.F.; Vaccaro, G.M.; Blanke, C.D. Phase 2 Study of MK-2206, an Allosteric Inhibitor of AKT, as Second-Line Therapy for Advanced Gastric and Gastroesophageal Junction Cancer: A SWOG Cooperative Group Trial (S1005). Cancer 2015, 121, 2193–2197. [Google Scholar] [CrossRef]
- Li, R.; Li, J.; Wang, X.; Liang, P.; Gao, J. Detection of Gastric Cancer and Its Histological Type Based on Iodine Concentration in Spectral CT. Cancer Imaging 2018, 18, 42. [Google Scholar] [CrossRef] [PubMed]
- Kwee, R.M.; Kwee, T.C. Imaging in Assessing Lymph Node Status in Gastric Cancer. Gastric Cancer 2009, 12, 6–22. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, J. The Role of MRI in the Diagnosis and Treatment of Gastric Cancer. Diagn. Interv. Radiol. 2020, 26, 182. [Google Scholar] [CrossRef] [PubMed]
- Fonocho, E.; Aydin, N.; Reddy, S.; Misra, S. Limitations in the Use of 18F-FDG PET in the Pre-Operative Staging of Gastric Cancer: A Case Series. Int. J. Surg. Case Rep. 2017, 36, 147–150. [Google Scholar] [CrossRef]
- Elingarami, S.; Liu, M.; Fan, J.; He, N. Applications of Nanotechnology in Gastric Cancer: Detection and Prevention by Nutrition. J. Nanosci. Nanotechnol. 2014, 14, 932–945. [Google Scholar] [CrossRef]
- IARC International Agency for Research on Cancer. Fruit and Vegetables. In IARC Handbooks of Cancer Prevention; IARC: Lyon, France, 2003; Volume 8, Available online: https://publications.iarc.fr/Book-And-Report-Series/Iarc-Handbooks-Of-Cancer-Prevention/Fruit-And-Vegetables-2003 (accessed on 3 March 2023).
- Ford, A.C.; Forman, D.; Hunt, R.H.; Yuan, Y.; Moayyedi, P. Helicobacter Pylori Eradication Therapy to Prevent Gastric Cancer in Healthy Asymptomatic Infected Individuals: Systematic Review and Meta-Analysis of Randomised Controlled Trials. BMJ 2014, 348, g3174. [Google Scholar] [CrossRef]
- Choi, J.; Kim, S.G.; Yoon, H.; Im, J.P.; Kim, J.S.; Kim, W.H.; Jung, H.C. Eradication of Helicobacter Pylori after Endoscopic Resection of Gastric Tumors Does Not Reduce Incidence of Metachronous Gastric Carcinoma. Clin. Gastroenterol. Hepatol. 2014, 12, 793–800.e1. [Google Scholar] [CrossRef]
- Fukase, K.; Kato, M.; Kikuchi, S.; Inoue, K.; Uemura, N.; Okamoto, S.; Terao, S.; Amagai, K.; Hayashi, S.; Asaka, M. Effect of Eradication of Helicobacter Pylori on Incidence of Metachronous Gastric Carcinoma after Endoscopic Resection of Early Gastric Cancer: An Open-Label, Randomised Controlled Trial. Lancet 2008, 372, 392–397. [Google Scholar] [CrossRef]
- Tsubono, Y.; Hisamichi, S. Screening for Gastric Cancer in Japan. Gastric Cancer 2000, 3, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Karimi, P.; Islami, F.; Anandasabapathy, S.; Freedman, N.D.; Kamangar, F. Gastric Cancer: Descriptive Epidemiology, Risk Factors, Screening, and Prevention. Cancer Epidemiol. Biomark. Prev. 2014, 23, 700–713. [Google Scholar] [CrossRef] [PubMed]
- Hamashima, C.; Ogoshi, K.; Okamoto, M.; Shabana, M.; Kishimoto, T.; Fukao, A. A Community-Based, Case-Control Study Evaluating Mortality Reduction from Gastric Cancer by Endoscopic Screening in Japan. PLoS ONE 2013, 8, e79088. [Google Scholar] [CrossRef] [PubMed]
- Kienesberger, S.; Cox, L.M.; Livanos, A.; Zhang, X.S.; Chung, J.; Perez-Perez, G.I.; Gorkiewicz, G.; Zechner, E.L.; Blaser, M.J. Gastric Helicobacter Pylori Infection Affects Local and Distant Microbial Populations and Host Responses. Cell Rep. 2016, 14, 1395–1407. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwu, C.D.; Iwu-Jaja, C.J. Gastric Cancer Epidemiology: Current Trend and Future Direction. Hygiene 2023, 3, 256-268. https://doi.org/10.3390/hygiene3030019
Iwu CD, Iwu-Jaja CJ. Gastric Cancer Epidemiology: Current Trend and Future Direction. Hygiene. 2023; 3(3):256-268. https://doi.org/10.3390/hygiene3030019
Chicago/Turabian StyleIwu, Chidozie Declan, and Chinwe Juliana Iwu-Jaja. 2023. "Gastric Cancer Epidemiology: Current Trend and Future Direction" Hygiene 3, no. 3: 256-268. https://doi.org/10.3390/hygiene3030019
APA StyleIwu, C. D., & Iwu-Jaja, C. J. (2023). Gastric Cancer Epidemiology: Current Trend and Future Direction. Hygiene, 3(3), 256-268. https://doi.org/10.3390/hygiene3030019





