Diet, DNA, and the Mesolithic–Neolithic Transition in Western Scotland
Abstract
1. Introduction
2. Archaeological and Archaeogenetic Background
3. Stable Isotope Analysis
- The Δ15N diet–consumer diet consumer offset used, i.e., +5.5 ± 0.5‰
- The Δ13C diet–consumer offset used, i.e., +1.0‰
- The inclusion of marine foods in the dietary model
- The omission of plant foods from the model
- Whether the faunal samples used to establish food source isotope values were contemporaneous with the human remains from the site.
3.1. Δ15Ndiet–Consumer Offset
3.2. Food Sources
3.3. Critique of Bayesian Models
4. Discussion
4.1. Revised Dietary Models
4.2. Diet, DNA, and the Mesolithic–Neolithic Transition
4.3. Shell Middens and Neolithic Burials
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ammerman, A. (2020). The Neolithic transition in Europe at 50 years. arXiv, arXiv:2012.11713. [Google Scholar] [CrossRef]
- Anderson, J. (1895). Notice of a cave recently discovered at Oban, containing human remains, and a refuse-heap of shells and bones of animals, and stone and bone implements. Proceedings of the Society of Antiquaries of Scotland, 29, 211–230. [Google Scholar] [CrossRef]
- Barrett, J. H., Orton, D., Johnstone, C., Harland, J., Van Neer, W., Ervynck, A., Roberts, C., Locker, A., Amundsen, C., Enghoff, I. B., Hamilton-Dyer, S., Heinrich, D., Hufthammer, A. K., Jones, A. K., Jonsson, L., Makowiecki, D., Pope, P., O’Connell, T. C., de Roo, T., & Richards, M. (2011). Interpreting the expansion of sea fishing in medieval Europe using stable isotope analysis of archaeological cod bones. Journal of Archaeological Science, 38(7), 1516–1524. [Google Scholar] [CrossRef]
- Bartosiewicz, L., Zapata, L., & Bonsall, C. (2010). A tale of two shell middens: The natural versus the cultural in ‘Obanian’ deposits at Carding Mill Bay, Oban, western Scotland. In A. VanDerwarker, & A. Peres (Eds.), Integrating zooarchaeology and paleoethnobotany: A consideration of issues, methods, and cases (pp. 205–225). Springer. [Google Scholar]
- Bergsvik, K. A., & Ritchie, K. (2020). Mesolithic fishing landscapes in western Norway. In A. Schülke (Ed.), Coastal landscapes of the Mesolithic. Human engagement with the coast from the Atlantic to the Baltic Sea (pp. 229–263). Routledge. [Google Scholar]
- Berryman, C. E., Lieberman, H. R., Fulgoni, V. L., & Pasiakos, S. M. (2018). Protein intake trends and conformity with the dietary reference intakes in the United States: Analysis of the National Health and Nutrition Examination Survey, 2001–2014. The American Journal of Clinical Nutrition, 108(2), 405–413. [Google Scholar] [CrossRef] [PubMed]
- Bickle, P. (2018). Stable isotopes and dynamic diets: The Mesolithic-Neolithic dietary transition in terrestrial Central Europe. Journal of Archaeological Science: Reports, 22, 444–451. [Google Scholar] [CrossRef]
- Bishop, R., Church, M., & Rowley-Conwy, P. (2010). Cereals, fruits and nuts in the Scottish Neolithic. Proceedings of the Society of Antiquaries of Scotland, 139, 47–103. [Google Scholar] [CrossRef]
- Bishop, R., Church, M., & Rowley-Conwy, P. (2014). Seeds, fruits and nuts in the Scottish Mesolithic. Proceedings of the Society of Antiquaries of Scotland, 143, 9–71. [Google Scholar] [CrossRef]
- Bishop, R., Gröcke, D. R., Ralston, I., Clarke, D., Lee, D. H. J., Shepherd, A., Thomas, A. S., Rowley-Conwy, P. A., & Church, M. J. (2022). Scotland’s first farmers: New insights into early farming practices in North-west Europe. Antiquity, 96(389), 1087–1104. [Google Scholar] [CrossRef]
- Bocherens, H. (2015). Isotopic tracking of large carnivore palaeoecology in the mammoth steppe. Quaternary Science Reviews, 117, 42–71. [Google Scholar] [CrossRef]
- Bocherens, H., & Drucker, D. (2003). Trophic level isotopic enrichment of carbon and nitrogen in bone collagen: Case studies from recent and ancient terrestrial ecosystems. International Journal of Osteoarchaeology, 13(1–2), 46–53. [Google Scholar] [CrossRef]
- Bollongino, R., Nehlich, O., Richards, M. P., Orschiedt, J., Thomas, M. G., Sell, C., Fajkošová, Z., Powell, A., & Burger, J. (2013). Years of parallel societies in Stone Age Central Europe. Science, 342(6157), 479–481. [Google Scholar] [CrossRef] [PubMed]
- Bonsall, C. (2000). Raschoille Cave, Oban. Discovery Excavation Scotland 1999, 112. [Google Scholar]
- Bonsall, C., Anderson, D., & Macklin, M. (2002). The Mesolithic–Neolithic transition in western Scotland and its European context. Documenta Praehistorica, 29, 1–25. [Google Scholar] [CrossRef]
- Bonsall, C., Pickard., C., & Ritchie, G. (2012). From Assynt to Oban: Some observations on prehistoric cave use in western Scotland. In K. A. Bergsvik, & R. Skeates (Eds.), Caves in context: The cultural significance of caves and rockshelters in Europe (pp. 10–21). Oxbow Books. [Google Scholar]
- Bonsall, C., & Smith, C. (1989). Late Palaeolithic and Mesolithic bone and antler artifacts from Britain: First reactions to accelerator dates. Mesolithic Miscellany, 10(1), 33–38. [Google Scholar]
- Bonsall, C., & Smith, C. (1992). New AMS 14C dates for antler and bone artifacts from Great Britain. Mesolithic Miscellany, 13(1), 28–34. [Google Scholar]
- Bownes, J. (2018). Reassessing the Scottish Mesolithic–Neolithic transition: Questions of diet and chronology [Ph.D. thesis, University of Glasgow]. Available online: https://theses.gla.ac.uk/8911/ (accessed on 19 February 2019).
- Bownes, J. M., Ascough, P. L., Cook, G. T., Murray, I., & Bonsall, C. (2017). Using stable isotopes and a Bayesian mixing model (FRUITS) to investigate diet at the Early Neolithic site of Carding Mill Bay, Scotland. Radiocarbon, 59(5), 1275–1294. [Google Scholar] [CrossRef]
- Brace, S., & Booth, T. J. (2023). The genetics of the inhabitants of Neolithic Britain: A review. In A. Whittle, J. Pollard, & S. Greaney (Eds.), Ancient DNA and the European Neolithic, relations and descent (pp. 123–146). Oxbow Books. [Google Scholar]
- Brace, S., Diekmann, Y., Booth, T. J., van Dorp, L., Faltyskova, Z., Rohland, N., Mallick, S., Olalde, I., Ferry, M., Michel, M., Oppenheimer, J., Broomandkhoshbacht, N., Stewardson, K., Martiniano, R., Walsh, S., Kayser, M., Charlton, S., Hellenthal, G., Armit, I., & Barnes, I. (2019). Ancient genomes indicate population replacement in Early Neolithic Britain. Nature Ecology & Evolution, 3(5), 765–771. [Google Scholar] [CrossRef]
- Bronk Ramsey, C. (2021). OxCal 4.4 online. Available online: https://c14.arch.ox.ac.uk/oxcal/OxCal.html (accessed on 30 November 2024).
- Brunel, S., Bennett, E. A., Cardin, L., Garraud, D., Emam, H. B., Beylier, A., Boulestin, B., Chenal, F., Ciesielski, E., Convertini, F., Dedet, B., Desbrosse-Degobertiere, S., Desenne, S., Dubouloz, J., Duday, H., Escalon, G., Fabre, V., Gailledrat, E., Gandelin, M., & Pruvost, M. (2020). Ancient genomes from present-day France unveil 7000 years of its demographic history. Proceedings of the National Academy of Sciences, 117(23), 12791–12798. [Google Scholar] [CrossRef]
- Caut, S., Angulo, E., & Courchamp, F. (2009). Variation in discrimination factors (Δ15N and Δ13C): The effect of diet isotopic values and applications for diet reconstruction. Journal of Applied Ecology, 46(2), 443–453. [Google Scholar] [CrossRef]
- Charlton, S., Alexander, M., Collins, M., Milner, N., Mellars, P., O’Connell, T. C., Stevens, R. E., & Craig, O. E. (2016). Finding Britain’s last hunter-gatherers: A new biomolecular approach to ‘unidentifiable’ bone fragments utilising bone collagen. Journal of Archaeological Science, 73, 55–61. [Google Scholar] [CrossRef]
- Cheung, C., & Szpak, P. (2021). Interpreting past human diets using stable isotope mixing models—Best practices for data acquisition. Journal of Archaeological Method and Theory, 29(1), 138–161. [Google Scholar] [CrossRef]
- Connock, K. D., Finlayson, B., Mills, A. C. M., Boardman, S. J., Crone, B. A., Hamilton-Dyer, S., McCormick, F., Lorimer, D. H., Morton, A., Russell, N. J., & Carter, S. (1991). Excavation of a shell midden site at Carding Mill Bay near Oban, Scotland. Glasgow Archaeological Journal, 17(1), 25–38. [Google Scholar] [CrossRef]
- Cramp, L. J. E., Jones, J., Sheridan, A., Smyth, J., Whelton, H., Mulville, J., Sharples, N., & Evershed, R. P. (2014). Immediate replacement of fishing with dairying by the earliest farmers of the northeast Atlantic archipelagos. Proceedings of The Royal Society B-Biological Sciences, 281(1780), 20132372. [Google Scholar] [CrossRef] [PubMed]
- Fairweather, A. D., & Ralston, I. B. M. (1993). The Neolithic timber hall at Balbridie, Grampian Region, Scotland: The building, the date, the plant macrofossils. Antiquity, 67(255), 313–323. [Google Scholar] [CrossRef]
- Fernandes, D. M., Strapagiel, D., Borówka, P., Marciniak, B., Żądzińska, E., Sirak, K., Siska, V., Grygiel, R., Carlsson, J., Manica, A., Lorkiewicz, W., & Pinhasi, R. (2018). A genomic Neolithic time transect of hunter-farmer admixture in central Poland. Scientific Reports, 8(1), 1–11. [Google Scholar] [CrossRef]
- Fernandes, R., Grootes, P. M., Nadeau, M.-J., & Nehlich, O. (2015). Quantitative diet reconstruction of a Neolithic population using a Bayesian mixing model (FRUITS): The case study of Ostorf (Germany). American Journal of Physical Anthropology, 158(2), 325–340. [Google Scholar] [CrossRef]
- Fernandes, R., Millard, A. R., Brabec, M., Nadeau, M.-J., & Grootes, P. (2014). Food Reconstruction Using Isotopic Transferred Signals (FRUITS): A Bayesian model for diet reconstruction. PLoS ONE, 9(2), e87436. [Google Scholar] [CrossRef]
- Fernandes, R., Nadeau, M.-J., & Grootes, P. M. (2012). Macronutrient-based model for dietary carbon routing in bone collagen and bioapatite. Archaeological and Anthropological Sciences, 4(4), 291–301. [Google Scholar] [CrossRef]
- Fornander, E. (2011). Consuming and communicating identities: Dietary diversity and interaction in middle neolithic sweden [Doctoral dissertation, Stockholm University]. Available online: https://www.diva-portal.org/smash/get/diva2:439410/FULLTEXT01.pdf (accessed on 2 February 2023).
- Fort, J. (2012). Synthesis between demic and cultural diffusion in the Neolithic transition in Europe. Proceedings of the National Academy of Sciences, 109(46), 18669–18673. [Google Scholar] [CrossRef]
- Fry, B. (2013). Alternative approaches for solving underdetermined isotope mixing problems. Marine Ecology Progress Series, 472, 1–13. [Google Scholar] [CrossRef]
- García-Escárzaga, A., & Gutiérrez-Zugasti, I. (2021). The role of shellfish in human subsistence during the Mesolithic of Atlantic Europe: An approach from meat yield estimations. Quaternary International, 584, 9–19. [Google Scholar] [CrossRef]
- Gron, K. J., & Sørensen, L. (2018). Cultural and economic negotiation: A new perspective on the Neolithic Transition of Southern Scandinavia. Antiquity, 92(364), 958–974. [Google Scholar] [CrossRef]
- Groom, P., Pickard, C., & Bonsall, C. (2019). Early Holocene sea fishing in western Scotland: An experimental study. The Journal of Island and Coastal Archaeology, 14(3), 426–450. [Google Scholar] [CrossRef]
- Haak, W., Balanovsky, O., Sanchez, J. J., Koshel, S., Zaporozhchenko, V., Adler, C. J., Der Sarkissian, C. S. I., Brandt, G., Schwarz, C., Nicklisch, N., Dresely, V., Fritsch, B., Balanovska, E., Villems, R., Meller, H., Alt, K. W., Cooper, A., & the Genographic Consortium. (2010). Ancient DNA from European Early Neolithic farmers reveals their Near Eastern affinities. PLoS Biology, 8(11), e1000536. [Google Scholar] [CrossRef]
- Halffman, C. M., Potter, B. A., McKinney, H. J., Tsutaya, T., Finney, B. P., Kemp, B. M., Bartelink, E. J., Wooller, M. J., Buckley, M., Clark, C. T., Johnson, J. J., Bingham, B. L., Lanoë, B., Sattler, R. A., & Reuther, J. D. (2020). Ancient Beringian paleodiets revealed through multiproxy stable isotope analyses. Science Advances, 6(36), eabc1968. [Google Scholar] [CrossRef] [PubMed]
- Hare, P. E., Fogel, M. L., Stafford, T. W., Mitchell, A. D., & Hoering, T. C. (1991). The isotopic composition of carbon and nitrogen in individual amino acids isolated from modern and fossil proteins. Journal of Archaeological Science, 18(3), 277–292. [Google Scholar] [CrossRef]
- Hedges, R. E. M. (2003). On bone collagen—apatite-carbonate isotopic relationships. International Journal of Osteoarchaeology, 13((1-2)), 66–79. [Google Scholar] [CrossRef]
- Hedges, R. E. M., & Reynard, L. M. (2007). Nitrogen isotopes and the trophic level of humans in archaeology. Journal of Archaeological Science, 34(8), 1240–1251. [Google Scholar] [CrossRef]
- Hildebrand, G. V., Farley, S. D., Robbins, C. T., Hanley, T. A., Titus, K., & Servheen, C. (1996). Use of stable isotopes to determine diets of living and extinct bears. Canadian Journal of Zoology, 74(11), 2080–2088. [Google Scholar] [CrossRef]
- Jørkov, M. L. S., Heinemeier, J., & Lynnerup, N. (2009). The petrous bone—A new sampling site for identifying early dietary patterns in stable isotopic studies. American Journal of Physical Anthropology, 138(2), 199–209. [Google Scholar] [CrossRef]
- Keeling, R. F., Graven, H. D., Welp, L. R., & Meijer, H. A. J. (2017). Atmospheric evidence for a global secular increase in carbon isotopic discrimination of land photosynthesis. Proceedings of the National Academy of Sciences, 114(39), 10361–10366. [Google Scholar] [CrossRef] [PubMed]
- Knight, M., Sheridan, A., Skoglund, P., Booth, T., Brown, L., Reich, D., Armit, I., Bonsall, C., Anastasiadou, K., Boyle, A., Büster, L. S., Oswald, M., Carver, H., Gilardet, A., Kelly, M., McCabe, J., Montgomery, J., Pickard, C., Rhodes, D., Silva, M., … Williams, M. (2021). A summary round-up list of Scottish archaeological human remains that have been sampled/analysed for DNA between January 2019 and November 2021. DES, 21, 201. [Google Scholar]
- Lipson, M., Szécsényi-Nagy, A., Mallick, S., Pósa, A., Stégmár, B., Keerl, V., Rohland, N., Stewardson, K., Ferry, M., Michel, M., Oppenheimer, J., Broomandkhoshbacht, N., Harney, E., Nordenfelt, S., Llamas, B., Mende, B. G., Köhler, K., Oross, K., Bondár, M., & Reich, D. (2017). Parallel palaeogenomic transects reveal complex genetic history of early European farmers. Nature, 551(7680), 368–372. [Google Scholar] [CrossRef]
- Longin, R. (1971). New Method of Collagen Extraction for Radiocarbon Dating. Nature, 230, 241–242. [Google Scholar] [CrossRef]
- Mathieson, I., Lazaridis, I., Rohland, N., Mallick, S., Patterson, N., Roodenberg, S. A., Harney, E., Stewardson, K., Fernandes, D., Novak, M., Sirak, K., Gamba, C., Jones, E. R., Llamas, B., Dryomov, S., Pickrell, J., Arsuaga, J. L., de Castro, J. M. B., Carbonell, E., & Reich, D. (2015). Genome-wide patterns of selection in 230 ancient Eurasians. Nature, 528(7583), 499–503. [Google Scholar] [CrossRef]
- Milner, N., & Craig, O. E. (2009). Mysteries of the middens: Change and continuity across the Mesolithic–Neolithic transition. In M. J. Allen, N. Sharples, & T. O’Connor (Eds.), Land and people. Papers in honour of John G. Evans. Prehistoric Society research paper no. 2 (pp. 169–180). Oxbow Books. [Google Scholar]
- Milner, N., & Craig, O. E. (2012). Isotope analyses. In A. Saville, K. Hardy, R. Miket, T. B. Ballin, & A. Corran (Eds.), Staffin, Skye: A rockshelter with Mesolithic and later occupation (pp. 77–79). SAIR. [Google Scholar] [CrossRef]
- Minagawa, M., & Wada, E. (1984). Stepwise enrichment of 15N along food chains: Further evidence and the relation between δ15N and animal age. Geochimica et Cosmochimica Acta, 48(5), 1135–1140. [Google Scholar] [CrossRef]
- Mithen, S. (2022). How long was the Mesolithic–Neolithic overlap in western Scotland? Evidence from the 4th millennium BC on the Isle of Islay and the evaluation of three scenarios for Mesolithic–Neolithic interaction. Proceedings of the Prehistoric Society, 88, 53–77. [Google Scholar] [CrossRef]
- Mithen, S., Pirie, A., Smith, S., & Wicks, K. (2007). The Mesolithic–Neolithic transition in western Scotland: A review and new evidence from Tiree. In A. Whittle, & V. Cummings (Eds.), Going over: The Mesolithic-Neolithic transition in North-West Europe (pp. 511–541). Oxford University Press. [Google Scholar]
- Montgomery, J., Beaumont, J., Jay, M., Keefe, K., Gledhill, A. R., Cook, G. T., Dockrill, S. J., & Melton, N. D. (2013). Strategic and sporadic marine consumption at the onset of the Neolithic: Increasing temporal resolution in the isotope evidence. Antiquity, 87(338), 1060–1072. [Google Scholar] [CrossRef]
- Newsome, S. D., Phillips, D. L., Culleton, B. J., Guilderson, T. P., & Koch, P. L. (2004). Dietary reconstruction of an Early to Middle Holocene human population from the central California coast: Insights from advanced stable isotope mixing models. Journal of Archaeological Science, 31(8), 1101–1115. [Google Scholar] [CrossRef]
- O’Connell, T. C., Kneale, C. J., Tasevska, N., & Kuhnle, G. G. C. (2012). The diet-body offset in human nitrogen isotopic values: A controlled dietary study. American Journal of Physical Anthropology, 149(3), 426–434. [Google Scholar] [CrossRef]
- Olalde, I., Brace, S., Allentoft, M. E., Armit, I., Kristiansen, K., Booth, T., Rohland, N., Mallick, S., Szécsényi-Nagy, A., Mittnik, A., Altena, E., Lipson, M., Lazaridis, I., Harper, T. K., Patterson, N., Broomandkhoshbacht, N., Diekmann, Y., Faltyskova, Z., Fernandes, D., & Reich, D. (2018). The Beaker phenomenon and the genomic transformation of Northwest Europe. Nature, 555(7695), 190–196. [Google Scholar] [CrossRef] [PubMed]
- Olalde, I., Mallick, S., Patterson, N., Rohland, N., Villalba-Mouco, V., Silva, M., Dulias, K., Edwards, C. J., Gandini, F., Pala, M., Soares, P., Ferrando-Bernal, M., Adamski, N., Broomandkhoshbacht, N., Cheronet, O., Culleton, B. J., Fernandes, D., Lawson, A. M., Mah, M., & Reich, D. (2019). The genomic history of the Iberian Peninsula over the past 8000 years. Science, 363(6432), 1230–1234. [Google Scholar] [CrossRef]
- Parnell, A. C., Inger, R., Bearhop, S., & Jackson, A. L. (2010). Source partitioning using stable isotopes: Coping with too much variation. PLoS ONE, 5(3), e9672. [Google Scholar] [CrossRef] [PubMed]
- Patterson, N., Isakov, M., Booth, T., Büster, L., Fischer, C., Olalde, I., Ringbauer, H., Akbari, A., Cheronet, O., Bleasdale, M., Adamski, N., Altena, E., Bernardos, R., Brace, S., Broomandkhoshbacht, N., Callan, K., Candilio, F., Culleton, B., Curtis, E., & Reich, D. (2022). Large-scale migration into Britain during the Middle to Late Bronze Age. Nature, 601(7894), 588–594. [Google Scholar] [CrossRef] [PubMed]
- Peterson, R. (2019). Neolithic cave burials. In Agency, structure and environment. Manchester University Press. [Google Scholar] [CrossRef]
- Phillips, D. L., Inger, R., Bearhop, S., Jackson, A. L., Moore, J. W., Parnell, A. C., Semmens, B. X., & Ward, E. J. (2014). Best practices for use of stable isotope mixing models in food-web studies. Canadian Journal of Zoology, 92(10), 823–835. [Google Scholar] [CrossRef]
- Phillips, D. L., & Koch, P. L. (2002). Incorporating concentration dependence in stable isotope mixing models. Oecologia, 130(1), 114–125. [Google Scholar] [CrossRef]
- Pickard, C., & Bonsall, C. (2020). Post-glacial hunter-gatherer subsistence patterns in Britain: Dietary reconstruction using FRUITS. Archaeological and Anthropological Sciences, 12(7), 1–22. [Google Scholar] [CrossRef]
- Pickard, C., & Bonsall, C. (2022). Reassessing Neolithic diets in western Scotland. Humans, 2(4), 226–250. [Google Scholar] [CrossRef]
- Piličiauskas, G., Jankauskas, R., Piličiauskienė, G., & Dupras, T. (2017). Reconstructing Subneolithic and Neolithic diets of the inhabitants of the SE Baltic coast (3100–2500 cal BC) using stable isotope analysis. Archaeological and Anthropological Sciences, 9(7), 1421–1437. [Google Scholar] [CrossRef]
- Post, D. M. (2002). Using stable isotopes to estimate trophic position: Models, methods, and assumptions. Ecology, 83, 703–718. [Google Scholar] [CrossRef]
- Reimer, P. J., Austin, W. E. N., Bard, E., Bayliss, A., Blackwell, P. G., Ramsey, C. B., Butzin, M., Cheng, H., Edwards, R. L., Friedrich, M., Grootes, P. M., Guilderson, T. P., Hajdas, I., Heaton, T. J., Hogg, A. G., A Hughen, K., Kromer, B., Manning, S. W., Muscheler, R., & Talamo, S. (2020). The IntCal20 northern hemisphere radiocarbon age calibration curve (0–55 cal kBP). Radiocarbon, 62(4), 725–757. [Google Scholar] [CrossRef]
- Richards, M., & Hedges, R. E. M. (1999). Stable isotope evidence for similarities in the types of marine foods used by Late Mesolithic humans at sites along the Atlantic coast of Europe. Journal of Archaeological Science, 26(6), 717–722. [Google Scholar] [CrossRef]
- Richards, M., & Mellars, P. (1998). Stable isotopes and the seasonality of the Oronsay middens. Antiquity, 72(275), 178–184. [Google Scholar] [CrossRef]
- Richards, M., Schulting, R., & Hedges, R. (2003). Sharp shift in diet at onset of Neolithic. Nature, 425(6956), 366. [Google Scholar] [CrossRef]
- Richards, M. P., & Schulting, R. (2006). Touch not the Fish: The Mesolithic–Neolithic change of diet and its significance. Antiquity, 80(308), 444–456. [Google Scholar] [CrossRef]
- Rivero, D. G., Taylor, R., Umbelino, C., Cubas, M., Barrera Cruz, M., & Díaz Rodríguez, M. J. (2021). Early Neolithic ritual funerary behaviours in the western-most regions of the Mediterranean: New insights from Dehesilla Cave (southern Iberian Peninsula). Documenta Praehistorica, 48, 298–327. [Google Scholar] [CrossRef]
- Rivollat, M., Jeong, C., Schiffels, S., Küçükkalıpçı, I., Pemonge, M., Rohrlach, A. B., Alt, K. W., Binder, D., Friederich, S., Ghesquière, E., Gronenborn, D., Laporte, L., Lefranc, P., Meller, H., Réveillas, H., Rosenstock, E., Rottier, S., Scarre, C., Soler, L., & Haak, W. (2020). Ancient genome-wide DNA from France highlights the complexity of interactions between Mesolithic hunter-gatherers and Neolithic farmers. Science Advances, 6(22), eaaz5344. [Google Scholar] [CrossRef] [PubMed]
- Robbins, C. T., Hilderbrand, G. V., & Farley, S. D. (2002). Incorporating concentration dependence in stable isotope mixing models: A response to Phillips and Koch (2002). Oecologia, 133(1), 10–13. [Google Scholar] [CrossRef]
- Russell, N., Bonsall, C., & Sutherland, D. G. (1995). The role of shellfish-gathering in the Mesolithic of western Scotland: The evidence from Ulva Cave, Inner Hebrides. In A. Fischer (Ed.), Man and sea in the Mesolithic. Coastal settlement above and below the present sea level (pp. 273–288). Oxbow Books. [Google Scholar]
- Saville, A., Hardy, K., Miket, R., & Ballin, T. B. (2012). An Corran, Staffin, Skye: A Rockshelter with Mesolithic and Later Occupation. Scottish Archaeological Internet Reports, 51, 1–101. [Google Scholar] [CrossRef]
- Sayle, K. L., Brodie, C. R., Cook, G. T., & Hamilton, W. D. (2019). Sequential measurement of δ15N, δ13C and δ34S values in archaeological bone collagen at the Scottish Universities Environmental Research Centre (SUERC): A new analytical frontier. Rapid Communications in Mass Spectrometry, 33(15), 1258–1266. [Google Scholar] [CrossRef]
- Schier, W. (2022). Modes and models of neolithization in Europe: Comments to an ongoing debate. In P. F. Biehl, & E. Rosenstock (Eds.), 6000 BC: Transformation and change in the Near East and Europe (pp. 372–392). Cambridge University Press. [Google Scholar]
- Schulting, R. J. (1998). Slighting the sea: Stable isotope evidence for the transition to farming in northwestern Europe. Documenta Praehistorica, 25, 203–218. [Google Scholar]
- Schulting, R. J., & Borić, D. (2017). A tale of two processes of Neolithisation: Southeast Europe and Britain/Ireland. In P. Bickle, V. Cummings, D. Hofmann, & J. Pollard (Eds.), The Neolithic of Europe: Papers in honour of Alasdair Whittle (pp. 82–106). Oxbow Books. [Google Scholar]
- Schulting, R. J., MacDonald, R., & Richards, M. P. (2023). FRUITS of the sea? A cautionary tale regarding Bayesian modelling of palaeodiets using stable isotope data. Quaternary International, 650, 52–61. [Google Scholar] [CrossRef]
- Schulting, R. J., & Richards, M. P. (2002). The wet, the wild and the domesticated: The Mesolithic–Neolithic transition on the west coast of Scotland. European Journal of Archaeology, 5(2), 147–189. [Google Scholar] [CrossRef]
- Schwarcz, H. P. (1991). Some theoretical aspects of isotope paleodiet studies. Journal of Archaeological Science, 18(3), 261–275. [Google Scholar] [CrossRef]
- Scott, J. G. (1963). The excavation of the chambered cairn at Crarae, Loch Fyneside, Mid Argyll. Proceedings of the Society of Antiquaries of Scotland, 94, 1–27. [Google Scholar] [CrossRef]
- Sheridan, A. (2010). The Neolithization of Britain and Ireland: The ‘Big Picture’. In B. Finlayson, & G. Warren (Eds.), Landscapes in transition (pp. 89–105). Oxbow Books. [Google Scholar]
- Sheridan, A. (2012). Contextualising Kilmartin: Building a narrative for developments in western Scotland and beyond, from the Early Neolithic to the Late Bronze Age. In A. M. Jones, J. Pollard, M. J. Allen, & J. Gardiner (Eds.), Image, memory and monumentality (pp. 163–183). Prehistoric Society Research Paper No. 5. Oxbow Books. [Google Scholar]
- Sheridan, A., & Whittle, A. (2023). aDNA and modelling the Mesolithic–Neolithic transition in Britain and Ireland. In A. Whittle, J. Pollard, & S. Greaney (Eds.), Ancient DNA and the European Neolithic, relations and descent (pp. 169–182). Oxbow Books. [Google Scholar]
- Sheridan, J. A., & Schulting, R. J. (2020). Making sense of Scottish Neolithic funerary monuments: Tracing trajectories and understanding their rationale. In A. B. Gebauer, L. Sørensen, A. Teather, & A. C. Valera (Eds.), Monumentalising life in the Neolithic: Narratives of change and continuity (pp. 195–215). Oxbow Books. [Google Scholar]
- Silvestri, L., Achino, K. F., Gatta, M., Rolfo, M. F., & Salari, L. (2020). Grotta Mora Cavorso: Physical, material and symbolic boundaries of life and death practices in a Neolithic cave of central Italy. Quaternary International, 539, 29–38. [Google Scholar] [CrossRef]
- Sparacello, V., Varalli, A., Rossi, S., Panelli, C., Goude, G., Palstra, S., Conventi, M., Del Lucchese, A., Arobba, D., De Pascale, A., Zavattaro, M., Garibaldi, P., Rossi, G., Molinari, I., Maggi, R., Moggi-Cecchi, J., Starnini, E., Biagi, P., & Dori, I. (2020). Dating the funerary use of caves in Liguria (northwestern Italy) from the Neolithic to historic times: Results from a large-scale AMS campaign on human skeletal series. Quaternary International, 536, 30–44. [Google Scholar] [CrossRef]
- Stevens, C. J., Crema, E. R., & Shoda, S. (2022). The importance of wild resources as a reflection of the resilience and changing nature of early agricultural systems in East Asia and Europe. Frontiers in Ecology and Evolution, 10, 1017909. [Google Scholar] [CrossRef]
- Stevens, C. J., & Fuller, D. Q. (2012). Did Neolithic farming fail? The case for a Bronze Age agricultural revolution in the British Isles. Antiquity, 86, 707–722. [Google Scholar] [CrossRef]
- Stevens, C. J., & Fuller, D. Q. (2015). Alternative strategies to agriculture: The evidence for climatic shocks and cereal declines during the British Neolithic and Bronze Age (a reply to Bishop). World Archaeology, 47, 856–875. [Google Scholar] [CrossRef]
- Tauber, H. (1981). 13C evidence for dietary habits of prehistoric man in Denmark. Nature, 292, 332–333. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J. (1991). Rethinking the Neolithic. Cambridge University Press. [Google Scholar]
- Thomas, J. (2004). Recent debates on the Mesolithic–Neolithic transition in Britain and Ireland. Documenta Praehistorica, 31, 113–130. [Google Scholar] [CrossRef]
- Thomas, J. (2013). The birth of Neolithic Britain: An interpretive account. Oxford University Press. [Google Scholar] [CrossRef]
- Turner, W. (1895). On human and animal remains found in caves at Oban, Argyllshire. Proceedings of the Society of Antiquaries of Scotland, 29, 410–438. [Google Scholar] [CrossRef]
- Vanderklift, M. A., & Ponsard, S. (2003). Sources of variation in consumer-diet δ15N enrichment: A meta-analysis. Oecologia, 136, 169–182. [Google Scholar] [CrossRef]
- Vogel, J. C., & van der Merwe, N. J. (1977). Isotopic evidence for early maize cultivation in New York State. American Antiquity, 42(2), 238–242. [Google Scholar] [CrossRef]
- Zerjal, T., Xue, Y., Bertorelle, G., Wells, R. S., Bao, W., Zhu, S., Qamar, R., Ayub, Q., Mohyuddin, A., Fu, S., & Li, P. (2003). The genetic legacy of the Mongols. American Journal of Human Genetics, 72(3), 717–721. [Google Scholar] [CrossRef]
- Zilhão, J. (2011). Time is on my side. In A. Hadjikoumis, E. Robinson, & S. Viner (Eds.), Dynamics of Neolithisation in Europe: Studies in honour of Andrew Sherratt (pp. 46–65). Oxbow Books. [Google Scholar]
- Zvelebil, M., & Rowley-Conwy, P. (1984). Transition to farming in Northern Europe: A hunter-gatherer perspective. Norwegian Archaeological Review, 17(2), 104–128. [Google Scholar] [CrossRef]

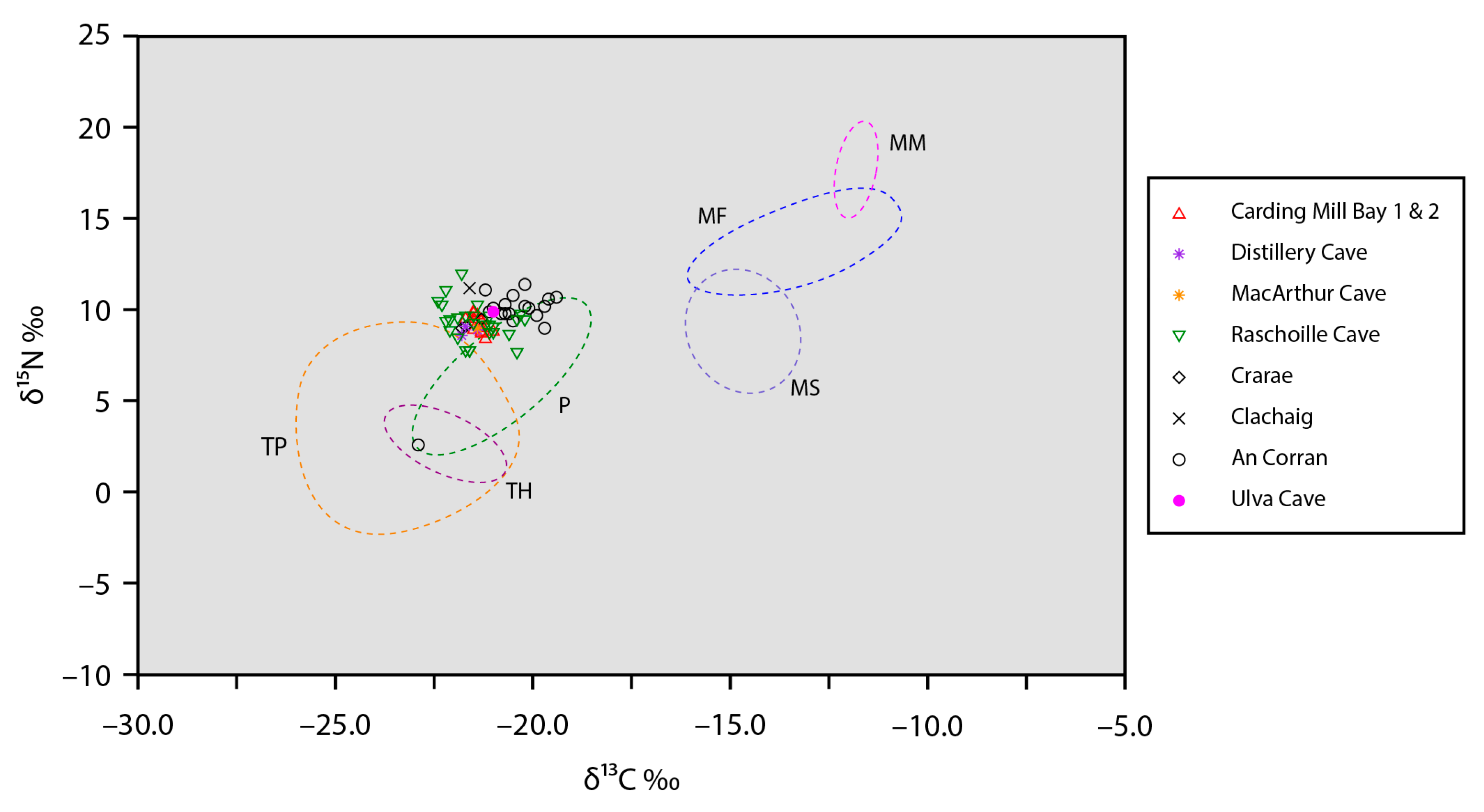
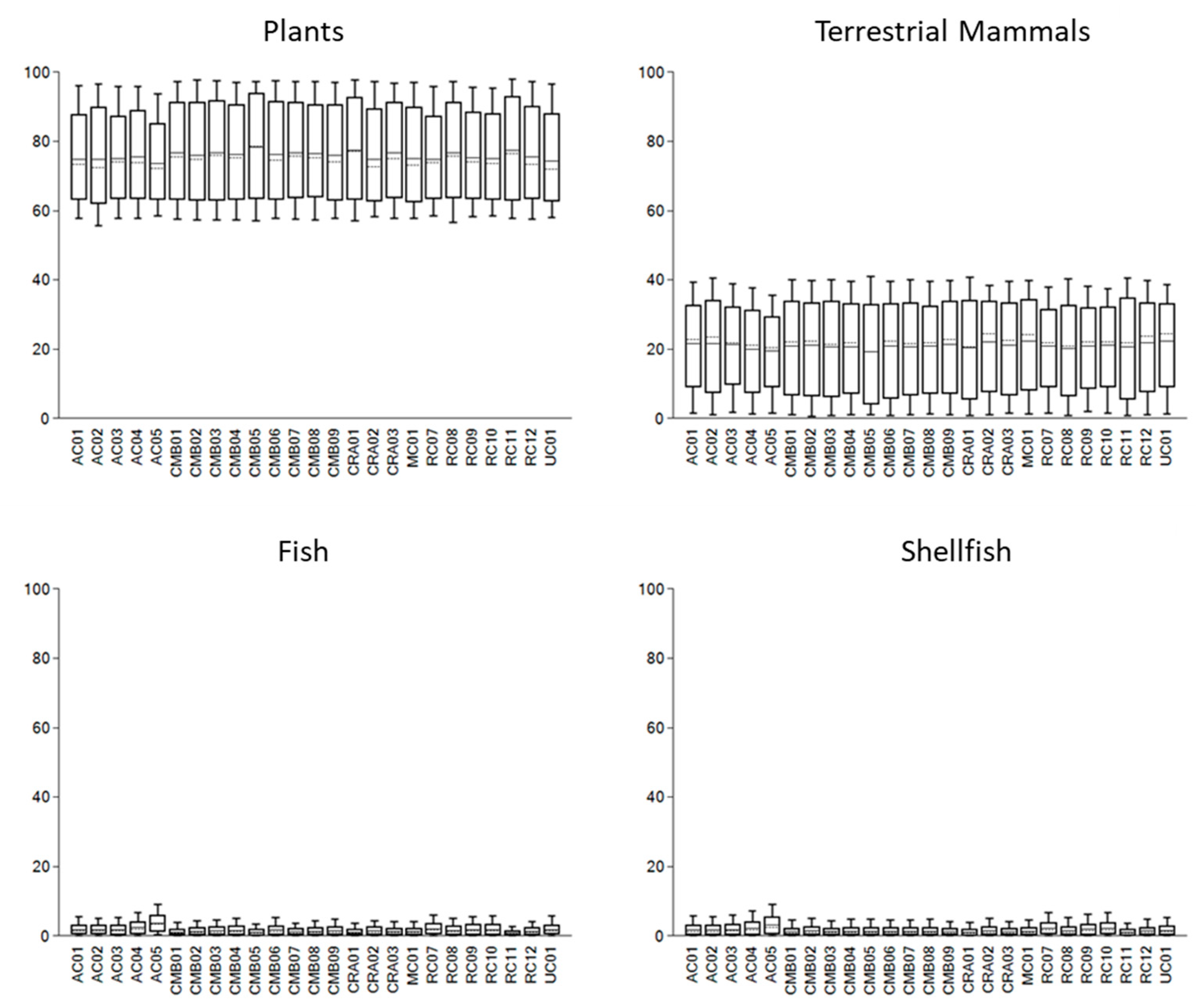

| Site | ID | Sex | Date Type | Lab Code | 14C (BP) | Calendar Years (Cal BCE/BCE) | Median (Cal BCE/BCE) | Ancestry | % WHG |
|---|---|---|---|---|---|---|---|---|---|
| MacArthur Cave | I2657 | M | 14C | SUERC-68701 | 5052 ± 30 | 3960–3770 | 3872 | Farmer | 21.8 |
| MacArthur Cave | I2658 | M | Contextual | n/a | n/a | 4000–3700 | 3850 | Farmer | 21.5 |
| Distillery Cave | I2659 | F | 14C | SUERC-68702 | 4914 ± 27 | 3770–3640 | 3686 | Farmer | 23.4 |
| Ulva Cave | I12312 | M | 14C | PSUAMS-5771 | 4895 ± 25 | 3760–3630 | 3679 | Admixed farmer/hunter-gatherer | 40.4 |
| Distillery Cave | I2691 | M | 14C | SUERC-68704 | 4881 ± 25 | 3710–3630 | 3653 | Admixed farmer/hunter-gatherer | 30.7 |
| Raschoille Cave | I5370 | F | Contextual | n/a | n/a | 4000–3300 | 3650 | Farmer | 25.1 |
| Raschoille Cave | I5371 | F | Contextual | n/a | n/a | 4000–3300 | 3650 | Admixed farmer/hunter-gatherer | 26.2 |
| Carding Mill Bay 2 | I12314 | F | 14C | PSUAMS-5772 PSUAMS-5773 PSUAMS-5774 PSUAMS-5776 | 4832 ± 14 | 3650–3530 | 3634 | Admixed farmer/hunter-gatherer | 48.8 |
| Raschoille Cave | I3135 | M | 14C | PSUAMS-2068 | 4770 ± 30 | 3640–3380 | 3576 | Admixed farmer/hunter-gatherer | 37.0 |
| Carding Mill Bay 2 | I12313 | F | Contextual | n/a | n/a | 3700–3350 | 3525 | Admixed farmer/hunter-gatherer | 26.8 |
| Raschoille Cave | I3134 | M | 14C | PSUAMS-2155 | 4730 ± 25 | 3630–3370 | 3524 | Farmer | 24.5 |
| Carding Mill Bay 2 | I12317 | M | 14C | PSUAMS-5775 | 4725 ± 25 | 3630–3370 | 3524 | Admixed farmer/hunter-gatherer | 30.0 |
| Raschoille Cave | I3133 | M | 14C | PSUAMS-2154 | 4725 ± 20 | 3630–3370 | 3514 | Farmer | 24.3 |
| Raschoille Cave | I3041 | M | Contextual | n/a | n/a | 3950–3030 | 3490 | Admixed farmer/hunter-gatherer | 31.0 |
| Raschoille Cave | I3136 | F | 14C | PSUAMS-2069 | 4665 ± 30 | 3520–3370 | 3450 | Farmer | 20.5 |
| Clachaig | I2988 | F | 14C | SUERC-68711 | 4645 ± 29 | 3520–3360 | 3458 | Farmer | 17.5 |
| Distillery Cave | I2660 | M | 14C | SUERC-68703 | 4631 ± 29 | 3520–3350 | 3462 | Admixed farmer/hunter-gatherer | 25.0 |
| Raschoille Cave | I3137 | M | Contextual | n/a | n/a | 3800–3000 | 3400 | Farmer | 23.6 |
| Raschoille Cave | I3139_d | F | Contextual | n/a | n/a | 3800–3000 | 3400 | Admixed farmer/hunter-gatherer | 45.0 |
| Raschoille Cave | I3138 | F | 14C | PSUAMS-2156 | 4415 ± 25 | 3320–2920 | 3040 | Farmer | 23.4 |
| Parameter | Model | Description |
|---|---|---|
| Δ15Ndiet–consumer | Both | 4.6 ± 0.5‰ |
| Δ13Cdiet–consumer | Both | 4.8 ± 0.5‰ |
| Manured cereals δ13C and δ15N | Model A | δ13Cprotein = 26 ± 1.0‰ and δ15Nmanured = 5.0 ± 2.0‰ |
| Unmanured cereals δ13C and δ15N | Model B | δ13Cprotein = 26 ± 1.0‰ and δ15Nmanured = 3.0 ± 1.0‰ |
| Terrestrial herbivores δ13C and δ15N | Both | Terrestrial herbivores δ13Cprotein = − 24.7 ± 0.1‰ and δ15N = 3.4 ± 0.1‰ |
| Young herbivores δ13C and δ15N | Both | Young herbivores δ13Cprotein = − 23.7 ± 0.1‰ and δ15N = 5.4 ± 0.1‰ |
| Site | Context | Individual | Terrestrial Herbivore | Young Herbivore | Cereals | |||
|---|---|---|---|---|---|---|---|---|
| Mean % | CI (68%) | Mean % | CI (68%) | Mean % | CI (68%) | |||
| CMB 1 | XXIII | OxA-7890 | 24 ± 16 | 7–41 | 18 ± 14 | 5–33 | 58 ± 17 | 41–76 |
| CMB 1 | VII | OxA-7665 | 22 ± 16 | 5–40 | 17 ± 13 | 4–31 | 60 ± 16 | 43–78 |
| CMB 1 | XV | OxA-7664 | 35 ± 22 | 11–58 | 20 ± 15 | 5–36 | 45 ± 21 | 23–68 |
| CMB 1 | XIV | OxA-7663 | 26 ± 19 | 7–46 | 16 ± 13 | 4–29 | 58 ± 19 | 38–77 |
| Site | Context | Individual | Terrestrial Herbivore | Young Herbivore | Cereals | |||
|---|---|---|---|---|---|---|---|---|
| Mean % | CI (68%) | Mean % | CI (68%) | Mean % | CI (68%) | |||
| CMB 1 | XXIII | OxA-7890 | 17 ± 14 | 4–31 | 27 ± 16 | 9–44 | 56 ± 17 | 41–76 |
| CMB 1 | VII | OxA-7665 | 17 ± 14 | 4–31 | 23 ± 15 | 4–31 | 60 ± 17 | 43–78 |
| CMB 1 | XV | OxA-7664 | 28 ± 20 | 7–49 | 25 ± 15 | 9–42 | 47 ± 18 | 29–66 |
| CMB 1 | XIV | OxA-7663 | 22 ± 17 | 5–41 | 20 ± 14 | 6–35 | 57 ± 17 | 40–76 |
| Site | Date | Species | Common Name | δ13C ‰ | δ15N ‰ | C:N | Lab/Specimen ID | Reference |
|---|---|---|---|---|---|---|---|---|
| TERRESTRIAL MAMMALS | ||||||||
| An Corran | Mesolithic/Neolithic | Bos | Cattle | −22.2 | 1.6 | Milner and Craig (2009) | ||
| An Corran | Mesolithic/Neolithic | Bos | Cattle | −22.0 | 1.7 | Milner and Craig (2009) | ||
| Carding Mill Bay 1 | Neolithic | Bos taurus (probable) | Cattle | −23.3 | 3.5 | 3.3 | GU39625 (XIV) | J. Bownes (2018) |
| Carding Mill Bay 1 | Neolithic | Bos taurus (probable) | Cattle | −23.2 | 3.3 | 3.3 | GU39626 (XIV) | J. Bownes (2018) |
| Carding Mill Bay 1 | Neolithic | Bos taurus (probable) | Cattle | −23.3 | 3.5 | 3.3 | GU39627 (XIV) | J. Bownes (2018) |
| Carding Mill Bay 1 | Neolithic | Bos taurus (probable) | Cattle | −23.4 | 3.4 | 3.3 | GU39628 (XIV) | J. Bownes (2018) |
| Carding Mill Bay 1 | Neolithic | Bos taurus (probable) | Cattle | −22.6 | 3.8 | 3.3 | GU39629 (XV) | J. Bownes (2018) |
| Carding Mill Bay 1 | Neolithic | Bos taurus (probable) | Cattle | −23.3 | 4.2 | 3.3 | GU39630 (XV) | J. Bownes (2018) |
| Carding Mill Bay 1 | Neolithic | Bos taurus (probable) | Cattle | −23.4 | 3.4 | 3.3 | GU39631 (XV) | J. Bownes (2018) |
| Carding Mill Bay 1 | Neolithic | Bos taurus (probable) | Cattle | −22.0 | 3.0 | 3.3 | GU39632 (XV) | J. Bownes (2018) |
| An Corran | Mesolithic/Neolithic | Cervus elaphus | Red deer | −21.2 | 1.0 | Milner and Craig (2009) | ||
| An Corran | Mesolithic/Neolithic | Cervus elaphus | Red deer | −22.5 | 2.4 | Milner and Craig (2009) | ||
| Carding Mill Bay 1 | Neolithic | Cervus elaphus | Red deer | −23.2 | 3.0 | 3.4 | GUsi3509 (XVII) | J. Bownes (2018) |
| Carding Mill Bay 1 | Neolithic | Cervus elaphus | Red deer | −21.9 | 2.0 | CVII:123 | Schulting and Richards (2002) | |
| Carding Mill Bay 1 | Neolithic | Cervus elaphus | Red deer | −22.9 | 2.5 | C XVII:4 | Schulting and Richards (2002) | |
| Raschoille Cave | 7640 ± 80 BP | Cervus elaphus | Red deer | −21.8 | 2.9 | OxA-8396 | Pickard and Bonsall (2022) | |
| Raschoille Cave | 7575 ± 75 BP | Cervus elaphus | Red deer | −21.5 | 2.8 | OxA-8397 | Pickard and Bonsall (2022) | |
| Raschoille Cave | 7480 ± 75 BP | Cervus elaphus | Red deer | −21.6 | 2.6 | OxA-8398 | Pickard and Bonsall (2022) | |
| Ulva Cave | Mesolithic | Cervus elaphus | Red deer | −2.8 | 2.6 | 3.4 | GUsi8894 | this study |
| Carding Mill Bay 1 | Neolithic | Cervus elaphus/Bos taurus | Red deer/Cattle | −23.2 | 3.7 | 3.3 | GUsi3505 | J. M. Bownes et al. (2017) |
| Carding Mill Bay 1 | Neolithic | Cervus elaphus/Bos taurus | Red deer/Cattle | −22.5 | 2.4 | 3.2 | GUsi3506 | J. M. Bownes et al. (2017) |
| Carding Mill Bay 1 | Neolithic | Cervus elaphus/Bos taurus | Red deer/Cattle | −23.2 | 2.3 | 3.2 | GUsi3508 | J. M. Bownes et al. (2017) |
| Carding Mill Bay 1 | Neolithic | Cervus elaphus/Bos taurus | Red deer/Cattle | −22.8 | 3.9 | 3.2 | GUsi3511 | J. M. Bownes et al. (2017) |
| Carding Mill Bay 1 | Neolithic | Ovis aries/Capreolus capreolus | Sheep/Roe deer | −21.6 | 3.5 | 3.2 | GUsi3497 | J. M. Bownes et al. (2017) |
| Carding Mill Bay 1 | Neolithic | Ovis aries/Capreolus capreolus | Sheep/Roe deer | −22.9 | 3.7 | 3.2 | GUsi3507 | J. M. Bownes et al. (2017) |
| Carding Mill Bay 1 | Neolithic | Ovis aries/Capreolus capreolus | Sheep/Roe deer | −23.3 | 3.4 | 3.2 | GUsi3498 | J. M. Bownes et al. (2017) |
| Carding Mill Bay 1 | Neolithic | Ovis aries/Capreolus capreolus | Sheep/Roe deer | −22.8 | 2.8 | 3.5 | GUsi3500 | J. M. Bownes et al. (2017) |
| Carding Mill Bay 1 | Neolithic | Ovis aries/Capreolus capreolus | Sheep/Roe deer | −23.1 | 3.1 | 3.5 | GUsi3501 | J. M. Bownes et al. (2017) |
| Carding Mill Bay 1 | Neolithic | Ovis aries/Capreolus capreolus | Sheep/Roe deer | −22.8 | 2.8 | 3.6 | GUsi3502 | J. M. Bownes et al. (2017) |
| Carding Mill Bay 1 | Neolithic | Ovis aries/Capreolus capreolus | Sheep/Roe deer | −22.5 | 2.7 | 3.4 | GUsi3503 | J. M. Bownes et al. (2017) |
| Carding Mill Bay 1 | Neolithic | Ovis aries/Capreolus capreolus | Sheep/Roe deer | −22.5 | 3.1 | 3.2 | GUsi3504 | J. M. Bownes et al. (2017) |
| Ulva Cave | Mesolithic | Ovis aries/Capreolus capreolus | Sheep/Roe deer | −22.8 | 2.3 | 3.4 | GUsi8895 | this study |
| An Corran | Mesolithic/Neolithic | Sus | Pig | −22.3 | 2.3 | Milner and Craig (2009) | ||
| An Corran | Mesolithic/Neolithic | Sus | Pig | −22.6 | 3.3 | Milner and Craig (2009) | ||
| An Corran | Mesolithic/Neolithic | Sus | Pig | −21.7 | 4.1 | Milner and Craig (2009) | ||
| Carding Mill Bay 1 | Neolithic | Sus | Pig | −21.9 | 3.2 | C XXIV:2 | Schulting and Richards (2002) | |
| Cnoc Coig | Mesolithic | Sus | Pig | −21.2 | 4.3 | Charlton et al. (2016) | ||
| Cnoc Coig | Mesolithic | Sus | Pig | −21.0 | 4.6 | Charlton et al. (2016) | ||
| Cnoc Coig | Mesolithic | Sus | Pig | −18.8 | 10.2 | Charlton et al. (2016) | ||
| Ulva Cave | Mesolithic | Sus | Pig | −21.9 | 6.5 | 3.3 | GUsi8896 | this study |
| MARINE RESOURCES-ARCHAEOLOGICAL SPECIMENS | ||||||||
| Ulva Cave | Mesolithic | Dicentrarchus labrax | Seabass | −12.6 | 13.4 | 3.2 | GUsi8897 | this study |
| An Corran | Mesolithic/Neolithic | Gadus morhua | Cod | −13.6 | 15.3 | 0055-r | Milner and Craig (2012) | |
| Bornish | 12th–13th C. AD | Gadus morhua | Cod | −12.9 | 14.5 | 703 | Barrett et al. (2011) | |
| Bornish | 13th C. AD | Gadus morhua | Cod | −11.3 | 15.4 | 706 | Barrett et al. (2011) | |
| Bornish | 13th C. AD | Gadus morhua | Cod | −13.1 | 13.8 | 708 | Barrett et al. (2011) | |
| Bornish | 12th–13th C. AD | Gadus morhua | Cod | −13.2 | 13.8 | 713 | Barrett et al. (2011) | |
| Ulva Cave | Mesolithic | Labrus bergylta | Ballan wrasse | −16.0 | 11.8 | 3.4 | GUsi8900 | this study |
| Ulva Cave | Mesolithic | Labrus sp. | Wrasse | −12.6 | 14.1 | 3.3 | GUsi8898 | this study |
| Ulva Cave | Mesolithic | Labrus sp. | Wrasse | −12.1 | 14.6 | 3.2 | GUsi8902 | this study |
| Ulva Cave | Mesolithic | Pollachius virens | Saithe | −12.2 | 13.6 | 3.2 | GUsi8899 | this study |
| Ulva Cave | Mesolithic | Pollachius virens | Saithe | −13.4 | 12.1 | 3.2 | GUsi8901 | this study |
| Cnoc Coig | Mesolithic | Pinniped | Seal | −11.6 | 18.8 | 10,502 | Charlton et al. (2016) | |
| Cnoc Coig | Mesolithic | Pinniped | Seal | −11.8 | 19.5 | 10,420 | Charlton et al. (2016) | |
| Airds Bay, Loch Etive | Modern flesh | Patella | Limpet | −14.1 | 6.3 | GUsi3201/3208 | J. Bownes (2018) | |
| Airds Bay, Loch Etive | Modern flesh | Patella | Limpet | −15.0 | 6.7 | GUsi3202/3209 | J. Bownes (2018) | |
| Airds Bay, Loch Etive | Modern flesh | Patella | Limpet | −15.1 | 6.3 | GUsi3204/3211 | J. Bownes (2018) | |
| Airds Bay, Loch Etive | Modern flesh | Patella | Limpet | −15.0 | 7.1 | GUsi3205/3212 | J. Bownes (2018) | |
| Airds Bay, Loch Etive | Modern flesh | Patella | Limpet | −14.0 | 7.0 | GUsi3206/3213 | J. Bownes (2018) | |
| Airds Bay, Loch Etive | Modern flesh | Patella | Limpet | −15.2 | 6.7 | GUsi3207/3214 | J. Bownes (2018) | |
| Oban, Scotland | Modern flesh | Patella | Limpet | −13.6 | 7.8 | GUsi3215/3221 | J. Bownes (2018) | |
| Oban, Scotland | Modern flesh | Patella | Limpet | −15.5 | 6.9 | GUsi3216/3222 | J. Bownes (2018) | |
| Oban, Scotland | Modern flesh | Patella | Limpet | −14.7 | 6.2 | GUsi3217/3223 | J. Bownes (2018) | |
| Oban, Scotland | Modern flesh | Littorina | Periwinkle | −15.3 | 11.7 | GUsi3446/3598 | J. Bownes (2018) | |
| Oban, Scotland | Modern flesh | Littorina | Periwinkle | −13.3 | 8.5 | GUsi3451/3603 | J. Bownes (2018) | |
| PLANTS | ||||||||
| Ulva Cave | Mesolithic | Corylus avellana | Hazelnut (shell) | −26.0 | 9.3 | UA1 | this study | |
| Ulva Cave | Mesolithic | Corylus avellana | Hazelnut (shell) | −24.9 | 7.4 | UA2 | this study | |
| Ulva Cave | Mesolithic | Corylus avellana | Hazelnut (shell) | −27.2 | 8.0 | UA3 | this study | |
| Ulva Cave | Mesolithic | Corylus avellana | Hazelnut (shell) | −25.6 | 5.6 | UA4 | this study | |
| Ulva Cave | Mesolithic | Corylus avellana | Hazelnut (shell) | −24.6 | 6.2 | UA5 | this study | |
| Ulva Cave | Mesolithic | Corylus avellana | Hazelnut (shell) | −25.1 | 5.7 | UMS1 RW | this study | |
| Ulva Cave | Mesolithic | Corylus avellana | Hazelnut (shell) | −23.9 | 0.9 | UMS2 RW | this study | |
| Ulva Cave | Mesolithic | Corylus avellana | Hazelnut (shell) | −25.0 | −1.2 | UMS3 RW | this study | |
| Ulva Cave | Mesolithic | Corylus avellana | Hazelnut (shell) | −27.9 | −1.0 | UMS4 RW | this study | |
| Ulva Cave | Mesolithic | Corylus avellana | Hazelnut (shell) | −27.0 | 0.5 | UMS5 RW | this study | |
| Ulva Cave | Mesolithic | Corylus avellana | Hazelnut (shell) | −25.0 | 3.4 | UMS6 RW | this study | |
| Balbridie | Neolithic | Hordeum vulgare | Naked barley | −24.6 | 0.6 | 24.4 | BB F40 IS.1 | Bishop et al. (2022) |
| Balbridie | Neolithic | Hordeum vulgare | Naked barley | −23.1 | 0.1 | 36.0 | BB F40 IS.2 | Bishop et al. (2022) |
| Balbridie | Neolithic | Hordeum vulgare | Naked barley | −24.5 | 0.8 | 32.0 | BB F40 IS.3 | Bishop et al. (2022) |
| Balbridie | Neolithic | Hordeum vulgare | Naked barley | −24.6 | −1.6 | 37.2 | BB F40 IS.4 | Bishop et al. (2022) |
| Balbridie | Neolithic | Hordeum vulgare | Naked barley | −23.8 | 2.3 | 30.2 | BB F40 IS.5 | Bishop et al. (2022) |
| Balbridie | Neolithic | Hordeum vulgare | Naked barley | −24.8 | 1.4 | 27.0 | BB F40 IS.6 | Bishop et al. (2022) |
| Balbridie | Neolithic | Hordeum vulgare | Naked barley | −24.4 | 1.1 | 25.4 | BB F40 IS.7 | Bishop et al. (2022) |
| Balbridie | Neolithic | Hordeum vulgare | Naked barley | −24.1 | 3.0 | 27.7 | BB F40 IS.8 | Bishop et al. (2022) |
| Balbridie | Neolithic | Hordeum vulgare | Naked barley | −25.2 | 1.4 | 29.3 | BB F40 IS.9 | Bishop et al. (2022) |
| Balbridie | Neolithic | Hordeum vulgare | Naked barley | −22.7 | 1.5 | 27.8 | BB F40 IS.10 | Bishop et al. (2022) |
| Balbridie | Neolithic | Hordeum vulgare | Naked barley | −24.4 | 0.4 | 30.0 | BB F294 IS.32 | Bishop et al. (2022) |
| Balbridie | Neolithic | Hordeum vulgare | Naked barley | −23.9 | 0.5 | 35.5 | BB F294 IS.33 | Bishop et al. (2022) |
| Balbridie | Neolithic | Hordeum vulgare | Naked barley | −25.2 | 0.5 | 26.1 | BB F294 IS.34 | Bishop et al. (2022) |
| Balbridie | Neolithic | Hordeum vulgare | Naked barley | −25.2 | 0.0 | 33.0 | BB F294 IS.35 | Bishop et al. (2022) |
| Balbridie | Neolithic | Hordeum vulgare | Naked barley | −25.2 | 1.3 | 31.0 | BB F294 IS.36 | Bishop et al. (2022) |
| Balbridie | Neolithic | Hordeum vulgare | Naked barley | −23.5 | −0.2 | 28.2 | BB F294 IS.37 | Bishop et al. (2022) |
| Balbridie | Neolithic | Hordeum vulgare | Naked barley | −23.5 | −1.6 | 27.5 | BB F294 IS.38 | Bishop et al. (2022) |
| Balbridie | Neolithic | Hordeum vulgare | Naked barley | −24.6 | 0.9 | 31.0 | BB F294 IS.39 | Bishop et al. (2022) |
| Balbridie | Neolithic | Hordeum vulgare | Naked barley | −24.6 | 2.4 | 27.7 | BB F294 IS.40 | Bishop et al. (2022) |
| Balbridie | Neolithic | Hordeum vulgare | Naked barley | −24.8 | −1.3 | 30.7 | BB F294 IS.41 | Bishop et al. (2022) |
| Skara Brae | Neolithic | Hordeum vulgare | Naked barley | −21.6 | 4.0 | 18.8 | SB C.168 IS.1 | Bishop et al. (2022) |
| Skara Brae | Neolithic | Hordeum vulgare | Naked barley | −23.0 | 2.5 | 25.9 | SB C.168 IS.2 | Bishop et al. (2022) |
| Skara Brae | Neolithic | Hordeum vulgare | Naked barley | −22.7 | 2.5 | 19.5 | SB C.168 IS.3 | Bishop et al. (2022) |
| Skara Brae | Neolithic | Hordeum vulgare | Naked barley | −23.1 | 4.0 | 17.7 | SB C.168 IS.4 | Bishop et al. (2022) |
| Skara Brae | Neolithic | Hordeum vulgare | Naked barley | −22.7 | 4.1 | 23.3 | SB C.168 IS.5 | Bishop et al. (2022) |
| Skara Brae | Neolithic | Hordeum vulgare | Naked barley | −21.0 | 3.1 | 12.3 | SB C.168 IS.6 | Bishop et al. (2022) |
| Skara Brae | Neolithic | Hordeum vulgare | Naked barley | −22.8 | 2.2 | 21.7 | SB C.168 IS.7 | Bishop et al. (2022) |
| Skara Brae | Neolithic | Hordeum vulgare | Naked barley | −22.4 | 5.3 | 17.0 | SB C.168 IS.8 | Bishop et al. (2022) |
| Skara Brae | Neolithic | Hordeum vulgare | Naked barley | −22.3 | 4.5 | 26.0 | SB C.168 IS.9 | Bishop et al. (2022) |
| Skara Brae | Neolithic | Hordeum vulgare | Naked barley | −23.8 | 3.2 | 19.7 | SB C.168 IS.10 | Bishop et al. (2022) |
| Braes of Ha’Breck | Neolithic | Hordeum vulgare | Naked barley | −23.1 | 7.3 | 17.0 | BOH S.506.8 IS1 | Bishop et al. (2022) |
| Braes of Ha’Breck | Neolithic | Hordeum vulgare | Naked barley | −22.1 | 0.2 | 24.7 | BOH S.506.8 IS2 | Bishop et al. (2022) |
| Braes of Ha’Breck | Neolithic | Hordeum vulgare | Naked barley | −24.0 | 1.1 | 22.7 | BOH S.506.8 IS3 | Bishop et al. (2022) |
| Braes of Ha’Breck | Neolithic | Hordeum vulgare | Naked barley | −24.3 | 1.2 | 27.0 | BOH S.506.8 IS4 | Bishop et al. (2022) |
| Braes of Ha’Breck | Neolithic | Hordeum vulgare | Naked barley | −24.1 | 3.8 | 19.8 | BOH S.506.8 IS5 | Bishop et al. (2022) |
| Braes of Ha’Breck | Neolithic | Hordeum vulgare | Naked barley | −24.3 | 1.5 | 30.8 | BOH S.506.8 IS6 | Bishop et al. (2022) |
| Braes of Ha’Breck | Neolithic | Hordeum vulgare | Naked barley | −23.4 | 3.9 | 17.8 | BOH S.506.8 IS7 | Bishop et al. (2022) |
| Braes of Ha’Breck | Neolithic | Hordeum vulgare | Naked barley | −23.3 | 5.9 | 20.1 | BOH S.506.8 IS8 | Bishop et al. (2022) |
| Braes of Ha’Breck | Neolithic | Hordeum vulgare | Naked barley | −25.3 | 5.9 | 28.7 | BOH S.506.8 IS9 | Bishop et al. (2022) |
| Braes of Ha’Breck | Neolithic | Hordeum vulgare | Naked barley | −24.1 | 3.3 | 18.7 | BOH S.506.8 IS10 | Bishop et al. (2022) |
| Braes of Ha’Breck | Neolithic | Hordeum vulgare | Naked barley | −24.0 | 1.7 | 31.7 | BOH S.124 IS1 | Bishop et al. (2022) |
| Braes of Ha’Breck | Neolithic | Hordeum vulgare | Naked barley | −23.2 | 5.1 | 28.1 | BOH S.124 IS2 | Bishop et al. (2022) |
| Braes of Ha’Breck | Neolithic | Hordeum vulgare | Naked barley | −22.5 | 4.0 | 27.6 | BOH S.124 IS3 | Bishop et al. (2022) |
| Braes of Ha’Breck | Neolithic | Hordeum vulgare | Naked barley | −23.2 | 3.5 | 25.2 | BOH S.124 IS4 | Bishop et al. (2022) |
| Braes of Ha’Breck | Neolithic | Hordeum vulgare | Naked barley | −23.9 | 2.6 | 31.3 | BOH S.124 IS5 | Bishop et al. (2022) |
| Braes of Ha’Breck | Neolithic | Hordeum vulgare | Naked barley | −23.2 | 3.0 | 22.8 | BOH S.124 IS6 | Bishop et al. (2022) |
| Braes of Ha’Breck | Neolithic | Hordeum vulgare | Naked barley | −23.9 | 4.1 | 30.5 | BOH S.124 IS7 | Bishop et al. (2022) |
| Braes of Ha’Breck | Neolithic | Hordeum vulgare | Naked barley | −24.2 | 2.2 | 22.1 | BOH S.124 IS8 | Bishop et al. (2022) |
| Braes of Ha’Breck | Neolithic | Hordeum vulgare | Naked barley | −23.9 | 1.0 | 21.8 | BOH S.124 IS9 | Bishop et al. (2022) |
| Braes of Ha’Breck | Neolithic | Hordeum vulgare | Naked barley | −24.0 | 2.7 | 21.9 | BOH S.124 IS10 | Bishop et al. (2022) |
| Braes of Ha’Breck | Neolithic | Hordeum vulgare | Naked barley | −23.1 | 5.7 | 14.0 | BOH S.168 IS1 | Bishop et al. (2022) |
| Braes of Ha’Breck | Neolithic | Hordeum vulgare | Naked barley | −22.9 | 3.8 | 21.4 | BOH S.168 IS2 | Bishop et al. (2022) |
| Braes of Ha’Breck | Neolithic | Hordeum vulgare | Naked barley | −24.2 | 6.1 | 19.3 | BOH S.168 IS3 | Bishop et al. (2022) |
| Braes of Ha’Breck | Neolithic | Hordeum vulgare | Naked barley | −24.9 | 4.5 | 27.3 | BOH S.168 IS4 | Bishop et al. (2022) |
| Braes of Ha’Breck | Neolithic | Hordeum vulgare | Naked barley | −24 | 3.5 | 11.6 | BOH S.168 IS5 | Bishop et al. (2022) |
| Braes of Ha’Breck | Neolithic | Hordeum vulgare | Naked barley | −24.5 | 3.8 | 22.7 | BOH S.168 IS6 | Bishop et al. (2022) |
| Braes of Ha’Breck | Neolithic | Hordeum vulgare | Naked barley | −23.8 | 7.6 | 15.7 | BOH S.168 IS7 | Bishop et al. (2022) |
| Braes of Ha’Breck | Neolithic | Hordeum vulgare | Naked barley | −25.1 | 4.1 | 28.8 | BOH S.168 IS8 | Bishop et al. (2022) |
| Braes of Ha’Breck | Neolithic | Hordeum vulgare | Naked barley | −22.9 | 2.3 | 27.9 | BOH S.168 IS9 | Bishop et al. (2022) |
| Braes of Ha’Breck | Neolithic | Hordeum vulgare | Naked barley | −23.9 | 1.7 | 29.7 | BOH S.168 IS10 | Bishop et al. (2022) |
| Braes of Ha’Breck | Neolithic | Hordeum vulgare | Naked barley | −24.1 | 0.3 | 23.6 | BOH S.24 IS1 | Bishop et al. (2022) |
| Braes of Ha’Breck | Neolithic | Hordeum vulgare | Naked barley | −23.1 | 3.9 | 27.5 | BOH S.24 IS2 | Bishop et al. (2022) |
| Braes of Ha’Breck | Neolithic | Hordeum vulgare | Naked barley | −23.2 | 3.9 | 21.4 | BOH S.24 IS3 | Bishop et al. (2022) |
| Braes of Ha’Breck | Neolithic | Hordeum vulgare | Naked barley | −23.9 | 4.5 | 18.7 | BOH S.24 IS4 | Bishop et al. (2022) |
| Braes of Ha’Breck | Neolithic | Hordeum vulgare | Naked barley | −25.2 | 2.7 | 19.9 | BOH S.24 IS5 | Bishop et al. (2022) |
| Braes of Ha’Breck | Neolithic | Hordeum vulgare | Naked barley | −24.1 | 6.3 | 30.2 | BOH S.24 IS6 | Bishop et al. (2022) |
| Braes of Ha’Breck | Neolithic | Hordeum vulgare | Naked barley | −25.4 | 7.0 | 28.2 | BOH S.24 IS7 | Bishop et al. (2022) |
| Braes of Ha’Breck | Neolithic | Hordeum vulgare | Naked barley | −24.1 | 3.6 | 29.4 | BOH S.24 IS8 | Bishop et al. (2022) |
| Braes of Ha’Breck | Neolithic | Hordeum vulgare | Naked barley | −22.4 | 8.0 | 25.0 | BOH S.24 IS9 | Bishop et al. (2022) |
| Braes of Ha’Breck | Neolithic | Hordeum vulgare | Naked barley | −22.9 | 1.9 | 25.9 | BOH S.24 IS10 | Bishop et al. (2022) |
| Braes of Ha’Breck | Neolithic | Hordeum vulgare | Naked barley | −25.2 | 4.5 | 20.3 | BOH S.41 IS1 | Bishop et al. (2022) |
| Braes of Ha’Breck | Neolithic | Hordeum vulgare | Naked barley | −23.6 | 4.2 | 26.7 | BOH S.41 IS2 | Bishop et al. (2022) |
| Braes of Ha’Breck | Neolithic | Hordeum vulgare | Naked barley | −24.7 | 8.3 | 31.3 | BOH S.41 IS3 | Bishop et al. (2022) |
| Braes of Ha’Breck | Neolithic | Hordeum vulgare | Naked barley | −23.4 | 2.8 | 25.6 | BOH S.41 IS4 | Bishop et al. (2022) |
| Braes of Ha’Breck | Neolithic | Hordeum vulgare | Naked barley | −23.8 | 13.7 | 20.4 | BOH S.41 IS5 | Bishop et al. (2022) |
| Braes of Ha’Breck | Neolithic | Hordeum vulgare | Naked barley | −22.3 | 8.8 | 24.2 | BOH S.41 IS6 | Bishop et al. (2022) |
| Braes of Ha’Breck | Neolithic | Hordeum vulgare | Naked barley | −22.5 | 1.4 | 24.1 | BOH S.41 IS7 | Bishop et al. (2022) |
| Braes of Ha’Breck | Neolithic | Hordeum vulgare | Naked barley | −23.8 | 2.9 | 24.0 | BOH S.41 IS8 | Bishop et al. (2022) |
| Braes of Ha’Breck | Neolithic | Hordeum vulgare | Naked barley | −23.7 | 2.6 | 28.0 | BOH S.41 IS9 | Bishop et al. (2022) |
| Braes of Ha’Breck | Neolithic | Hordeum vulgare | Naked barley | −23.4 | 4.2 | 28.6 | BOH S.41 IS10 | Bishop et al. (2022) |
| Braes of Ha’Breck | Neolithic | Hordeum vulgare | Naked barley | −23.1 | 3.4 | 14.2 | BOH S.112 IS1 | Bishop et al. (2022) |
| Braes of Ha’Breck | Neolithic | Hordeum vulgare | Naked barley | −24.3 | 1.2 | 32.2 | BOH S.112 IS2 | Bishop et al. (2022) |
| Braes of Ha’Breck | Neolithic | Hordeum vulgare | Naked barley | −24.0 | 4.7 | 22.0 | BOH S.112 IS3 | Bishop et al. (2022) |
| Braes of Ha’Breck | Neolithic | Hordeum vulgare | Naked barley | −22.6 | 3.7 | 20.2 | BOH S.112 IS4 | Bishop et al. (2022) |
| Braes of Ha’Breck | Neolithic | Hordeum vulgare | Naked barley | −22.8 | 3.1 | 19.0 | BOH S.112 IS5 | Bishop et al. (2022) |
| Braes of Ha’Breck | Neolithic | Hordeum vulgare | Naked barley | −25.7 | 2.8 | 33.7 | BOH S.112 IS6 | Bishop et al. (2022) |
| Braes of Ha’Breck | Neolithic | Hordeum vulgare | Naked barley | −24.2 | 2.4 | 22.2 | BOH S.112 IS7 | Bishop et al. (2022) |
| Braes of Ha’Breck | Neolithic | Hordeum vulgare | Naked barley | −24.5 | 3.1 | 19.9 | BOH S.112 IS8 | Bishop et al. (2022) |
| Braes of Ha’Breck | Neolithic | Hordeum vulgare | Naked barley | −23.3 | 2.9 | 19.6 | BOH S.112 IS9 | Bishop et al. (2022) |
| Braes of Ha’Breck | Neolithic | Hordeum vulgare | Naked barley | −24.4 | 2.7 | 21.7 | BOH S.112 IS10 | Bishop et al. (2022) |
| Dubton Farm | Neolithic | Hordeum vulgare | Naked barley twisted grain | −26.6 | 4.4 | 15.2 | BREW S.235 IS.11 | Bishop et al. (2022) |
| Dubton Farm | Neolithic | Hordeum vulgare | Naked barley twisted grain | −25.1 | 3.3 | 24.7 | BREW S.235 IS.12 | Bishop et al. (2022) |
| Dubton Farm | Neolithic | Hordeum vulgare | Naked barley twisted grain | −24.3 | 3.0 | 15.6 | BREW S.235 IS.13 | Bishop et al. (2022) |
| Dubton Farm | Neolithic | Hordeum vulgare | Naked barley twisted grain | −24.9 | 2.4 | 26.1 | BREW S.235 IS.14 | Bishop et al. (2022) |
| Dubton Farm | Neolithic | Hordeum vulgare | Naked barley twisted grain | −24.2 | 3.9 | 18.5 | BREW S.235 IS.15 | Bishop et al. (2022) |
| Dubton Farm | Neolithic | Hordeum vulgare | Naked barley twisted grain | −24.8 | −0.9 | 19.2 | BREW S.235 IS.16 | Bishop et al. (2022) |
| Dubton Farm | Neolithic | Hordeum vulgare | Naked barley twisted grain | −24.4 | −0.2 | 28.1 | BREW S.235 IS.17 | Bishop et al. (2022) |
| Dubton Farm | Neolithic | Hordeum vulgare | Naked barley twisted grain | −23.9 | 3.1 | 22.2 | BREW S.235 IS.18 | Bishop et al. (2022) |
| Dubton Farm | Neolithic | Hordeum vulgare | Naked barley twisted grain | −26.4 | 2.8 | 25.9 | BREW S.235 IS.19 | Bishop et al. (2022) |
| Dubton Farm | Neolithic | Hordeum vulgare | Naked barley twisted grain | −25.0 | 3.4 | 26.1 | BREW S.235 IS.20 | Bishop et al. (2022) |
| Dubton Farm | Neolithic | Hordeum vulgare | Naked barley twisted grain | −22.9 | 3.8 | 21.6 | BREW S.214 IS.69 | Bishop et al. (2022) |
| Dubton Farm | Neolithic | Hordeum vulgare | Naked barley grain | −24.3 | 2.5 | 27.1 | BREW S.374 IS.56 | Bishop et al. (2022) |
| Dubton Farm | Neolithic | Hordeum vulgare | Naked barley grain | −25.0 | 1.3 | 23.3 | BREW S.374 IS.57 | Bishop et al. (2022) |
| Dubton Farm | Neolithic | Hordeum vulgare | Naked barley grain | −24.4 | 2.5 | 23.2 | BREW S.374 IS.58 | Bishop et al. (2022) |
| Dubton Farm | Neolithic | Hordeum vulgare | Naked barley grain | −22.7 | 3.2 | 27.3 | BREW S.374 IS.59 | Bishop et al. (2022) |
| Dubton Farm | Neolithic | Hordeum vulgare | Naked barley grain | −26.0 | 4.5 | 31.0 | BREW S.214 IS.68 | Bishop et al. (2022) |
| Dubton Farm | Neolithic | Hordeum vulgare | Naked barley grain | −23.8 | 5.8 | 21.3 | BREW S.214 IS.70 | Bishop et al. (2022) |
| Dubton Farm | Neolithic | Hordeum vulgare | Naked barley straight grain | −26.9 | 4.3 | 33.4 | BREW S.65 IS.51 | Bishop et al. (2022) |
| Dubton Farm | Neolithic | Hordeum vulgare | Naked barley straight grain | −24.2 | 1.9 | 14.7 | BREW S.374 IS.60 | Bishop et al. (2022) |
| Dubton Farm | Neolithic | Hordeum vulgare | Naked barley straight grain | −24.9 | 4.8 | 22.4 | BREW S.374 IS.61 | Bishop et al. (2022) |
| Dubton Farm | Neolithic | Hordeum vulgare | Naked barley straight grain | −24.2 | 2.1 | 23.8 | BREW S.374 IS.62 | Bishop et al. (2022) |
| Dubton Farm | Neolithic | Hordeum vulgare | Naked barley twisted grain | −25.5 | 2.5 | 23.4 | BREW S.65 IS.52 | Bishop et al. (2022) |
| Dubton Farm | Neolithic | Hordeum vulgare | Naked barley twisted grain | −26.2 | 0.7 | 36.0 | BREW S.65 IS.53 | Bishop et al. (2022) |
| Dubton Farm | Neolithic | Hordeum vulgare | Naked barley twisted grain | −24.9 | 3.3 | 25.5 | BREW S.65 IS.54 | Bishop et al. (2022) |
| Dubton Farm | Neolithic | Hordeum vulgare | Naked barley twisted grain | −25.1 | 4.6 | 24.6 | BREW S.65 IS.55 | Bishop et al. (2022) |
| Dubton Farm | Neolithic | Hordeum vulgare | Naked barley twisted grain | −23.7 | 1.6 | 18.3 | BREW S.374 IS.63 | Bishop et al. (2022) |
| Dubton Farm | Neolithic | Hordeum vulgare | Naked barley twisted grain | −26.0 | 5.9 | 27.3 | BREW S.214 IS.67 | Bishop et al. (2022) |
| Dubton Farm | Neolithic | Hordeum vulgare | Naked barley twisted grain | −25.2 | 5.8 | 26.2 | BREW S.214 IS.71 | Bishop et al. (2022) |
| Balbridie | Neolithic | Triticum dicoccon | Emmer wheat grain | −23.7 | 1.4 | 28.8 | BB F40 IS.11 | Bishop et al. (2022) |
| Balbridie | Neolithic | Triticum dicoccon | Emmer wheat grain | −23.2 | 0.9 | 27.3 | BB F40 IS.12 | Bishop et al. (2022) |
| Balbridie | Neolithic | Triticum dicoccon | Emmer wheat grain | −23.6 | 1.0 | 23.7 | BB F40 IS.13 | Bishop et al. (2022) |
| Balbridie | Neolithic | Triticum dicoccon | Emmer wheat grain | −23.2 | −0.7 | 24.4 | BB F40 IS.14 | Bishop et al. (2022) |
| Balbridie | Neolithic | Triticum dicoccon | Emmer wheat grain | −23.4 | 0.5 | 26.0 | BB F40 IS.15 | Bishop et al. (2022) |
| Balbridie | Neolithic | Triticum dicoccon | Emmer wheat grain | −22.0 | −0.8 | 27.0 | BB F40 IS.16 | Bishop et al. (2022) |
| Balbridie | Neolithic | Triticum dicoccon | Emmer wheat grain | −23.2 | 1.0 | 34.0 | BB F40 IS.17 | Bishop et al. (2022) |
| Balbridie | Neolithic | Triticum dicoccon | Emmer wheat grain | −23.1 | 0.6 | 25.6 | BB F40 IS.18 | Bishop et al. (2022) |
| Balbridie | Neolithic | Triticum dicoccon | Emmer wheat grain | −23.2 | −0.5 | 25.8 | BB F40 IS.19 | Bishop et al. (2022) |
| Balbridie | Neolithic | Triticum dicoccon | Emmer wheat grain | −23.4 | 3.1 | 25.1 | BB F40 IS.20 | Bishop et al. (2022) |
| Balbridie | Neolithic | Triticum dicoccon | Emmer wheat grain | −23.1 | 0.6 | 25.1 | BB F294 IS.42 | Bishop et al. (2022) |
| Balbridie | Neolithic | Triticum dicoccon | Emmer wheat grain | −23.4 | 1.0 | 28.7 | BB F294 IS.43 | Bishop et al. (2022) |
| Balbridie | Neolithic | Triticum dicoccon | Emmer wheat grain | −22.7 | 0.8 | 27.1 | BB F294 IS.44 | Bishop et al. (2022) |
| Balbridie | Neolithic | Triticum dicoccon | Emmer wheat grain | −24.0 | −0.6 | 27.5 | BB F294 IS.45 | Bishop et al. (2022) |
| Balbridie | Neolithic | Triticum dicoccon | Emmer wheat grain | −23.4 | 0.7 | 24.3 | BB F294 IS.46 | Bishop et al. (2022) |
| Balbridie | Neolithic | Triticum dicoccon | Emmer wheat grain | −22.9 | 1.1 | 23.8 | BB F294 IS.47 | Bishop et al. (2022) |
| Balbridie | Neolithic | Triticum dicoccon | Emmer wheat grain | −22.6 | 1.4 | 24.4 | BB F294 IS.48 | Bishop et al. (2022) |
| Balbridie | Neolithic | Triticum dicoccon | Emmer wheat grain | −23.7 | −0.6 | 24.8 | BB F294 IS.49 | Bishop et al. (2022) |
| Balbridie | Neolithic | Triticum dicoccon | Emmer wheat grain | −23.5 | −0.2 | 28.0 | BB F294 IS.50 | Bishop et al. (2022) |
| Balbridie | Neolithic | Triticum dicoccon | Emmer wheat grain | −23.5 | 1.1 | 22.1 | BB F294 IS.51 | Bishop et al. (2022) |
| Dubton Farm | Neolithic | Triticum dicoccon | Emmer wheat grain | −25.0 | 1.7 | 13.4 | BREW S.235 IS.1 | Bishop et al. (2022) |
| Dubton Farm | Neolithic | Triticum dicoccon | Emmer wheat grain | −24.1 | 5.5 | 16.2 | BREW S.235 IS.2 | Bishop et al. (2022) |
| Dubton Farm | Neolithic | Triticum dicoccon | Emmer wheat grain | −24.9 | 5.4 | 16.4 | BREW S.235 IS.3 | Bishop et al. (2022) |
| Dubton Farm | Neolithic | Triticum dicoccon | Emmer wheat grain | −25.1 | 5.0 | 15.4 | BREW S.235 IS.4 | Bishop et al. (2022) |
| Dubton Farm | Neolithic | Triticum dicoccon | Emmer wheat grain | −25.0 | 4.8 | 11.1 | BREW S.235 IS.5 | Bishop et al. (2022) |
| Dubton Farm | Neolithic | Triticum dicoccon | Emmer wheat grain | −24.6 | 4.2 | 15.5 | BREW S.235 IS.6 | Bishop et al. (2022) |
| Dubton Farm | Neolithic | Triticum dicoccon | Emmer wheat grain | −25.0 | 5.3 | 10.3 | BREW S.235 IS.7 | Bishop et al. (2022) |
| Dubton Farm | Neolithic | Triticum dicoccon | Emmer wheat grain | −24.3 | 1.7 | 13.4 | BREW S.235 IS.8 | Bishop et al. (2022) |
| Dubton Farm | Neolithic | Triticum dicoccon | Emmer wheat grain | −24.3 | 2.4 | 13.0 | BREW S.235 IS.9 | Bishop et al. (2022) |
| Dubton Farm | Neolithic | Triticum dicoccon | Emmer wheat grain | −24.7 | 3.9 | 15.0 | BREW S.235 IS.10 | Bishop et al. (2022) |
| Dubton Farm | Neolithic | Triticum dicoccon | Emmer wheat grain | −24.0 | 0.2 | 18.7 | BREW S.65 IS.21 | Bishop et al. (2022) |
| Dubton Farm | Neolithic | Triticum dicoccon | Emmer wheat grain | −24.1 | 0.8 | 15.7 | BREW S.65 IS.22 | Bishop et al. (2022) |
| Dubton Farm | Neolithic | Triticum dicoccon | Emmer wheat grain | −24.0 | 3.8 | 15.9 | BREW S.374 IS.31 | Bishop et al. (2022) |
| Dubton Farm | Neolithic | Triticum dicoccon | Emmer wheat grain | −24.7 | 3.4 | 25.3 | BREW S.374 IS.32 | Bishop et al. (2022) |
| Dubton Farm | Neolithic | Triticum dicoccon | Emmer wheat grain | −25.3 | 3.7 | 19.1 | BREW S.214 IS.65 | Bishop et al. (2022) |
| Dubton Farm | Neolithic | Triticum dicoccon | Emmer wheat grain | −25.2 | 1.6 | 22.7 | BREW S.65 IS.23 | Bishop et al. (2022) |
| Dubton Farm | Neolithic | Triticum dicoccon | Emmer wheat grain | −23.7 | 4.8 | 23.6 | BREW S.65 IS.24 | Bishop et al. (2022) |
| Dubton Farm | Neolithic | Triticum dicoccon | Emmer wheat grain | −23.5 | 4.9 | 16.0 | BREW S.65 IS.25 | Bishop et al. (2022) |
| Dubton Farm | Neolithic | Triticum dicoccon | Emmer wheat grain | −25.0 | 3.0 | 20.0 | BREW S.65 IS.26 | Bishop et al. (2022) |
| Dubton Farm | Neolithic | Triticum dicoccon | Emmer wheat grain | −24.6 | 1.5 | 24.4 | BREW S.65 IS.27 | Bishop et al. (2022) |
| Dubton Farm | Neolithic | Triticum dicoccon | Emmer wheat grain | −25.3 | 3.7 | 15.6 | BREW S.65 IS.28 | Bishop et al. (2022) |
| Dubton Farm | Neolithic | Triticum dicoccon | Emmer wheat grain | −22.8 | 6.5 | 17.4 | BREW S.65 IS.29 | Bishop et al. (2022) |
| Dubton Farm | Neolithic | Triticum dicoccon | Emmer wheat grain | −23.7 | 3.2 | 13.8 | BREW S.65 IS.30 | Bishop et al. (2022) |
| Dubton Farm | Neolithic | Triticum dicoccon | Emmer wheat grain | −24.2 | 1.6 | 13.4 | BREW S.374 IS.33 | Bishop et al. (2022) |
| Dubton Farm | Neolithic | Triticum dicoccon | Emmer wheat grain | −24.6 | 1.3 | 20.2 | BREW S.374 IS.34 | Bishop et al. (2022) |
| Dubton Farm | Neolithic | Triticum dicoccon | Emmer wheat grain | −25.0 | 2.6 | 17.4 | BREW S.374 IS.35 | Bishop et al. (2022) |
| Dubton Farm | Neolithic | Triticum dicoccon | Emmer wheat grain | −23.9 | 3.8 | 10.6 | BREW S.374 IS.36 | Bishop et al. (2022) |
| Dubton Farm | Neolithic | Triticum dicoccon | Emmer wheat grain | −24.4 | 1.5 | 17.3 | BREW S.374 IS.37 | Bishop et al. (2022) |
| Dubton Farm | Neolithic | Triticum dicoccon | Emmer wheat grain | −25.4 | 2.5 | 21.8 | BREW S.374 IS.38 | Bishop et al. (2022) |
| Dubton Farm | Neolithic | Triticum dicoccon | Emmer wheat grain | −25.3 | 2.8 | 16.2 | BREW S.374 IS.39 | Bishop et al. (2022) |
| Dubton Farm | Neolithic | Triticum dicoccon | Emmer wheat grain | −23.4 | 1.6 | 13.0 | BREW S.374 IS.40 | Bishop et al. (2022) |
| Dubton Farm | Neolithic | Triticum dicoccon | Emmer wheat grain | −24.8 | 2.9 | 16.7 | BREW S.214 IS.64 | Bishop et al. (2022) |
| Dubton Farm | Neolithic | Triticum dicoccon | Emmer wheat grain | −25.4 | 5.3 | 16.8 | BREW S.214 IS.66 | Bishop et al. (2022) |
| Skara Brae | Neolithic | Triticum dicoccon | Emmer wheat grain | −20.7 | 3.8 | 15.6 | SB C.168 IS.11 | Bishop et al. (2022) |
| Skara Brae | Neolithic | Triticum dicoccon | Emmer wheat grain | −20.8 | 1.6 | 16.0 | SB C.168 IS.12 | Bishop et al. (2022) |
| Skara Brae | Neolithic | Triticum dicoccon | Emmer wheat grain | −22.2 | 3.5 | 15.3 | SB C.168 IS.13 | Bishop et al. (2022) |
| Skara Brae | Neolithic | Triticum dicoccon | Emmer wheat grain | −22.5 | 0.9 | 13.7 | SB C.168 IS.14 | Bishop et al. (2022) |
| Skara Brae | Neolithic | Triticum dicoccon | Emmer wheat grain | −21.5 | 3.1 | 15.0 | SB C.168 IS.15 | Bishop et al. (2022) |
| Balbridie | Neolithic | Triticum | Free-threshing wheat | −24.1 | 0.5 | 32.2 | BB F40 IS.21 | Bishop et al. (2022) |
| Balbridie | Neolithic | Triticum | Free-threshing wheat | −22.7 | 0.2 | 25.0 | BB F40 IS.22 | Bishop et al. (2022) |
| Balbridie | Neolithic | Triticum | Free-threshing wheat | −22.7 | −0.3 | 25.6 | BB F40 IS.23 | Bishop et al. (2022) |
| Balbridie | Neolithic | Triticum | Free-threshing wheat | −23.1 | 0.1 | 26.8 | BB F40 IS.24 | Bishop et al. (2022) |
| Balbridie | Neolithic | Triticum | Free-threshing wheat | −22.9 | 1.2 | 29.0 | BB F40 IS.25 | Bishop et al. (2022) |
| Balbridie | Neolithic | Triticum | Free-threshing wheat | −23.8 | −0.6 | 25.8 | BB F40 IS.26 | Bishop et al. (2022) |
| Balbridie | Neolithic | Triticum | Free-threshing wheat | −23.1 | 0.6 | 28.2 | BB F40 IS.27 | Bishop et al. (2022) |
| Balbridie | Neolithic | Triticum | Free-threshing wheat | −23.5 | 0.2 | 29.6 | BB F40 IS.28 | Bishop et al. (2022) |
| Balbridie | Neolithic | Triticum | Free-threshing wheat | −22.8 | −0.7 | 30.8 | BB F40 IS.29 | Bishop et al. (2022) |
| Balbridie | Neolithic | Triticum | Free-threshing wheat | −22.4 | 2.0 | 27.9 | BB F40 IS.30 | Bishop et al. (2022) |
| Balbridie | Neolithic | Triticum | Free-threshing wheat | −22.5 | 0.8 | 28.4 | BB F294 IS.52 | Bishop et al. (2022) |
| Balbridie | Neolithic | Triticum | Free-threshing wheat | −23.5 | 0.5 | 30.6 | BB F294 IS.53 | Bishop et al. (2022) |
| Balbridie | Neolithic | Triticum | Free-threshing wheat | −22.9 | 0.8 | 21.6 | BB F294 IS.54 | Bishop et al. (2022) |
| Balbridie | Neolithic | Triticum | Free-threshing wheat | −23.0 | −0.3 | 23.5 | BB F294 IS.55 | Bishop et al. (2022) |
| Balbridie | Neolithic | Triticum | Free-threshing wheat | −22.7 | −0.7 | 30.3 | BB F294 IS.56 | Bishop et al. (2022) |
| Balbridie | Neolithic | Triticum | Free-threshing wheat | −22.2 | 0.3 | 21.9 | BB F294 IS.57 | Bishop et al. (2022) |
| Balbridie | Neolithic | Triticum | Free-threshing wheat | −22.9 | −0.2 | 24.2 | BB F294 IS.58 | Bishop et al. (2022) |
| Balbridie | Neolithic | Triticum | Free-threshing wheat | −22.6 | 0.0 | 22.8 | BB F294 IS.59 | Bishop et al. (2022) |
| Balbridie | Neolithic | Triticum | Free-threshing wheat | −24.5 | 3.7 | 24.1 | BB F294 IS.60 | Bishop et al. (2022) |
| Balbridie | Neolithic | Triticum | Free-threshing wheat | −22.9 | 2.3 | 33.8 | BB F294 IS.61 | Bishop et al. (2022) |
| ID | Site | Lab/Context ID | Skeletal Element | Sex | Age | δ13C ‰ | δ15N ‰ | C/N | 14C Age BP | References |
|---|---|---|---|---|---|---|---|---|---|---|
| AC.01 | An Corran | Small adult | −20.7 | 9.8 | Milner and Craig (2009) | |||||
| AC.02 | An Corran | Mature adult (<35) | −20.6 | 9.8 | Milner and Craig (2009) | |||||
| AC.03 | An Corran | Small adult | −20.5 | 9.4 | Milner and Craig (2009) | |||||
| AC.04 | An Corran | Mature adult (>40) | −20.2 | 10.2 | Milner and Craig (2009) | |||||
| AC.05 | An Corran | Mature adult | −19.4 | 10.7 | Milner and Craig (2009) | |||||
| AC.06 | An Corran | n.d. | −21.2 | 11.1 | Milner and Craig (2009) | |||||
| AC.07 | An Corran | n.d. | −21.1 | 9.9 | Milner and Craig (2009) | |||||
| AC.08 | An Corran | n.d. | −21.0 | 10.1 | Milner and Craig (2009) | |||||
| AC.09 | An Corran | n.d. | −20.7 | 10.3 | Milner and Craig (2009) | |||||
| AC.10 | An Corran | n.d. | −20.8 | 9.8 | Milner and Craig (2009) | |||||
| AC.11 | An Corran | n.d. | −20.5 | 10.8 | Milner and Craig (2009) | |||||
| AC.12 | An Corran | n.d. | −20.2 | 11.4 | Milner and Craig (2009) | |||||
| AC.13 | An Corran | n.d. | −20.1 | 10.1 | Milner and Craig (2009) | |||||
| AC.14 | An Corran | n.d. | −19.9 | 9.7 | Milner and Craig (2009) | |||||
| AC.15 | An Corran | n.d. | −19.7 | 9.0 | Milner and Craig (2009) | |||||
| AC.16 | An Corran | n.d. | −19.7 | 10.2 | Milner and Craig (2009) | |||||
| AC.17 | An Corran | n.d. | −19.6 | 10.6 | Milner and Craig (2009) | |||||
| AC.18 | An Corran | n.d. | −22.9 | 2.6 | Milner and Craig (2009) | |||||
| CMB.01 | Carding Mill Bay 1 | C XIV:1 | Phalanx | Adult | −21.5 | 9.0 | 3.2 | Schulting and Richards (2002) | ||
| CMB.02 | Carding Mill Bay 1 | C XV:1 | Metacarpal | Adult | −21.0 | 8.9 | 3.1 | Schulting and Richards (2002) | ||
| CMB.03 | Carding Mill Bay 1 | C VII:130 | Parietal | Adult | −21.5 | 9.6 | 3.2 | Schulting and Richards (2002) | ||
| CMB.04 | Carding Mill Bay 1 | C XXIII | Metatarsus | Adult | −21.4 | 9.8 | 3.1 | Schulting and Richards (2002) | ||
| CMB.05 | Carding Mill Bay 1 | C III:74 | Humerus | Adult | −21.3 | 8.8 | 3.2 | Schulting and Richards (2002) | ||
| CMB.06 | Carding Mill Bay 1 | C IV:94 | Phalanx | Adult | −21.5 | 10.0 | 3.1 | Schulting and Richards (2002) | ||
| CMB.07 | Carding Mill Bay 1 | C V:105 | Femur | Adult | −21.3 | 8.9 | 3.2 | Schulting and Richards (2002) | ||
| CMB.08 | Carding Mill Bay 1 | C VII:112 | Metatarsal | Adult | −21.3 | 9.1 | 3.2 | Schulting and Richards (2002) | ||
| CMB.09 | Carding Mill Bay 1 | C XVII:1 | Phalanx | Adult | −21.5 | 9.9 | 3.1 | Schulting and Richards (2002) | ||
| CMB.10 | Carding Mill Bay 1 | C X:1 | Scapula | Non-adult | −21.3 | 9.5 | 3.1 | Schulting and Richards (2002) | ||
| CMB.11 | Carding Mill Bay 2 | Incisor | Non-adult | −21.2 | 8.5 | 3.3 | Patterson et al. (2022) | |||
| CMB.12 | Carding Mill Bay 2 | PSUAMS-5772/5773/5774/5776 | Phalanx | F | Adult | −21.7 | 9.6 | 3.3 | 4832 ± 14 | Patterson et al. (2022) |
| CLA.01 | Clachaig | SUERC-68711/I2988 | Petrous | F | n.d. | −21.6 | 11.2 | 3.3 | 4645 ± 29 | Olalde et al. (2018) |
| CRA.01 | Crarae | 45 NN 1186.1 | Pelvis | Adult | −21.8 | 9.0 | 3.3 | Schulting and Richards (2002) | ||
| CRA.02 | Crarae | 45 NN 1186.2 | Phalanx | Adult | −21.3 | 9.5 | 3.3 | Schulting and Richards (2002) | ||
| CRA.03 | Crarae | 59C NN 1123 | Patella | Adult? | −21.7 | 9.1 | 3.5 | Schulting and Richards (2002) | ||
| DC.01 | Distillery Cave | SUERC-68702/I2659 | Petrous | F | n.d. | −21.4 | 8.9 | 3.2 | 4914 ± 27 | Olalde et al. (2018) |
| DC.02 | Distillery Cave | SUERC-68703/I2660 | Petrous | n.d. | −21.7 | 9.1 | 3.2 | 4631 ± 29 | Olalde et al. (2018) | |
| DC.03 | Distillery Cave | SUERC-68704/I2691 | Petrous | M | n.d. | −21.8 | 8.6 | 3.2 | 4881 ± 25 | Olalde et al. (2018) |
| MC.01 | MacArthur Cave | SUERC-68701/I2657 | Phalanx | M | Adult | −21.4 | 9.0 | 3.3 | 5052 ± 30 | Olalde et al. (2018) |
| RC.01 | Raschoille Cave | OxA-8537 | L Humerus | 1–2 yr | −21.8 | 11.9 | 3.3 | 4535 ± 50 | Bonsall (2000); Pickard & Bonsall (2022) | |
| RC.02 | Raschoille Cave | OxA-8434 | R Femur | c. 3 yr | −21.1 | 8.7 | 3.4 | 4720 ± 50 | Bonsall (2000); Pickard & Bonsall (2022) | |
| RC.03 | Raschoille Cave | OxA-8431 | L Femur | ?3–5 yr | −20.6 | 8.6 | 3.3 | 4930 ± 50 | Bonsall (2000); Pickard & Bonsall (2022) | |
| RC.04 | Raschoille Cave | OxA-8399/I3137 | Cervical vertebra | M | 3–7 yr | −21.4 | 10.2 | 3.4 | 4630 ± 65 | Bonsall (2000); Pickard & Bonsall (2022); Patterson et al. (2022) |
| RC.05 | Raschoille Cave | OxA-8432 | R Humerus | 8–10 yr | −20.4 | 7.6 | 3.3 | 4980 ± 50 | Bonsall (2000); Pickard & Bonsall (2022) | |
| RC.06 | Raschoille Cave | OxA-8401 | L Femur | ?10 yr | −21.1 | 9.1 | 3.3 | 4565 ± 65 | Bonsall (2000); Pickard & Bonsall (2022) | |
| RC.07 | Raschoille Cave | OxA-8400 | Rib | Adult | −20.3 | 9.7 | 3.2 | 4640 ± 65 | Bonsall (2000); Pickard & Bonsall (2022) | |
| RC.08 | Raschoille Cave | OxA-8444 | R Humerus | F | Adult | −21.1 | 9.6 | 3.5 | 4715 ± 45 | Bonsall (2000); Pickard & Bonsall (2022) |
| RC.09 | Raschoille Cave | OxA-8443 | R Humerus | Adult | −20.4 | 9.4 | 3.2 | 4825 ± 55 | Bonsall (2000); Pickard & Bonsall (2022) | |
| RC.10 | Raschoille Cave | OxA-8433 | L Humerus | M | Adult | −20.2 | 9.4 | 3.2 | 4920 ± 50 | Bonsall (2000); Pickard & Bonsall (2022) |
| RC.11 | Raschoille Cave | OxA-8404 | R Humerus | Adult | −21.6 | 7.7 | 3.2 | 4850 ± 70 | Bonsall (2000); Pickard & Bonsall (2022) | |
| RC.12 | Raschoille Cave | OxA-8441 | R Humerus | Adult? | −21.2 | 9.1 | 3.3 | 4900 ± 45 | Bonsall (2000); Pickard & Bonsall (2022) | |
| RC.13 | Raschoille Cave | OxA-8435 | Humerus | −22.5 | 10.3 | 3.4 | 4680 ± 50 | Bonsall (2000); Pickard & Bonsall (2022) | ||
| RC.14 | Raschoille Cave | OxA-8442 | R Humerus | −21.0 | 8.7 | 3.3 | 4890 ± 45 | Bonsall (2000); Pickard & Bonsall (2022) | ||
| RC.15 | Raschoille Cave | PSUAMS-2068/I3135 | Petrous | M | −21.5 | 9.6 | 3.2 | 4770 ± 30 | Olalde et al. (2018) | |
| RC.16 | Raschoille Cave | PSUAMS-2069/I3136 | Petrous | F | −21.0 | 9.0 | 3.3 | 4665 ± 30 | Olalde et al. (2018) | |
| RC.17 | Raschoille Cave | PSUAMS-2154/I3133 | Petrous | M | −21.7 | 9.6 | 3.0 | 4725 ± 20 | Olalde et al. (2018) | |
| RC.18 | Raschoille Cave | PSUAMS-2155/I3134 | Petrous | M | −22.2 | 11.0 | 3.2 | 4730 ± 25 | Olalde et al. (2018) | |
| RC.19 | Raschoille Cave | PSUAMS-2156/I3138 | Petrous | F | −22.1 | 9.4 | 3.2 | 4415 ± 25 | Olalde et al. (2018) | |
| RC.20 | Raschoille Cave | GU-40818/SB 513A2/I3041 | Petrous | M | Adult | −21.9 | 8.4 | 3.5 | 4550 ± 29 | J. M. Bownes et al. (2017); Knight et al. (2021) |
| RC.21 | Raschoille Cave | GU-40819/SUERC-67265/SB525A2 | Petrous | F | Adult? | −21.7 | 7.7 | 3.3 | 4738 ± 31 | J. M. Bownes et al. (2017); Knight et al. (2021) |
| RC.22 | Raschoille Cave | GU-40820/SUERC-67266/SB526A | Petrous | F? | Adult | −21.6 | 7.7 | 3.3 | 4817 ± 31 | J. M. Bownes et al. (2017); Knight et al. (2021) |
| RC.23 | Raschoille Cave | GU-40821/SUERC-67267/SB528A2/I5371 | Petrous | F | Indeterminate | −22.1 | 8.8 | 3.3 | 4490 ± 29 | J. M. Bownes et al. (2017); Knight et al. (2021) |
| RC.24 | Raschoille Cave | GU-40822/SUERC-67268/SB527A1/I5370 | Petrous | F | Adult? | −21.5 | 9.2 | 3.3 | 4499 ± 29 | J. M. Bownes et al. (2017); Knight et al. (2021) |
| RC.25 | Raschoille Cave | GU-40823 | −21.9 | 9.5 | 3.3 | 4668 ± 29 | J. M. Bownes et al. (2017); Knight et al. (2021) | |||
| RC.26 | Raschoille Cave | GU-40824 | −22.3 | 10.2 | 3.2 | 4432 ± 31 | J. M. Bownes et al. (2017) | |||
| RC.27 | Raschoille Cave | GU-40825/SUERC-67271 | Petrous | Non-adult | −22.4 | 10.4 | 3.3 | 4731 ± 29 | J. M. Bownes et al. (2017); Knight et al. (2021) | |
| RC.28 | Raschoille Cave | GU-40826/SUERC-67275 | Petrous | Non-adult | −22.2 | 9.3 | 3.3 | 4638 ± 31 | J. M. Bownes et al. (2017); Knight et al. (2021) | |
| UC.01 | Ulva Cave | PSUAMS-5771 | Long bone | M | Adult | −21.0 | 9.9 | 3.2 | 4895 ± 25 | Patterson et al. (2022) |
| Parameter | Description |
|---|---|
| Δ15Ndiet–consumer | 5.5 ± 0.5‰ |
| Δ13Cdiet–consumer | 4.8 ± 0.5‰ |
| Food source isotope values—Plants | δ13Cprotein = −26.4 ± 1.0‰; δ13Cenergy= −24.1 ± 1.0‰; δ15N = 2.8 ± 1.0‰ |
| Food source isotope values—Terrestrial animals | δ13Cprotein = −24.4 ± 1.0‰, δ13Cenergy= −30.4 ± 1.0‰; δ15N = 3.3 ± 1.0‰ |
| Food source isotope values—Fish | δ13Cprotein = −14.0 ± 1.0‰, δ13Cenergy= −20.0 ± 1.0‰; δ15N = 15.4 ± 1.0‰ |
| Food source isotope values—Shellfish | δ13Cprotein = −14.6 ± 1.0‰, δ13Cenergy= −18.1 ± 1.0‰; δ15N = 7.4 ± 1.0‰ |
| Food source nutrient concentrations—Plants | Protein–energy—10:90 |
| Food source nutrient concentrations—Terrestrial animals | Protein–energy—70:30 |
| Food source nutrient concentrations—Fish | Protein–energy—75:25 |
| Food source nutrient concentrations—Shellfish | Protein–energy—90:10 |
| Prior | 0.05 ≤ protein intake ≥ 0.35 |
| Sample | δ13C | δ15N | PLANTS | TERRESTRIAL ANIMAL | FISH | SHELLFISH | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ME (Cal) | CI (68%) | ME (Pro) | ME (Cal) | CI (68%) | ME (Pro) | ME (Cal) | CI (68%) | ME (Pro) | ME (Cal) | CI (68%) | ME (Pro) | |||
| AC.01 | −20.7 | 9.8 | 75 ± 11 | 63–88 | 34 ± 17 | 22 ± 11 | 9–34 | 54 ± 19 | 2 ± 1 | 0–3 | 5 ± 4 | 2 ± 2 | 0–3 | 6 ± 5 |
| AC.02 | −20.6 | 9.8 | 75 ± 12 | 62–90 | 34 ± 20 | 22 ± 12 | 7–34 | 54 ± 21 | 2 ± 1 | 0–3 | 5 ± 4 | 2 ± 2 | 0–3 | 6 ± 5 |
| AC.03 | −20.5 | 9.4 | 75 ± 11 | 63–87 | 34 ± 17 | 21 ± 10 | 10–32 | 54 ± 19 | 2 ± 1 | 0–3 | 5 ± 4 | 2 ± 2 | 0–3 | 6 ± 5 |
| AC.04 | −20.2 | 10.2 | 75 ± 11 | 63–89 | 35 ± 17 | 20 ± 11 | 7–31 | 50 ± 19 | 2 ± 2 | 1–4 | 7 ± 5 | 2 ± 2 | 0–4 | 8 ± 6 |
| AC.05 | −19.4 | 10.7 | 73 ± 10 | 63–85 | 31 ± 14 | 20 ± 9 | 9–29 | 48 ± 17 | 4 ± 2 | 1–6 | 10 ± 6 | 3 ± 3 | 1–6 | 10 ± 8 |
| CMB.01 | −21.5 | 9.0 | 77 ± 12 | 63–91 | 38 ± 20 | 21 ± 12 | 7–34 | 54 ± 22 | 1 ± 1 | 0–2 | 4 ± 3 | 1 ± 1 | 0–2 | 5 ± 4 |
| CMB.02 | −21.0 | 8.9 | 76 ± 12 | 63–91 | 37 ± 21 | 21 ± 12 | 6–34 | 53 ± 22 | 1 ± 1 | 0–2 | 4 ± 3 | 1 ± 1 | 0–3 | 5 ± 5 |
| CMB.03 | −21.5 | 9.6 | 77 ± 12 | 63–92 | 38 ± 20 | 21 ± 12 | 6–34 | 52 ± 22 | 1 ± 1 | 0–3 | 5 ± 4 | 1 ± 1 | 0–2 | 5 ± 4 |
| CMB.04 | −21.4 | 9.8 | 76 ± 12 | 63–91 | 37 ± 20 | 21 ± 11 | 7–33 | 53 ± 21 | 2 ± 1 | 0–3 | 5 ± 4 | 1 ± 1 | 0–3 | 5 ± 4 |
| CMB.05 | −21.3 | 8.8 | 78 ± 13 | 64–94 | 42 ± 22 | 19 ± 12 | 4–33 | 50 ± 23 | 1 ± 1 | 0–2 | 3 ± 3 | 1 ± 1 | 0–2 | 5 ± 4 |
| CMB.06 | −21.5 | 10.0 | 76 ± 12 | 63–92 | 38 ± 21 | 21 ± 12 | 6–33 | 52 ± 22 | 2 ± 1 | 0–3 | 5 ± 4 | 1 ± 1 | 0–3 | 5 ± 4 |
| CMB.07 | −21.3 | 8.9 | 77 ± 12 | 64–91 | 38 ± 20 | 21 ± 12 | 7–33 | 53 ± 21 | 1 ± 1 | 0–2 | 4 ± 3 | 1 ± 1 | 0–2 | 5 ± 4 |
| CMB.08 | −21.3 | 9.1 | 76 ± 12 | 64–91 | 38 ± 19 | 21 ± 11 | 7–34 | 53 ± 20 | 1 ± 1 | 0–2 | 4 ± 3 | 1 ± 1 | 0–3 | 5 ± 4 |
| CMB.09 | −21.5 | 9.9 | 76 ± 12 | 63–91 | 37 ± 20 | 21 ± 12 | 7–34 | 54 ± 21 | 1 ± 1 | 0–3 | 4 ± 4 | 1 ± 1 | 0–2 | 4 ± 4 |
| CRA.01 | −21.8 | 9.0 | 77 ± 13 | 63–93 | 40 ± 21 | 20 ± 12 | 6–34 | 52 ± 22 | 1 ± 1 | 0–2 | 3 ± 3 | 1 ± 1 | 0–2 | 4 ± 4 |
| CRA.02 | −21.3 | 9.5 | 75 ± 12 | 63–90 | 36 ± 20 | 22 ± 11 | 8–34 | 55 ± 21 | 1 ± 1 | 0–3 | 4 ± 3 | 1 ± 1 | 0–3 | 5 ± 5 |
| CRA.03 | −21.7 | 9.1 | 77 ± 12 | 64–91 | 38 ± 20 | 21 ± 12 | 7–33 | 54 ± 21 | 1 ± 1 | 0–2 | 4 ± 3 | 1 ± 1 | 0–2 | 4 ± 4 |
| MC.01 | −21.4 | 9.0 | 75 ± 12 | 62–90 | 36 ± 19 | 22 ± 11 | 8–34 | 56 ± 21 | 1 ± 1 | 0–2 | 4 ± 3 | 1 ± 1 | 0–2 | 5 ± 4 |
| RC.07 | −20.3 | 9.7 | 75 ± 11 | 64–87 | 34 ± 17 | 21 ± 10 | 9–32 | 53 ± 19 | 2 ± 2 | 0–4 | 6 ± 4 | 2 ± 2 | 0–4 | 7 ± 6 |
| RC.08 | −21.1 | 9.6 | 77 ± 12 | 64–91 | 38 ± 20 | 21 ± 12 | 7–33 | 52 ± 21 | 2 ± 1 | 0–3 | 5 ± 4 | 2 ± 1 | 0–3 | 5 ± 5 |
| RC.09 | −20.4 | 9.4 | 75 ± 11 | 64–88 | 35 ± 17 | 21 ± 10 | 9–32 | 53 ± 19 | 2 ± 2 | 0–3 | 5 ± 4 | 2 ± 2 | 0–4 | 7 ± 6 |
| RC.10 | −20.2 | 9.4 | 75 ± 11 | 63–88 | 34 ± 17 | 21 ± 10 | 9–32 | 53 ± 18 | 2 ± 2 | 0–3 | 5 ± 4 | 2 ± 2 | 0–1 | 7 ± 6 |
| RC.11 | −21.6 | 7.7 | 77 ± 13 | 63–93 | 41 ± 22 | 21 ± 12 | 5–35 | 53 ± 23 | 1 ± 1 | 0–2 | 2 ± 2 | 1 ± 1 | 0–2 | 4 ± 4 |
| RC.12 | −21.2 | 9.1 | 75 ± 12 | 63–90 | 36 ± 19 | 21 ± 11 | 8–33 | 55 ± 20 | 1 ± 1 | 0–2 | 4 ± 3 | 1 ± 1 | 0–3 | 5 ± 5 |
| UC.01 | −21 | 9.9 | 74 ± 11 | 63–88 | 34 ± 18 | 22 ± 11 | 9–33 | 55 ± 19 | 2 ± 2 | 0–3 | 5 ± 4 | 2 ± 1 | 0–3 | 6 ± 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pickard, C.; Greenberg, E.; Smith, E.; Barlow, A.; Bonsall, C. Diet, DNA, and the Mesolithic–Neolithic Transition in Western Scotland. Humans 2025, 5, 8. https://doi.org/10.3390/humans5010008
Pickard C, Greenberg E, Smith E, Barlow A, Bonsall C. Diet, DNA, and the Mesolithic–Neolithic Transition in Western Scotland. Humans. 2025; 5(1):8. https://doi.org/10.3390/humans5010008
Chicago/Turabian StylePickard, Catriona, Elizabeth Greenberg, Emma Smith, Andy Barlow, and Clive Bonsall. 2025. "Diet, DNA, and the Mesolithic–Neolithic Transition in Western Scotland" Humans 5, no. 1: 8. https://doi.org/10.3390/humans5010008
APA StylePickard, C., Greenberg, E., Smith, E., Barlow, A., & Bonsall, C. (2025). Diet, DNA, and the Mesolithic–Neolithic Transition in Western Scotland. Humans, 5(1), 8. https://doi.org/10.3390/humans5010008






