Trends of Major Foodborne Outbreaks in the European Union during the Years 2015–2019
Abstract
:1. Introduction
2. The EFSA-ECDC Annual Reports on Zoonoses and Foodborne Outbreaks
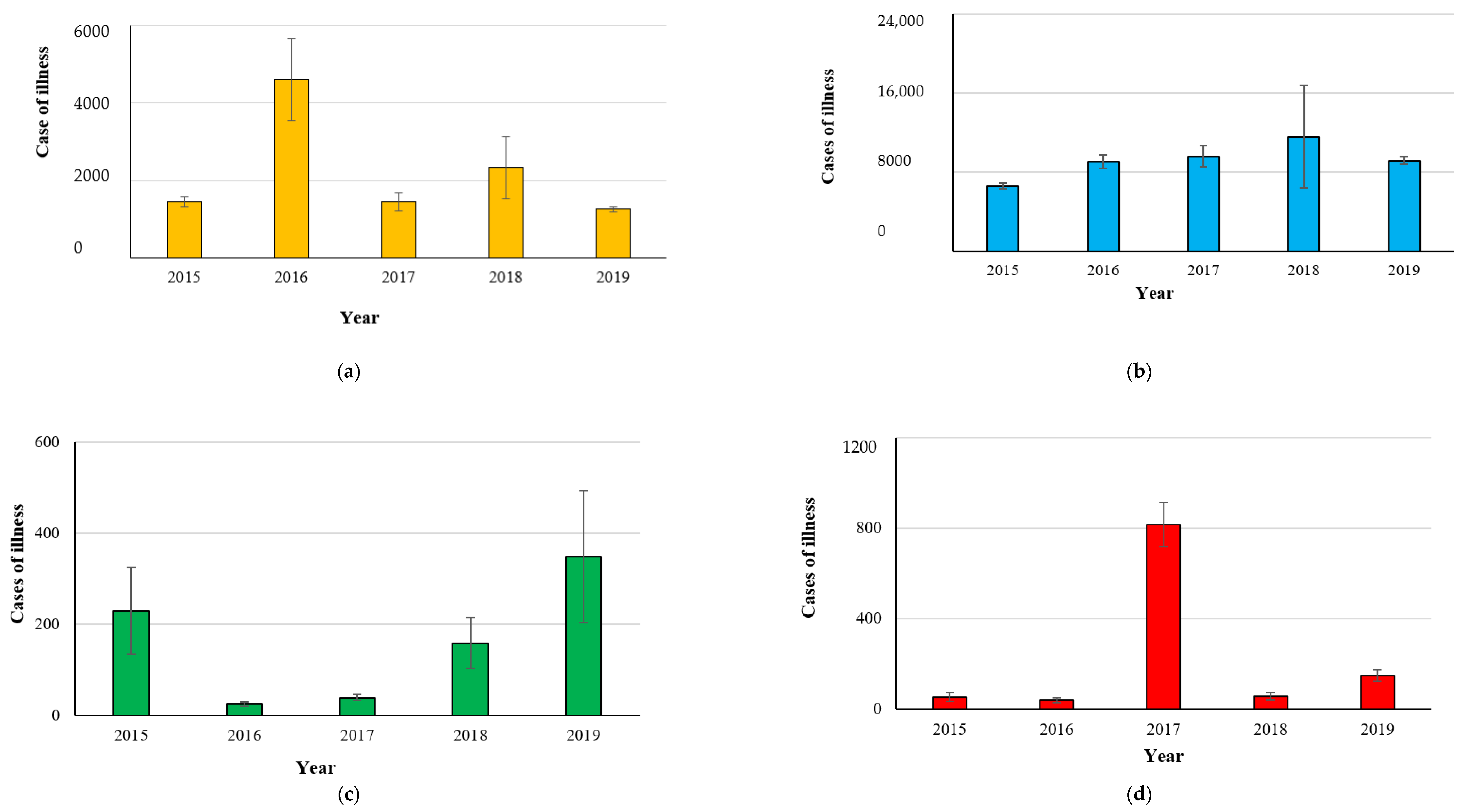
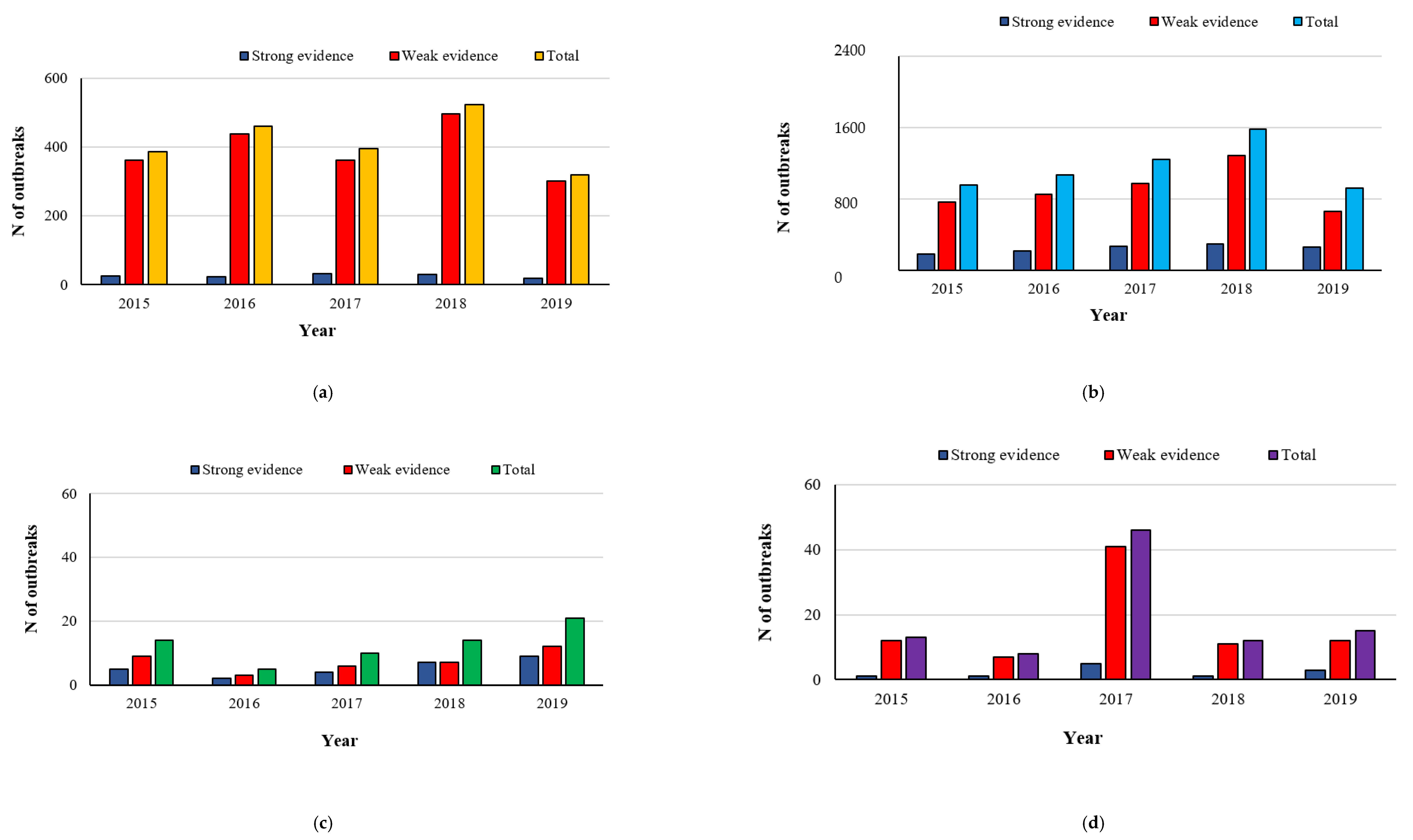
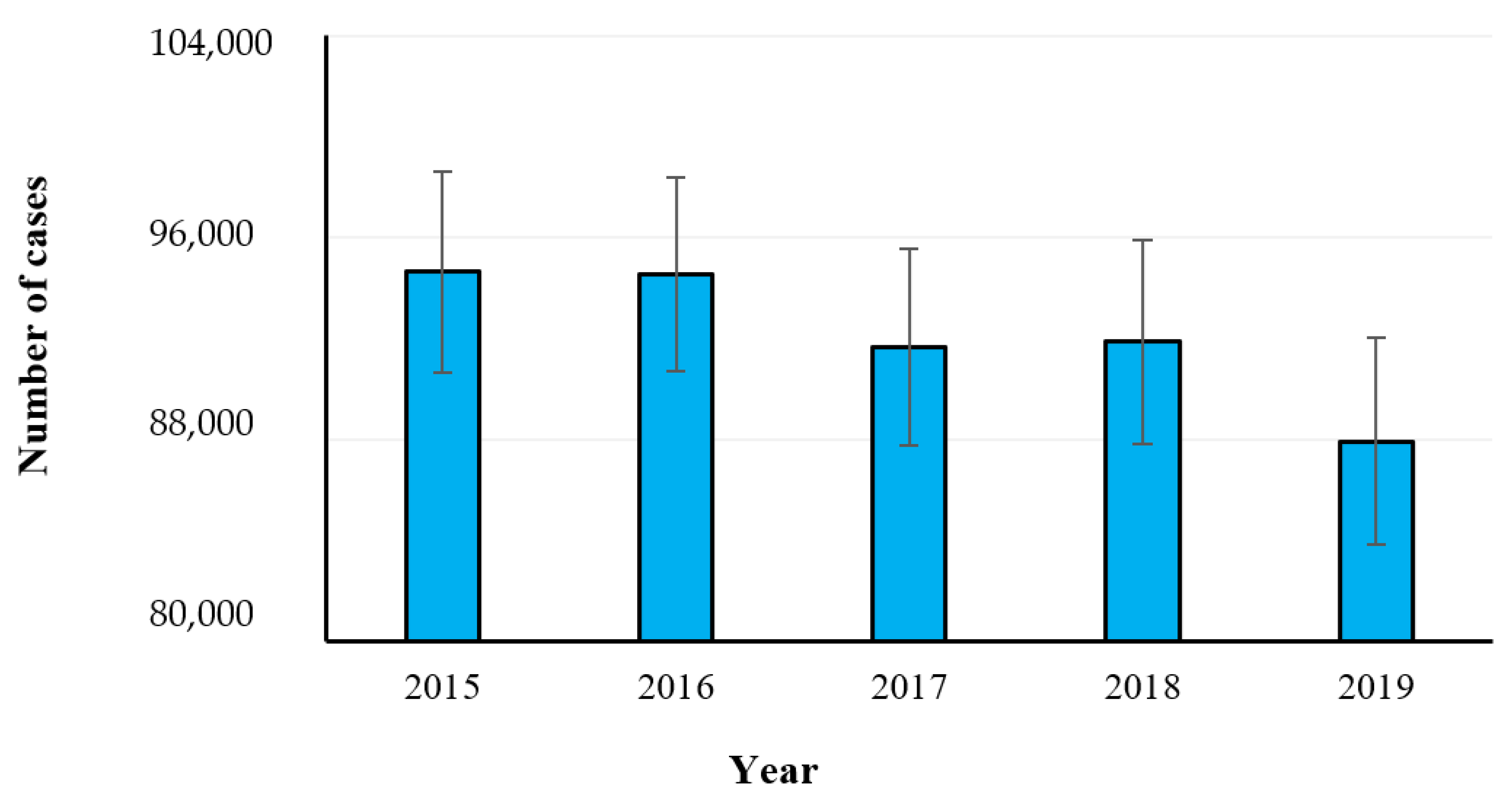
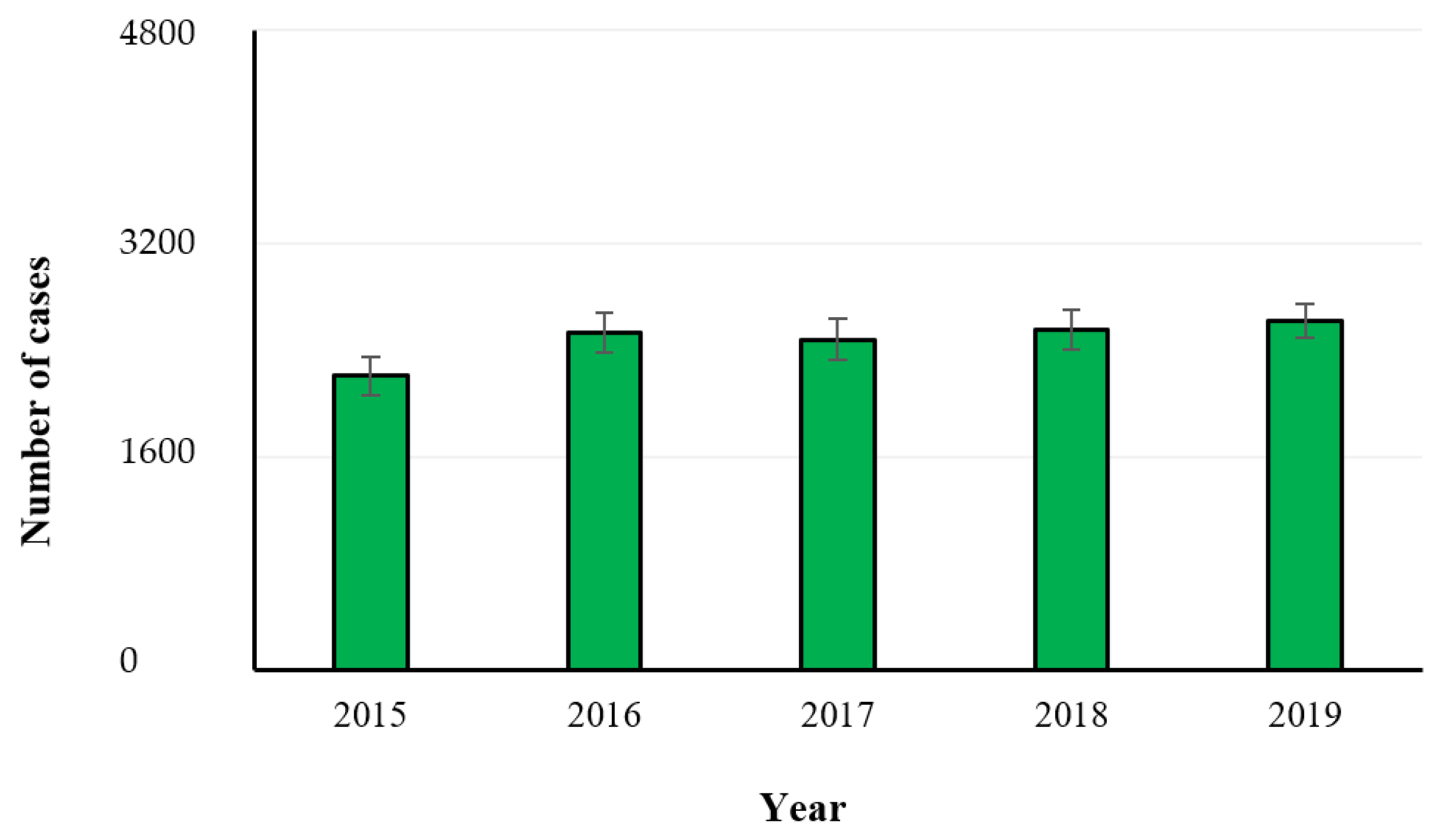
2.1. Campylobacteriosis
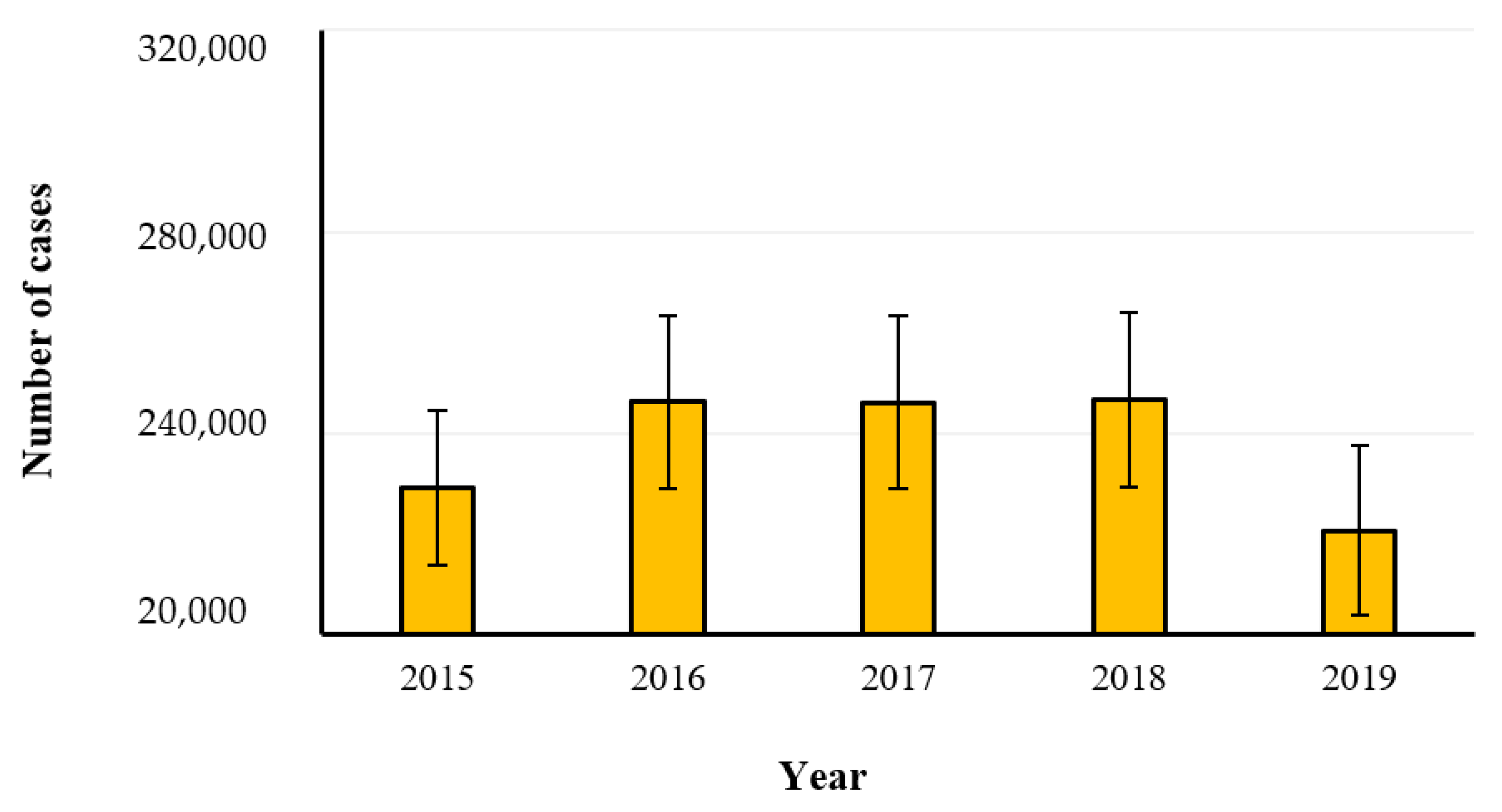
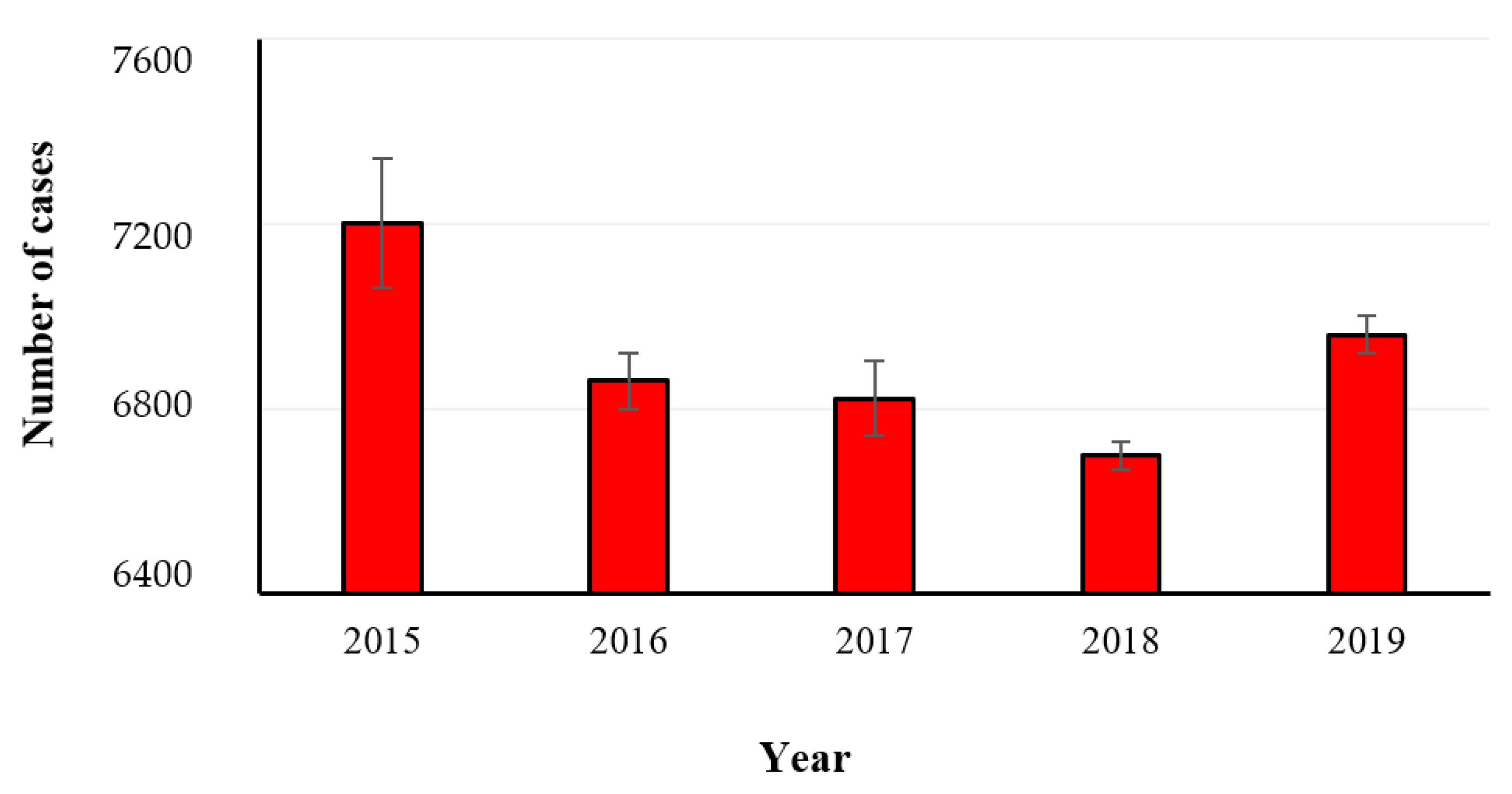
2.2. Salmonellosis
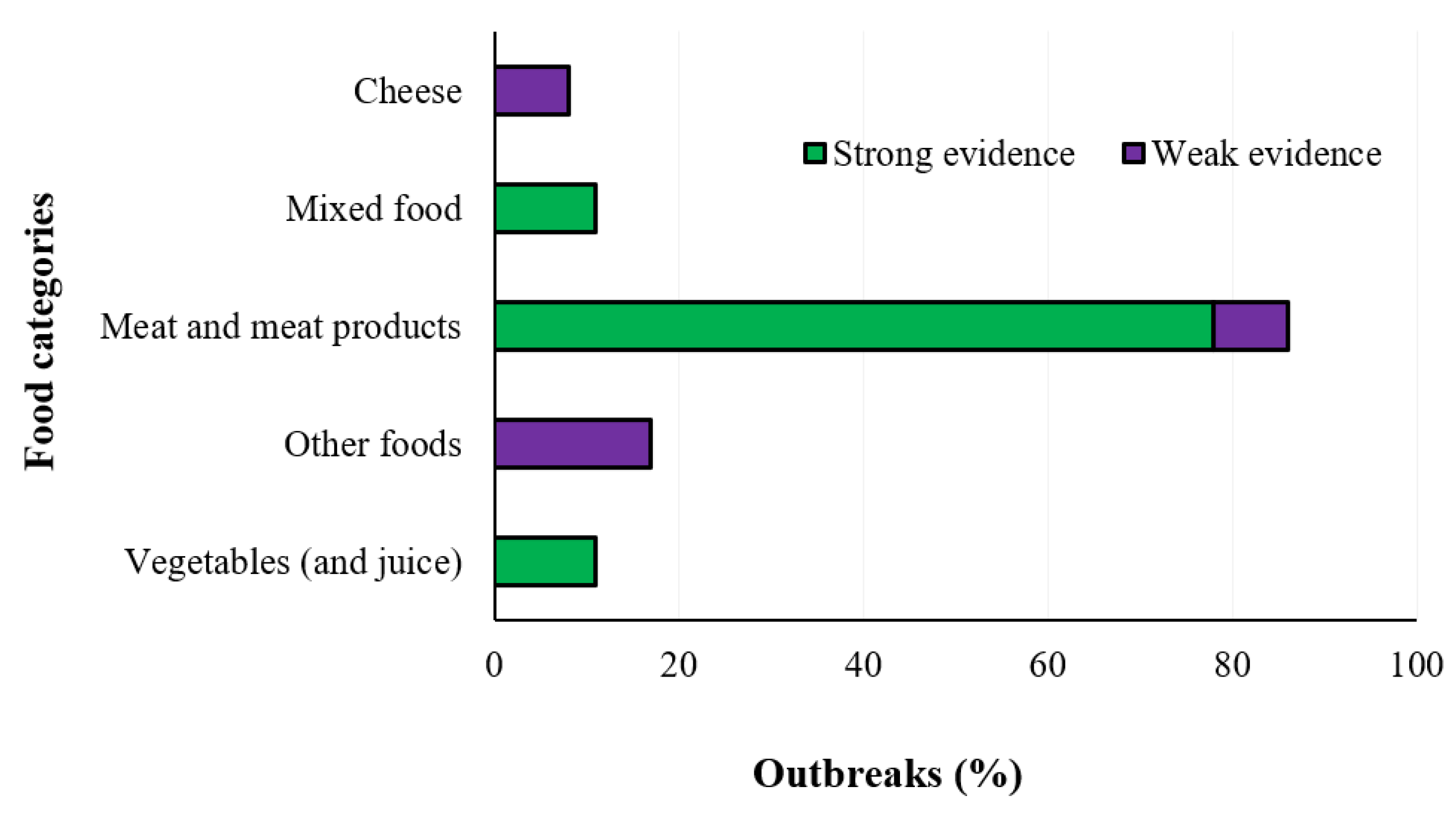
2.3. Listeriosis
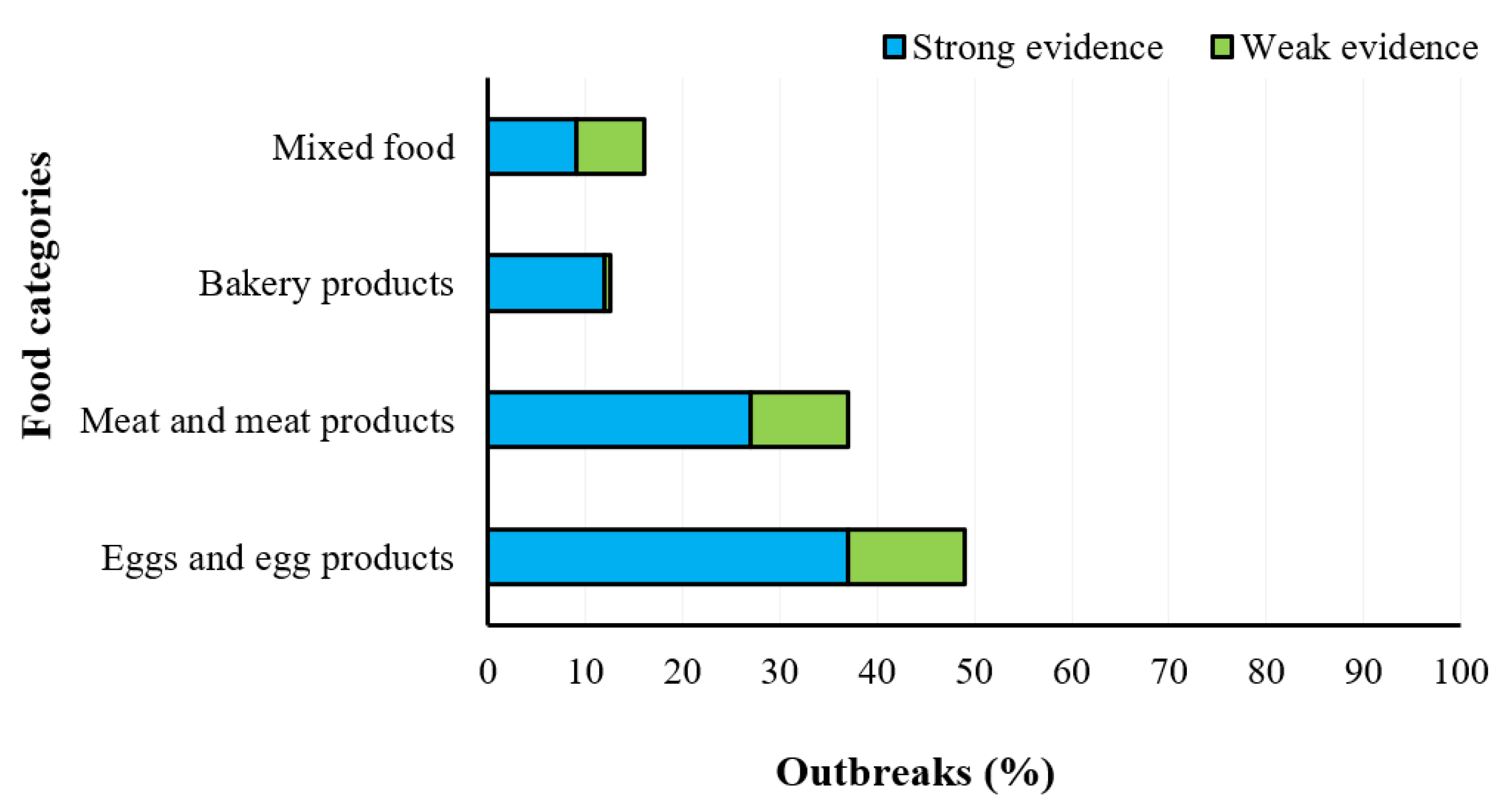
2.4. Yersiniosis
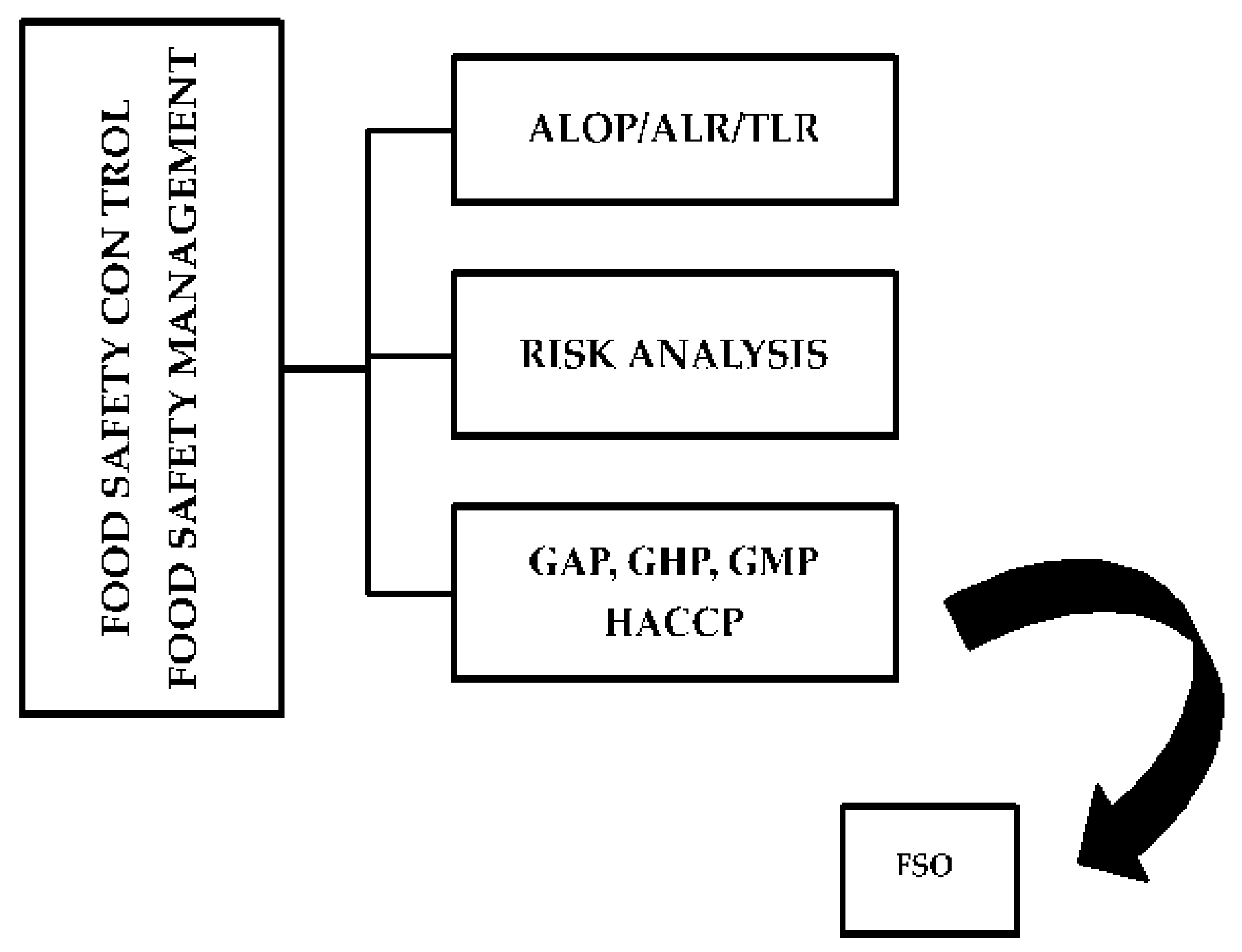
3. Prevention and Control Strategies
Author Contributions
Funding
Conflicts of Interest
References
- AL-Mamun, M.; Chowdhury, T.; Biswas, B.; Absar, N. Food Poisoning and Intoxication: A Global Leading Concern for Human Health. In Food Safety and Preservation. Modern Biological Approaches to Improving Consumer Health; Grumezescu, A.M., Holban, A.M., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; Chapter 11; pp. 307–352. [Google Scholar] [CrossRef]
- Chaves, R.D.; Alvarenga, V.O.; Campagnollo, F.B.; Rodriguez Caturla, M.Y.; Oteiza, J.M.; Sant’Ana, A.S. Food Safety. In Current Developments in Biotechnology and Bioengineering-Food and Beverages Industry; Part 1: Food and, Fermentation; Pandey, A., Sanroman, R.A., Du, G., Soccol, C.R., Dussap, C.-G., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2017; Chapter 9; pp. 245–259. [Google Scholar]
- Mota, J.D.O.; Boué, G.; Prévost, H.; Maillet, A.; Jaffres, E.; Maignien, T.; Arnich, N.; Sanaa, M.; Federighi, M. Environmental monitoring program to support food microbiological safety and quality in food industries: A scoping review of the research and guidelines. Food Control. 2021, 130, 108283. [Google Scholar] [CrossRef]
- Yu, H.; Guo, W.; Lu, X.; Xu, H.; Yang, Q.; Tan, J.; Zhang, W. Reduced graphene oxide nanocomposite based electrochemical biosensors for monitoring foodborne pathogenic bacteria: A review. Food Control. 2021, 127, 108117. [Google Scholar] [CrossRef]
- Shan, L.; Wang, S.; Wu, L.; Tsai, F.-S. Cognitive Biases of Consumers’ Risk Perception of Foodborne Diseases in China: Examining Anchoring Effect. Int. J. Environ. Res. Public Health 2019, 16, 2268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebel, E.D.; Williams, M.S.; Ward-Gokhale, L.A.; Kisselburgh, H.M. Assessing the maximum size of annual foodborne outbreaks in the United States: An analysis of 1973–2016 outbreaks. Microb. Risk Anal. 2019, 12, 20–26. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority). Update of the technical specifications for harmonised reporting of food-borne outbreaks through the European Union reporting system in accordance with Directive 2003/99/EC. EFSA J. 2014, 12, 3598. [Google Scholar]
- EFSA-ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control). The European Union One Health 2019 Zoonoses Report. EFSA J. 2021, 19, 6406. [Google Scholar]
- Kaakoush, N.O.; Castaño-Rodríguez, N.; Mitchell, H.M.; Man, S.M. Global Epidemiology of Campylobacter Infection. Clin. Microbiol. Rev. 2015, 28, 687–720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuhn, K.G.; Nygård, K.M.; Guzman-Herrador, B.; Sunde, L.S.; Rimhanen-Finne, R.; Trönnberg, L.; Jepsen, M.R.; Ruuhela, R.; Wong, W.K.; Ethelberg, S. Campylobacter infections expected to increase due to climate change in Northern Europe. Sci. Rep. 2020, 10, 13874. [Google Scholar] [CrossRef]
- Sher, A.A.; Ashraf, M.A.; Mustafa, B.E.; Raza, M.M. Epidemiological trends of foodborne Campylobacter outbreaks in the United States of America, 1998–2016. Food Microbiol. 2021, 97, 103751. [Google Scholar] [CrossRef]
- Igwaran, A.; Okoh, A. Human campylobacteriosis: A public health concern of global importance. Heliyon 2019, 5, e02814. [Google Scholar] [CrossRef] [PubMed]
- García-Fernández, A.; Dionisi, A.M.; Arena, S.; Iglesias-Torrens, Y.; Carattoli, A.; Luzzi, I. Human Campylobacteriosis in Italy: Emergence of Multi-Drug Resistance to Ciprofloxacin, Tetracycline, and Erythromycin. Front. Microbiol. 2018, 9, 1906. [Google Scholar] [CrossRef]
- Endtz, H.P. Campylobacter infections. In Hunter’s Tropical Medicine and Emerging Infectious Disease, 10th ed.; Philadelphia, P.A., Ryan, E.T., Hill, D.R., Solomon, T., Aaronson, N.E., Endy, T.P., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2020; Chapter 50; pp. 507–511. [Google Scholar] [CrossRef]
- Sher, A.A.; Mustafa, B.E.; Grady, S.C.; Gardiner, J.C.; Saeed, A.M. Outbreaks of foodborne Salmonella enteritidis in the United States between 1990 and 2015: An analysis of epidemiological and spatial-temporal trends. Int. J. Infect. Dis. 2021, 105, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Agregán, R.; Munekata, P.E.; Zhang, W.; Zhang, J.; Pérez-Santaescolástica, C.; Lorenzo, J.M. High-pressure processing in inactivation of Salmonella spp. in food products. Trends Food Sci. Technol. 2021, 107, 31–37. [Google Scholar] [CrossRef]
- Nadi, Z.R.; Salehi, T.Z.; Tamai, I.A.; Foroushani, A.R.; Sillanpaa, M.; Dallal, M.M.S. Evaluation of antibiotic resistance and prevalence of common Salmonella enterica serovars isolated from foodborne outbreaks. Microchem. J. 2020, 155, 104660. [Google Scholar] [CrossRef]
- Dhaliwal, H.K.; Gänzle, M.; Roopesh, M. Influence of drying conditions, food composition, and water activity on the thermal resistance of Salmonella enterica. Food Res. Int. 2021, 147, 110548. [Google Scholar] [CrossRef] [PubMed]
- Aljasir, S.F.; D’Amico, D.J. Effect of pre-exposure to protective bacterial cultures in food on Listeria monocytogenes virulence. LWT Food Sci. Technol. 2021, 152, 112373. [Google Scholar] [CrossRef]
- Desai, A.N.; Anyoha, A.; Madoff, L.C.; Lassmann, B. Changing epidemiology of Listeria monocytogenes outbreaks, sporadic cases, and recalls globally: A review of ProMED reports from 1996 to 2018. Int. J. Infect. Dis. 2019, 84, 48–53. [Google Scholar] [CrossRef] [Green Version]
- Evans, E.; Samuel, E.; Redmond, E.; Taylor, H. Exploring Listeria monocytogenes perceptions in small and medium sized food manufacturers: Technical leaders’ perceptions of risk, control and responsibility. Food Control. 2021, 126, 108078. [Google Scholar] [CrossRef]
- Wang, J.; Liu, M.; Wang, H.; Wu, Q.; Ding, Y.; Xu, T.; Ma, G.; Zhong, Y.; Zhang, J.; Chen, M.; et al. Occurrence, molecular characterization, and antimicrobial susceptibility of Yersinia enterocolitica isolated from retail food samples in China. LWT Food Sci. Technol. 2021, 150, 111876. [Google Scholar] [CrossRef]
- Riahi, S.M.; Ahmadi, E.; Zeinali, T. Global prevalence of Yersinia enterocolitica in cases of gastroenteritis: A systematic review and meta-analysis. Int. J. Microbiol. 2021, 2021, 1499869. [Google Scholar] [CrossRef]
- Lambertini, E.; Ruzante, J.M.; Chew, R.; Apodaca, V.L.; Kowalcyk, B.B. The public health impact of different microbiological criteria approaches for Salmonella in chicken parts. Microb. Risk Anal. 2019, 12, 44–59. [Google Scholar] [CrossRef]
- New Zealand Ministry for Primary Industries. Campylobacter Risk Management Strategy 2017–2020; Ministry for Primary Industries: Wellington, New Zealand, 2017.
- Paparella, A. Food safety: Definitions and aspects. In Food Safety Hazards; Al-Rub, F.A., Shibhab, P., Al-Rub, S.A., Pittia, P., Paparella, A., Eds.; Gavin Ebooks: Lisle, IL, USA, 2020; pp. 1–5. [Google Scholar] [CrossRef]
- De Filippis, F.; Valentino, V.; Alvarez-Ordóñez, A.; Cotter, P.D.; Ercolini, D. Environmental microbiome mapping as a strategy to improve quality and safety in the food industry. Curr. Opin. Food Sci. 2021, 38, 168–176. [Google Scholar] [CrossRef]
- Hou, J.; Luo, R.; Ni, H.; Li, K.; Mgomi, F.C.; Fan, L.; Yuan, L. Antimicrobial potential of kombucha against foodborne pathogens: A review. Qual. Assur. Saf. Crop. Foods 2021, 13, 53–61. [Google Scholar] [CrossRef]





| Country | Campylobacteriosis | Salmonellosis | Yersiniosis | Listeriosis | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2015 | 2016 | 2017 | 2018 | 2019 | 2015 | 2016 | 2017 | 2018 | 2019 | 2015 | 2016 | 2017 | 2018 | 2019 | 2015 | 2016 | 2017 | 2018 | 2019 | |
| Austria | ||||||||||||||||||||
| Belgium | ||||||||||||||||||||
| Bulgaria | ||||||||||||||||||||
| Croatia | ||||||||||||||||||||
| Cyprus | ||||||||||||||||||||
| Czech Republic | ||||||||||||||||||||
| Denmark | ||||||||||||||||||||
| Estonia | ||||||||||||||||||||
| Finland | ||||||||||||||||||||
| France | ||||||||||||||||||||
| Germany | ||||||||||||||||||||
| Greece | ||||||||||||||||||||
| Hungary | ||||||||||||||||||||
| Ireland | ||||||||||||||||||||
| Italy | ||||||||||||||||||||
| Latvia | ||||||||||||||||||||
| Lithuania | ||||||||||||||||||||
| Luxembourg | ||||||||||||||||||||
| Malta | ||||||||||||||||||||
| Netherlands | ||||||||||||||||||||
| Poland | ||||||||||||||||||||
| Portugal | ||||||||||||||||||||
| Romania | ||||||||||||||||||||
| Slovakia | ||||||||||||||||||||
| Slovenia | ||||||||||||||||||||
| Spain | ||||||||||||||||||||
| Sweden | ||||||||||||||||||||
| United Kingdom | ||||||||||||||||||||
| Total | 17 | 20 | 19 | 17 | 18 | 23 | 25 | 25 | 24 | 23 | 9 | 6 | 15 | 7 | 7 | 9 | 2 | 6 | 7 | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schirone, M.; Visciano, P. Trends of Major Foodborne Outbreaks in the European Union during the Years 2015–2019. Hygiene 2021, 1, 106-119. https://doi.org/10.3390/hygiene1030010
Schirone M, Visciano P. Trends of Major Foodborne Outbreaks in the European Union during the Years 2015–2019. Hygiene. 2021; 1(3):106-119. https://doi.org/10.3390/hygiene1030010
Chicago/Turabian StyleSchirone, Maria, and Pierina Visciano. 2021. "Trends of Major Foodborne Outbreaks in the European Union during the Years 2015–2019" Hygiene 1, no. 3: 106-119. https://doi.org/10.3390/hygiene1030010
APA StyleSchirone, M., & Visciano, P. (2021). Trends of Major Foodborne Outbreaks in the European Union during the Years 2015–2019. Hygiene, 1(3), 106-119. https://doi.org/10.3390/hygiene1030010






