1. Introduction
The diamino oxidase (DAO) enzyme plays a key role in breaking down histamine from many common foods in the gastrointestinal tract. Reduced DAO levels or impaired activity (DAO deficiency) can result in excessive unmetabolized histamine, which has been linked to various symptoms of histamine intolerance (HIT), such as migraines, gastrointestinal issues, and allergic processes [
1,
2,
3,
4]. Indeed, HIT is a multifactorial disorder primarily resulting from an impaired degradation of dietary histamine at the intestinal level via an impaired DAO activity [
1]. DAO deficiency can have different origins or factors influencing the activity of the enzyme. On one side, DAO deficiency can originate from pathological conditions in which an intestinal mucosal damage derived from certain inflammatory bowel diseases, such as gastroenteritis and irritable bowel syndrome, may interfere with DAO activity in the gut level [
5,
6]. On the other side, DAO activity can be impaired by alcohol consumption and several commonly prescribed pharmacological drugs [
1]. In general terms, these molecules bind to the DAO enzyme due to structural similarity to the histamine molecule or due to affinity with the active site of the enzyme, causing a decline in the activity of the available DAO. This DAO deficiency is temporary and reversible after stopping the consumption of these substances.
Finally, genetic predisposition is of relevance when it comes to DAO deficiency. A reduced DAO activity in the blood and the subset of symptoms of HIT have been associated with SNPs in four gene variants in the gene encoding for the DAO enzyme,
AOC1 [
7]. A recent study in a cohort of healthy newborns reported that 66% of them presented at least one of these four SNPs associated with a reduced DAO activity [
8], suggesting a high prevalence of genetic predisposition to DAO deficiency in the general population. The predominance of heterozygous genotypes and a 19% rate of homozygosity in any of these variants indicate that a substantial portion of the population may have reduced histamine degradation capacity from birth. In view of this high potential prevalence, there is now a reinforced need to find ways to manage this metabolic impairment.
The first line of strategy to mitigate HIT is following a low-histamine diet. In order to prevent symptoms of HIT, the recommendation is to eliminate specific foods from the diet which may contain high amounts of histamine. These are mainly cured, canned, preserved, and fermented foods, especially cheese-, meat-, and fish-based products, but also fermented alcoholic beverages like wine and beer, and certain fruits such as citrus and tomatoes, among others [
1]. As can be deduced, the complete exclusion of these foods can come with detrimental health consequences if followed long-term or without clinical supervision. Not only that, but in some people, it could have a strong social and psychological impact, potentially leading to food anxiety and disordered eating patterns, affecting quality of life and mental health. As a complementary approach to the low-histamine diet, oral supplementation of the DAO enzyme is currently being used to enhance histamine elimination.
Exogenous supplementation with the DAO enzyme could increase histamine-degrading activity in the small intestine, promoting local histamine breakdown and preventing or alleviating symptoms caused by food-related histamine intolerance. Histamine from the diet is mainly metabolized by DAO in the digestive system, and several studies have shown that DAO supplementation can effectively treat histamine-induced intestinal issues [
9,
10] and other symptoms associated with histamine intolerance [
11,
12,
13,
14], such as migraines, gastrointestinal disturbances, or skin symptoms, with no adverse effects.
Current recommended posology for pig kidney-sourced DAO supplementation is 4.2 mg of a protein extract rich in DAO enzyme, three times a day, for its use in dietary supplements and food for special medical purposes [
15]. A self-affirmed as Generally Recognized as Safe (GRAS) evaluation has been made for the plant-based version, obtained from pea sprouts, for use in food supplements and specific food categories including dairy products, processed fruits, vegetables and juices, according to the FDA regulation 21 CFR 170.30 (a) and 21 CFR 170.30 (b) [
16]. DAO supplementation has shown clinical efficacy in reducing symptoms of histamine intolerance, demonstrating good tolerability and safety [
10]. In addition, already commercially available supplements in capsules and mini-tablet form containing DAO also use the recommended posology, with servings containing 4.2 mg of DAO extract. Although this is the recommended dose for DAO, in some cases, physiological and pathological processes may require higher doses of the extract.
A standard meal in the Mediterranean diet may contain up to 50 mg of histamine, which does not cause any harm to non-sensitive patients. However, it is recognized by EFSA that, in a subgroup of the population, higher histamine content in the diet may cause undesirable reactions, which may be due to lower activity of DAO, and which may be resolved with DAO supplementation [
17].
Endogenous DAO levels can increase in certain physiological conditions, such as pregnancy [
18,
19,
20], but this does not necessarily prevent histamine accumulation, which may persist or even worsen in pathological contexts. Therefore, assessing the safety and tolerability of exogenous DAO at ascending oral doses in healthy volunteers is essential to support the safe use of higher doses than those currently established.
2. Materials and Methods
2.1. Study Design
This was a randomized, double blinded, placebo-controlled, single-centre, single ascending dose (SAD) study to evaluate the safety and tolerability of three different doses of DAO. Three parallel groups were included in this study, one for each dose (verum or placebo). There was no patient or public involvement in the design, conduct, or reporting of the trial.
The aim of this study was to evaluate the safety and tolerability of the administered DAO product, at three different ascending doses. As the primary endpoint, the safety and tolerability of the treatments were evaluated by assessing vital signs, laboratory analyses, electrocardiogram (ECG), and incidence of adverse events (AEs).
2.2. Ethics Approval
The study was performed in accordance with the Declaration of Helsinki. The study protocol was approved by the local ethics committee of Fundació de Gestió Sanitària de l’Hospital de la Santa Creu i Sant Pau de Barcelona (code DAO-MAX2024, approved on 3 July 2024) and was registered in the ClinicalTrials.gov website (NCT06715163) on 19 November 2024, where the trial protocol description and statistical analysis plan can be accessed. The study was performed at the Phase I Clinical Research Centre CIM-IR Sant Pau, Barcelona (Spain). At the initial screening visit, before any procedure was performed, the subjects were thoroughly informed on the nature of the study (aims, type of treatment, methodology) and the tested product (expected benefits, possible side effects, and adverse reactions) in a language understandable by them, both verbally and in written form. They were clearly informed that they could withdraw from the study at any time without any effect on their medical care. Information about the confidentiality of their personal data was also provided. All the subjects were recruited between 8 July and 18 July 2024, gave their consent to participate in the study, and signed an informed consent form (ICF) prior to performing any intervention specified in the clinical trial protocol. They received a signed and dated copy of this document. A complete medical check-up was conducted to verify that the subject met all the inclusion criteria and none of the exclusion criteria. Eligible subjects who were randomized were informed about their responsibilities during the study: not to drink alcohol from 48 h before the first Investigational Product administration until the end of study, not to drink stimulant beverages (coffee, cola, tea, or chocolate) from 24 h before their arrival to the study site and up to 24 h after Investigational Product administration, not to take any other medication (except for use of paracetamol in short-term symptomatic treatments, according to the investigator criteria) 14 days prior to the administration of the study Investigational Product and/or non-prescription medication nor over-the-counter products (including natural food supplements, vitamins and medicinal plants products) within 7 days prior to the administration of the study product until the end of study. The subjects were also required to inform the investigator about any medication taken, not to perform intense physical exercise during the treatment period until the end of study, not to perform risk activities, e.g., operating hazardous machinery or performing extreme sports before the first drug Investigational Product administration until the end of study, and to inform the investigators about the occurrence of any adverse events.
2.3. Study Treatment
Each mini tablet contained 4.2 mg of adiDAO® Veg, dehydrated powder of pea (Pisum sativum) sprouts, rich in DAO, standardized at an enzymatic activity against histamine of >14.500 KHDU/g DAO (supplied by DR Healthcare, Barcelona, Spain).
This DAO extract of pea sprout origin is not considered a Novel Food. The reason being is because it is considered a plant-based ingredient sourced from a natural process and the final extract is simply obtained by the dehydration of bean sprouts. Therefore, there are no limitations established by the European regulatory authorities regarding a maximum oral administration of this plant-based ingredient. Moreover, due to its nature, it is not considered a medical drug.
The following single-dose treatments were evaluated: 42 mg of DAO extract, once/day, 10 tablets with 240 mL of water administration (Dose 1) or placebo; 84 mg of DAO extract, once/day, 20 tablets with 240 mL of water administration (Dose 2) or placebo; 210 mg of DAO extract, once/day, 50 tablets with 240 mL of water administration (Dose 3), or a placebo. All administrations were performed under fasted conditions. Ten healthy volunteers (HVs) were enrolled in each dose level and were randomly assigned to DAO or the placebo in an 8:2 ratio (eight received active treatment, and two received the placebo). Thirty volunteers were enrolled in total. Therefore, 24 received active DAO formulation and 6 of them received the placebo. In order to guarantee double-blind conditions, all products (active and placebo) were of the same colour, size, and appearance. The composition of the active and placebo tablets only differed in the presence and absence, respectively, of DAO of plant-based origin (i.e., placebo tablets contained only the excipient formulation without DAO and the active tablets contained the excipient formulation with DAO of plant-based origin). The study product was taken under direct supervision of the investigator or staff member.
The study product and placebo were randomly assigned using the programme SPSS v. 26.0. At the beginning of the study, the subjects were allocated to a randomization number following a procedure of consecutive assignment. Each randomization number corresponded to a sequence of treatment administration according to the previously generated randomization table. The randomization schedule was generated in a balanced way (an equal number of subjects in each treatment sequence) and was kept strictly confidential, accessible only to the statistician and specified pharmacy personnel (both of which had no role in study assessments or interaction with participants), until the time of unblinding. All volunteers went to the centre the day before the product administration and were on site under surveillance, until +24 h of the product administration.
2.4. Study Population
Participants included in the study were healthy male and female subjects aged 18–50 years, with negative drug screening tests and an agreement to refrain from any other drugs (including natural food supplements, vitamins, and medicinal plants) within 14 days prior to taking the study supplement.
All healthy volunteers were determined to be healthy based on their medical history, physical examination results, virology testing (Hepatitis B, C and HIV) results, and routine laboratory hematology and biochemistry results before enrolment in the study.
2.5. Safety Assessment
Monitoring of adverse events (AEs) was performed in a systematic manner through assessment of vital signs, physical examination results, electrocardiograms (ECGs), safety laboratory variables, and self-administered AEs forms throughout the study and at the end the study, following the study schedule and evaluation times. Laboratory measures included hematology (hemoglobin, hematocrit, white blood cell count, red blood cell count, and platelet count), clinical chemistry (glucose, urea, creatinine, total bilirubin, triglycerides, cholesterol, GOT (AST), GPT (ALT), GGT, alkaline phosphatase, and albumin), coagulation, and urinalysis (after fasting for 10 h performed at screening visit and at End of Study (+24 h).
Vital signs and ECG (systolic, diastolic blood pressure, and heart rate), assessment of adverse events and review of concomitant medication were performed at baseline [pre-dose], [+1 h], [+2 h], [+4 h], [+8 h], and [+24 h] post administration. A follow-up visit via phone call was performed at 6–8 days after product intake to ensure the safety of the volunteers and to check possible AEs during that time. This visit was performed remotely by contacting the volunteers via phone call.
2.6. Statistical Analysis
The statistical analysis and data management were carried out at the CIM-IR Sant Pau. The analysis was performed using the programme IBM-SPSS (v26.0). Statistical summaries were descriptive in nature. In general, data obtained in the trial are summarized descriptively and interpreted in an exploratory way. Descriptive statistics were provided by treatment group and scheduled visit. There was no formal sample size calculation for this clinical trial. However, the number of participants selected is considered sufficient to allow for the detection and observation of relevant differences between participants and across the studied dose levels. The default summary statistics for quantitative (continuous) variables are the number of observations (N), mean, standard deviation (SD), median, minimum (min), and maximum (max), for those subjects with data. Safety analyses were performed in all randomized subjects who received at least one dose of the study supplementation and had at least one follow-up visit or safety assessment.
3. Results
3.1. Participants Characteristics
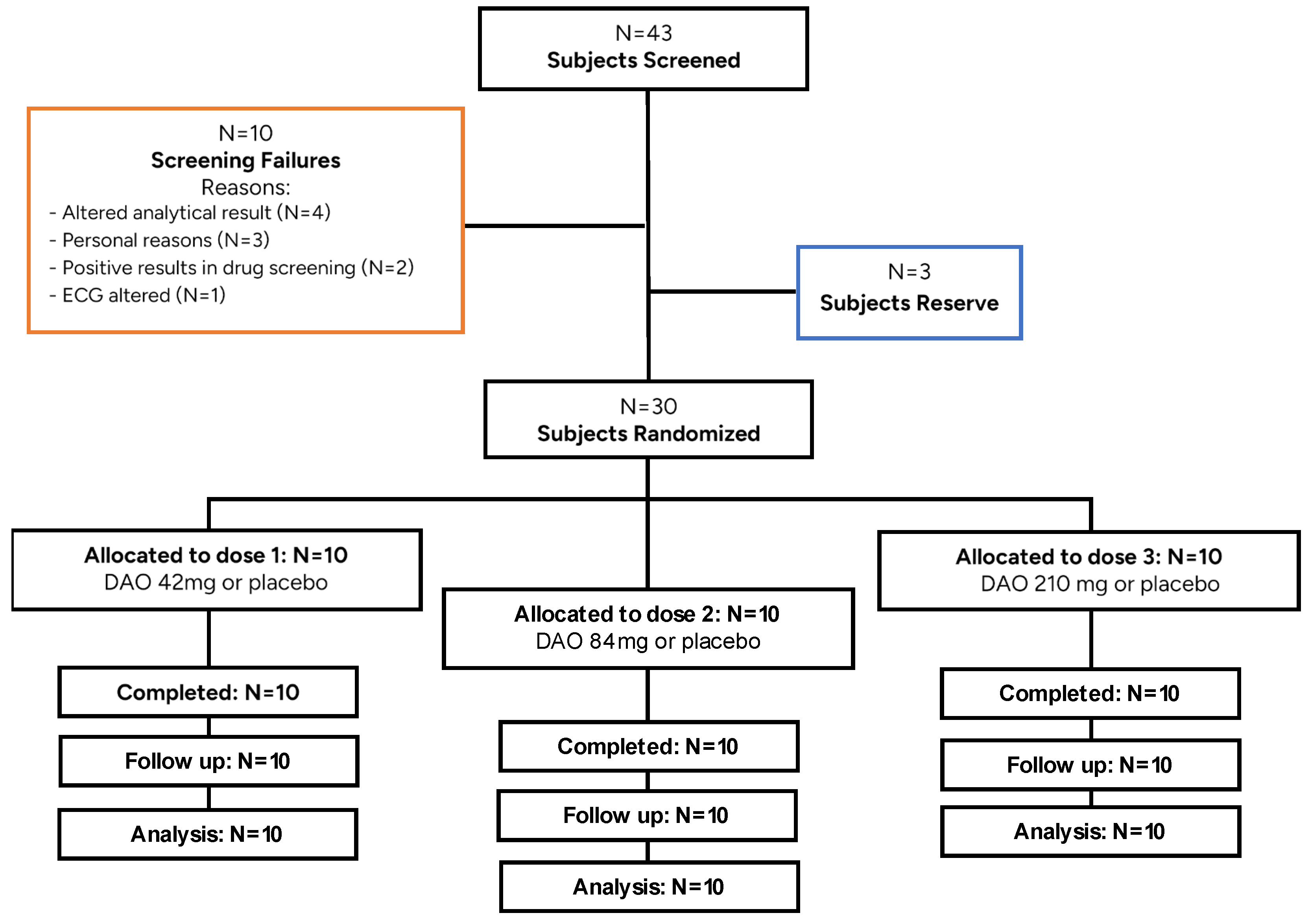
A total of 43 subjects were screened, and 30 were randomized (
Figure 1) [
21]. From the subjects that were not included in the study, three were maintained as reserves. Three volunteers were left out of the study for personal reasons, and seven did not fulfil the inclusion criteria. All subjects randomized in the study met the inclusion criteria and none of the exclusion criteria at the beginning of the trial. The study was performed according to the procedures specified in the protocol. A total of 30 HVs (15 males and 15 females) were eligible for safety evaluations and therefore were enrolled in the study. All 30 HVs received either a single dose of DAO or the placebo and completed all the phases of the study. None of the HVs discontinued the study. The characteristics of the observed HVs, including demographic data, are listed in
Table 1. No relevant differences were observed between groups, and demographic data were homogeneous across all treatment arms.
The randomized study population (N = 30) was composed of Caucasian volunteers with a body weight within the normal range, without clinical evidence of laboratory, ECG, or vital sign abnormalities. Twenty-six of the randomized subjects were non-smokers and the remaining four subjects stopped smoking at least 6 months prior to the study treatment phase.
Regarding alcohol consumption, 18 subjects did not drink alcoholic beverages at all, and the remaining 12 subjects consumed a maximum of 10 g of alcohol per day (mean 3.26 g/daily). Regarding xanthine consumption, 8 subjects participating in the study did not consume xanthines at all, and the remaining 22 subjects consumed xanthines (tea, coffee, chocolate, etc.) daily (mean 1.42 units/day). All the physical examinations performed during the screening (Skin-annexes, oropharynx, respiratory, cardio-vascular, digestive, neurology) were considered normal. No subjects discontinued the study.
The median (range) values for the demographic characteristics of HVs who were enrolled in the study were as follows: 30 (19–47) years old, 170 (151–190) cm tall, 71.80 (50–86) kg, and a body mass index (BMI) of 24.9 (19–29) kg/m2.
3.2. Adverse Events
Adverse events were coded according to the Medical Dictionary for Regulatory Activities (MedDRA version 27.0) and were summarized by system organ class (SOC) and preferred term (PT). A total of two treatment-emergent adverse events (TEAEs) were reported after DAO 210 mg administration, both classified under the SOC Nervous System Disorders, and both identified as headache (100%). These events were resolved without sequelae. The single non-treatment-emergent adverse event (non-TEAE) reported (100%) was recorded before DAO administration and was classified under the SOC Reproductive System and Breast Disorders, specifically as dysmenorrhoea. Paracetamol was used as concomitant medication in all three cases to manage the reported symptoms. No statistical comparison of the frequency of AEs onset was performed due to the scarce number of events. No serious adverse events (SAEs) were recorded during the study. All participants tolerated the supplementation well at all administered doses, with no observed differences between male and female participants, nor between the various dose levels, which were all found to be safe. Safety examinations based on clinical and laboratory parameters (hematology and biochemistry) did not show clinically relevant changes from baseline.
3.3. Hematological Parameters
Hematological values remained within normal ranges throughout the study, with only minor variations observed between the screening phase and the end of the study. A slight increase was noted in hemoglobin (from 139.47 ± 12.11 to 142.13 ± 12.33 g/L) and haematocrit levels (from 0.41 ± 0.03 to 0.42 ± 0.03 L/L), as well as in red blood cell count (from 4.65 ± 0.39 to 4.71 ± 0.38 × 1012/L). Mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and red cell distribution width (RDW) showed minimal changes. Platelet count showed a modest decrease (from 265.37 ± 43.19 to 248.23 ± 40.83 × 109/L), accompanied by a small reduction in plateletcrit. Leukocyte levels remained stable (6.00 ± 1.39 to 6.25 ± 1.16 × 109/L), with minor fluctuations in subpopulations, including lymphocytes and monocytes, which slightly increased by the end of the study, all within normal physiological ranges.
3.4. Biochemical Parameters
Biochemical assessments also showed no clinically relevant changes over the course of the study. Blood glucose levels increased marginally from 4.73 ± 0.42 to 4.83 ± 0.20 mmol/L, while urea and creatinine levels decreased slightly, indicating stable renal function. Liver function markers such as AST (GOT) and alkaline phosphatase showed a mild decrease (AST from 25.27 ± 4.60 to 21.13 ± 4.00 U/L; alkaline phosphatase from 79.77 ± 15.28 to 75.43 ± 14.28 U/L), suggesting no hepatic stress. Albumin levels declined modestly (from 45.93 ± 2.68 to 43.90 ± 2.91 g/L), while lipid parameters remained relatively constant, with a slight increase in triglycerides and a minor reduction in total cholesterol. Overall, the biochemical profile remained stable and within expected physiological ranges for healthy volunteers.
3.5. Electrocardiographic Parameters
Electrocardiographic (ECG) measurements remained within normal clinical limits throughout the study period. A decrease in ventricular rate was observed from baseline (67.17 ± 8.46 bpm) to the end of the study (58.23 ± 7.26 bpm), although this change was not associated with any reported clinical symptoms. The PR interval showed a slight increase (from 151.27 ± 21.92 to 157.47 ± 20.20 ms), while QRS duration remained essentially unchanged. The QT interval increased from 390.93 ± 25.82 to 410.13 ± 22.91 ms; however, the heart rate-corrected QT (QTc) interval slightly decreased (from 410.83 ± 20.93 to 401.53 ± 17.35 ms), suggesting no prolongation-related safety concerns. These variations are considered within the range of normal physiological variability in healthy subjects.
The changes observed in the vital signs (systolic and diastolic blood pressure and heart rate) did not show any clinically relevant abnormalities and all the parameters were within the normal range. No clinically significant changes were observed in the electrocardiogram recordings carried out during the study, nor did the physical examinations show any clinically important changes. All in all, the supplement was well-tolerated at all administered doses, with no observed differences between male and female participants, nor between the various dose levels, which were all found to be safe. There were no deaths or other significant/serious adverse events during the study.
4. Discussion
DAO is an enzyme endogenously produced by the human body that has a crucial role in maintaining gut health through the metabolization of histamine. Local DAO in the gut lumen is responsible for the breakdown of histamine contained in numerous common foods, consumed daily by the general population, such as fruits, nuts, cereals, fish, meat, and dairy products. People with a DAO deficiency are often recommended to follow a low-histamine diet and try to avoid histamine-rich foods, which might be unsustainable in the long term and lead to detrimental health effects, such as vitamin or mineral deficiencies. For this reason, finding alternative and complementary strategies to manage DAO deficiency is crucial.
Exogenous DAO supplementation available in the market has already shown promising results in supporting enzyme activity in the small intestine, breaking down dietary histamine locally and, therefore, alleviating HIT symptoms without adverse effects, offering a good option to support people with DAO deficiency. The DAO extract used in this study is a plant-based ingredient obtained from pea sprout dehydrated powder, covered with a gastro-resistant coating that protects the active ingredient from gastric acid. It is important to consider that there are no established limits for plant-sourced DAO supplementation.
Reported animal studies (acute oral study and a 90-day sub-chronic toxicity study) in rats corroborate the safety of orally administered diamine oxidase extract [
22]. The acute study investigated the oral toxicity of diamine oxidase at doses of 0.5 mL or 5.0 mL DAO/kg body weight (bw), equivalent to 7.85 mg and 78.5 mg DAO/kg bw, respectively. These represent over 600 and 6000-fold the daily intake of DAO present in the currently commercialized product (12.6 mg of extract daily, of which 7.14% corresponds to DAO enzyme = 0.013 mg DAO/kg for a 70 kg adult). No signs of clinical toxicity were noted in any of the animals [
22].
The sub-chronic study investigated the potential toxicity of diamine oxidase preparation in rats following repeated oral exposure for over 90 days. In this study, Wistar rats (8/sex/group) were administered diamine oxidase preparation suspended in saline at doses of 0 (control), 80 (low), 400 (mid), and 800 (high) µL/kg bw/day via gavage for 90 days. These daily doses corresponded to 1.256, 6.28, and 12.56 mg DAO/kg bw, respectively. The enzymatic activity and protein concentration of the diamine oxidase preparation in both the acute and sub-chronic studies were reported as 560,000 HDU (histamine degrading unit)/mL and 15.7 mg/mL, respectively [
22]. The doses used in this in vivo study represent almost 100-, 500-, and 1000-times the dose of DAO in the commercialized product at a standard dose of 12.6 mg extract/day. Based on findings from this in vivo study, the no observed adverse effect level (NOAEL) is determined as at least 800 µL DAO/kg bw/day, equivalent to 12.56 mg DAO/kg bw/day. Intake of 12.6 mg of marketed diamine oxidase supplement containing 7.14% DAO enzyme remains almost 1000 times below the NOAEL, further confirming a wide safety margin.
In the present clinical study, we administer daily doses of 42 mg, 84 mg, and 210 mg, respectively, which are significantly lower than the NOAEL. Apart from the animal toxicity data, some clinical studies have already used doses of 25.2 mg of oral DAO daily without any record of adverse events. A clinical study in patients with migraine and cefalea used daily doses of 25.2 mg of pig kidney protein extract standardized at the same concentration of the adiDAO
® Veg supplement tested in our study for a three-month period. No side effects were identified [
14]. It has also been published that during pregnancy the placenta secretes up to 1000 times more DAO than in non-pregnant periods [18, 20]. It is assumed to be a protection to the fetus and seems to be associated with a normal progression of pregnancy. Not only no adverse events have been linked to that physiological increase, but on the contrary, a lack of capacity to increase DAO levels during pregnancy is associated with some disorders (pre-eclampsia) and miscarriage [
19]. AdiDAO
® Veg has been on the market in Europe as a dietary supplement since 2022, with recommended daily doses of 12.6 mg of the pea sprout dehydrated extract, without any side effects reported so far.
In the current clinical study, thirty subjects were included in the safety population; twenty-four received active DAO formulation and six of them received placebo formulation. DAO enzyme supplementation appears safe and well-tolerated in the doses tested.
Based on the results obtained in our study, adiDAO® Veg is a safe, well-tolerated, and valid alternative to pig kidney protein extract rich in DAO. There was no difference between treatments and the placebo nor between treatment doses, demonstrating that all doses were safe.
The product’s safety and tolerability were thoroughly evaluated at three different doses, and no serious adverse events were observed, even after increasing the dose by 50-fold, as assessed by thorough physical evaluations, a comprehensive battery of laboratory tests (including hematology and biochemistry), vital signs monitoring, electrocardiograms (ECG), and detailed physical examinations. No relevant or consistent patterns of change were observed across these assessments. No serious adverse events (SAEs) were recorded during the study. These findings suggest that the product is highly safe for its potential application for managing DAO deficiency in healthy individuals and those with diagnosed histamine intolerance.
A practical limitation of the study is the administration of a high number of tablets to test the active dose. Alternative formats, such as sachets, have been developed to allow for higher doses of DAO in a single administration, which could improve ease of intake in future studies. Additionally, this study did not assess the effects of chronic high-dose DAO supplementation, but the findings demonstrate that acute high-dose intake is safe and does not pose any risk to individuals. For future research, a longer study duration could provide further insights on the safety of DAO consumption.