Shaping the Landscape of Lung Cancer: The Role and Therapeutic Potential of Matrix Metalloproteinases
Abstract
1. Introduction
2. MMPs
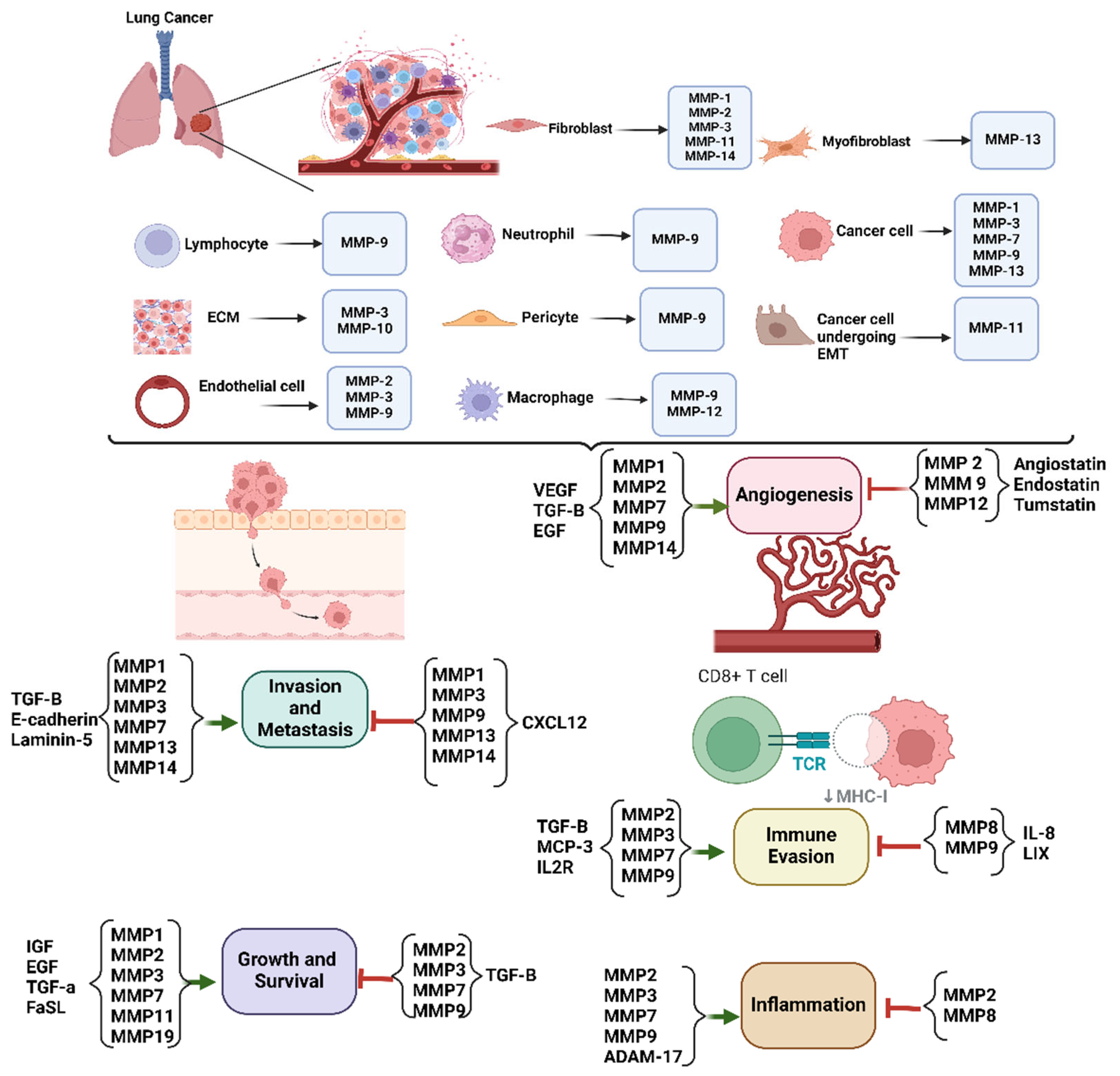
3. The Role of MMPs in Lung Cancer
3.1. Tumor Invasion and Metastasis
3.2. Angiogenesis
3.3. Tumor Microenvironment Modulation
3.4. Immune Evasion
3.5. Inflammation
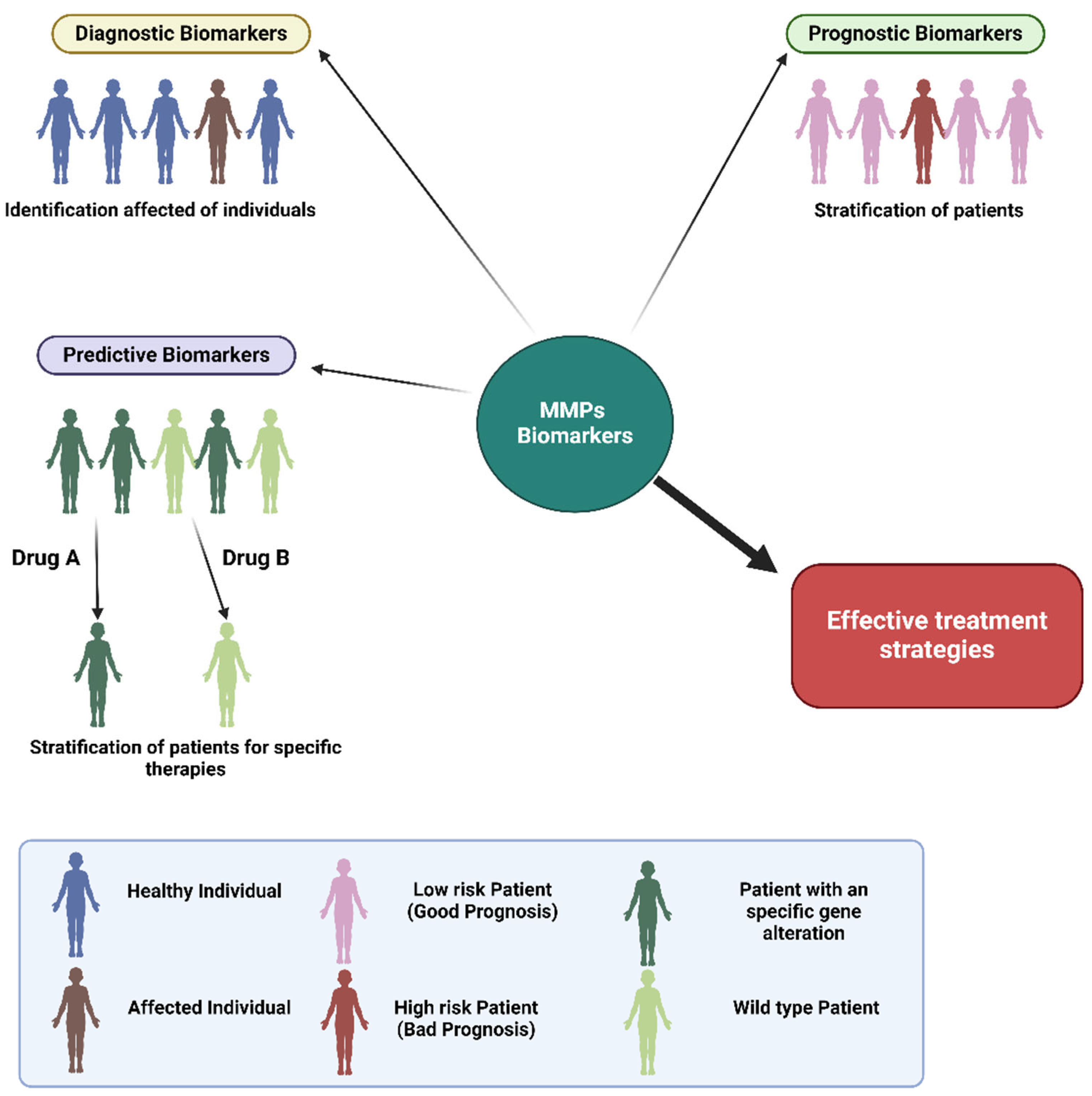
4. The Diagnostic and Prognostic Role of MMP Biomarkers in Lung Cancer
5. MMPs as Potential Targets for Therapy
6. Future Perspective
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Thandra, K.C.; Barsouk, A.; Saginala, K.; Aluru, J.S.; Barsouk, A. Epidemiology of lung cancer. Contemp. Oncol. 2021, 25, 45–52. [Google Scholar] [CrossRef]
- Chen, Z.; Fillmore, C.M.; Hammerman, P.S.; Kim, C.F.; Wong, K.K. Non-small-cell lung cancers: A heterogeneous set of diseases. Nat. Rev. Cancer 2014, 14, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Molina, J.R.; Yang, P.; Cassivi, S.D.; Schild, S.E.; Adjei, A.A. Non-small cell lung cancer: Epidemiology, risk factors, treatment, and survivorship. Mayo Clin. Proc. 2008, 83, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Dela Cruz, C.S.; Tanoue, L.T.; Matthay, R.A. Lung cancer: Epidemiology, etiology, and prevention. Clin. Chest Med. 2011, 32, 605–644. [Google Scholar] [CrossRef] [PubMed]
- Kusnierczyk, P. Genetic differences between smokers and never-smokers with lung cancer. Front. Immunol. 2023, 14, 1063716. [Google Scholar] [CrossRef]
- Shankar, A.; Dubey, A.; Saini, D.; Singh, M.; Prasad, C.P.; Roy, S.; Bharati, S.J.; Rinki, M.; Singh, N.; Seth, T.; et al. Environmental and occupational determinants of lung cancer. Transl. Lung Cancer Res. 2019, 8, S31–S49. [Google Scholar] [CrossRef]
- Dubin, S.; Griffin, D. Lung Cancer in Non-Smokers. Mo. Med. 2020, 117, 375–379. [Google Scholar]
- Kiyohara, C.; Otsu, A.; Shirakawa, T.; Fukuda, S.; Hopkin, J.M. Genetic polymorphisms and lung cancer susceptibility: A review. Lung Cancer 2002, 37, 241–256. [Google Scholar] [CrossRef]
- Islami, F.; Torre, L.A.; Jemal, A. Global trends of lung cancer mortality and smoking prevalence. Transl. Lung Cancer Res. 2015, 4, 327–338. [Google Scholar] [CrossRef]
- Shreves, A.H.; Buller, I.D.; Chase, E.; Creutzfeldt, H.; Fisher, J.A.; Graubard, B.I.; Hoover, R.N.; Silverman, D.T.; Devesa, S.S.; Jones, R.R. Geographic Patterns in U.S. Lung Cancer Mortality and Cigarette Smoking. Cancer Epidemiol. Biomark. Prev. 2023, 32, 193–201. [Google Scholar] [CrossRef]
- Yang, P. Epidemiology of lung cancer prognosis: Quantity and quality of life. Methods Mol. Biol. 2009, 471, 469–486. [Google Scholar] [CrossRef] [PubMed]
- Xing, P.Y.; Zhu, Y.X.; Wang, L.; Hui, Z.G.; Liu, S.M.; Ren, J.S.; Zhang, Y.; Song, Y.; Liu, C.C.; Huang, Y.C.; et al. What are the clinical symptoms and physical signs for non-small cell lung cancer before diagnosis is made? A nation-wide multicenter 10-year retrospective study in China. Cancer Med. 2019, 8, 4055–4069. [Google Scholar] [CrossRef] [PubMed]
- Nooreldeen, R.; Bach, H. Current and Future Development in Lung Cancer Diagnosis. Int. J. Mol. Sci. 2021, 22, 8661. [Google Scholar] [CrossRef] [PubMed]
- Schabath, M.B.; Cote, M.L. Cancer Progress and Priorities: Lung Cancer. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1563–1579. [Google Scholar] [CrossRef]
- Onwuzo, C.N.; Olukorode, J.; Sange, W.; Orimoloye, D.A.; Udojike, C.; Omoragbon, L.; Hassan, A.E.; Falade, D.M.; Omiko, R.; Odunaike, O.S.; et al. A Review of Smoking Cessation Interventions: Efficacy, Strategies for Implementation, and Future Directions. Cureus 2024, 16, e52102. [Google Scholar] [CrossRef]
- Normanno, N.; Rachiglio, A.M.; Roma, C.; Fenizia, F.; Esposito, C.; Pasquale, R.; La Porta, M.L.; Iannaccone, A.; Micheli, F.; Santangelo, M.; et al. Molecular diagnostics and personalized medicine in oncology: Challenges and opportunities. J. Cell Biochem. 2013, 114, 514–524. [Google Scholar] [CrossRef]
- Li, M.S.C.; Mok, K.K.S.; Mok, T.S.K. Developments in targeted therapy & immunotherapy-how non-small cell lung cancer management will change in the next decade: A narrative review. Ann. Transl. Med. 2023, 11, 358. [Google Scholar] [CrossRef]
- Niland, S.; Riscanevo, A.X.; Eble, J.A. Matrix Metalloproteinases Shape the Tumor Microenvironment in Cancer Progression. Int. J. Mol. Sci. 2021, 23, 146. [Google Scholar] [CrossRef]
- Merchant, N.; Nagaraju, G.P.; Rajitha, B.; Lammata, S.; Jella, K.K.; Buchwald, Z.S.; Lakka, S.S.; Ali, A.N. Matrix metalloproteinases: Their functional role in lung cancer. Carcinogenesis 2017, 38, 766–780. [Google Scholar] [CrossRef]
- Wei, C. The multifaceted roles of matrix metalloproteinases in lung cancer. Front. Oncol. 2023, 13, 1195426. [Google Scholar] [CrossRef]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; Castruita-De la Rosa, C.; Ramirez-Acuna, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef] [PubMed]
- Radisky, E.S. Extracellular proteolysis in cancer: Proteases, substrates, and mechanisms in tumor progression and metastasis. J. Biol. Chem. 2024, 300, 107347. [Google Scholar] [CrossRef] [PubMed]
- Shoari, A. Potential of MMP-2 and MMP-9 Gelatinase Blockade as a Therapeutic Strategy in Fibrosarcoma Treatment: A Decadal Review. Targets 2024, 2, 104–125. [Google Scholar] [CrossRef]
- Ra, H.J.; Parks, W.C. Control of matrix metalloproteinase catalytic activity. Matrix Biol. 2007, 26, 587–596. [Google Scholar] [CrossRef]
- Laronha, H.; Caldeira, J. Structure and Function of Human Matrix Metalloproteinases. Cells 2020, 9, 1076. [Google Scholar] [CrossRef]
- Shoari, A.; Khalili-Tanha, G.; Coban, M.A.; Radisky, E.S. Structure and computation-guided yeast surface display for the evolution of TIMP-based matrix metalloproteinase inhibitors. Front. Mol. Biosci. 2023, 10, 1321956. [Google Scholar] [CrossRef]
- Moliere, S.; Jaulin, A.; Tomasetto, C.L.; Dali-Youcef, N. Roles of Matrix Metalloproteinases and Their Natural Inhibitors in Metabolism: Insights into Health and Disease. Int. J. Mol. Sci. 2023, 24, 10649. [Google Scholar] [CrossRef]
- Gho, W.G.; Choi, Y.; Park, K.H.; Huh, J.K. Expression of collagenases (matrix metalloproteinase-1, 8, 13) and tissue inhibitor of metalloproteinase-1 of retrodiscal tissue in temporomandibular joint disorder patients. J. Korean Assoc. Oral. Maxillofac. Surg. 2018, 44, 120–127. [Google Scholar] [CrossRef]
- Nikolov, A.; Popovski, N. Role of Gelatinases MMP-2 and MMP-9 in Healthy and Complicated Pregnancy and Their Future Potential as Preeclampsia Biomarkers. Diagnostics 2021, 11, 480. [Google Scholar] [CrossRef]
- Bord, S.; Horner, A.; Hembry, R.M.; Compston, J.E. Stromelysin-1 (MMP-3) and stromelysin-2 (MMP-10) expression in developing human bone: Potential roles in skeletal development. Bone 1998, 23, 7–12. [Google Scholar] [CrossRef]
- Piskor, B.M.; Przylipiak, A.; Dabrowska, E.; Niczyporuk, M.; Lawicki, S. Matrilysins and Stromelysins in Pathogenesis and Diagnostics of Cancers. Cancer Manag. Res. 2020, 12, 10949–10964. [Google Scholar] [CrossRef] [PubMed]
- Itoh, Y. Membrane-type matrix metalloproteinases: Their functions and regulations. Matrix Biol. 2015, 44–46, 207–223. [Google Scholar] [CrossRef]
- Chou, P.H.; Lai, S.C. Elevated concentrations of matrix metalloproteinase-12 and elastin degradation products in the sera of pregnant women infected with Toxoplasma gondii. Ann. Trop. Med. Parasitol. 2011, 105, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Stracke, J.O.; Hutton, M.; Stewart, M.; Pendas, A.M.; Smith, B.; Lopez-Otin, C.; Murphy, G.; Knauper, V. Biochemical characterization of the catalytic domain of human matrix metalloproteinase 19. Evidence for a role as a potent basement membrane degrading enzyme. J. Biol. Chem. 2000, 275, 14809–14816. [Google Scholar] [CrossRef] [PubMed]
- Sternlicht, M.D.; Werb, Z. How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Dev. Biol. 2001, 17, 463–516. [Google Scholar] [CrossRef] [PubMed]
- Löffek, S.; Schilling, O.; Franzke, C.W. Biological role of matrix metalloproteinases: A critical balance. Eur. Respir. J. 2011, 38, 191–208. [Google Scholar] [CrossRef]
- Guo, C.B.; Wang, S.; Deng, C.; Zhang, D.L.; Wang, F.L.; Jin, X.Q. Relationship between matrix metalloproteinase 2 and lung cancer progression. Mol. Diagn. Ther. 2007, 11, 183–192. [Google Scholar] [CrossRef]
- Han, L.P.; Sheng, B.W.; Zeng, Q.D.; Yao, W.; Jiang, Q.F. Correlation between MMP2 expression in lung cancer tissues and clinical parameters: A retrospective clinical analysis. BMC Pulm. Med. 2020, 20, 283. [Google Scholar] [CrossRef]
- Morishita, A.; Gerber, A.; Gow, C.H.; Zelonina, T.; Chada, K.; D’Armiento, J. Cell Specific Matrix Metalloproteinase-1 Regulates Lung Metastasis Synergistically with Smoke Exposure. J. Cancer Res. Forecast. 2018, 1, 1014. [Google Scholar]
- Liu, D.; Nakano, J.; Ishikawa, S.; Yokomise, H.; Ueno, M.; Kadota, K.; Urushihara, M.; Huang, C.L. Overexpression of matrix metalloproteinase-7 (MMP-7) correlates with tumor proliferation, and a poor prognosis in non-small cell lung cancer. Lung Cancer 2007, 58, 384–391. [Google Scholar] [CrossRef]
- Stawowczyk, M.; Wellenstein, M.D.; Lee, S.B.; Yomtoubian, S.; Durrans, A.; Choi, H.; Narula, N.; Altorki, N.K.; Gao, D.; Mittal, V. Matrix Metalloproteinase 14 promotes lung cancer by cleavage of Heparin-Binding EGF-like Growth Factor. Neoplasia 2017, 19, 55–64. [Google Scholar] [CrossRef]
- Deryugina, E.I.; Quigley, J.P. Tumor angiogenesis: MMP-mediated induction of intravasation- and metastasis-sustaining neovasculature. Matrix Biol. 2015, 44–46, 94–112. [Google Scholar] [CrossRef] [PubMed]
- Nishida, N.; Yano, H.; Nishida, T.; Kamura, T.; Kojiro, M. Angiogenesis in cancer. Vasc. Health Risk Manag. 2006, 2, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Quintero-Fabian, S.; Arreola, R.; Becerril-Villanueva, E.; Torres-Romero, J.C.; Arana-Argaez, V.; Lara-Riegos, J.; Ramirez-Camacho, M.A.; Alvarez-Sanchez, M.E. Role of Matrix Metalloproteinases in Angiogenesis and Cancer. Front. Oncol. 2019, 9, 1370. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, H.Y.; Yan, L.X.; Du, W.; Zhang, M.; Chen, H.; Zhang, L.X.; Li, G.Q.; Li, J.J.; Dong, Y.C.; et al. MMP-2 and MMP-9 contribute to the angiogenic effect produced by hypoxia/15-HETE in pulmonary endothelial cells. J. Mol. Cell. Cardiol. 2018, 121, 36–50. [Google Scholar] [CrossRef]
- Rao, J.S.; Gondi, C.; Chetty, C.; Chittivelu, S.; Joseph, P.A.; Lakka, S.S. Inhibition of invasion, angiogenesis, tumor growth, and metastasis by adenovirus-mediated transfer of antisense uPAR and MMP-9 in non-small cell lung cancer cells. Mol. Cancer Ther. 2005, 4, 1399–1408. [Google Scholar] [CrossRef]
- Cao, C.; Xu, N.; Zheng, X.; Zhang, W.; Lai, T.; Deng, Z.; Huang, X. Elevated expression of MMP-2 and TIMP-2 cooperatively correlates with risk of lung cancer. Oncotarget. 2017, 8, 80560–80567. [Google Scholar] [CrossRef][Green Version]
- Ding, B.S.; Nolan, D.J.; Guo, P.; Babazadeh, A.O.; Cao, Z.; Rosenwaks, Z.; Crystal, R.G.; Simons, M.; Sato, T.N.; Worgall, S.; et al. Endothelial-derived angiocrine signals induce and sustain regenerative lung alveolarization. Cell 2011, 147, 539–553. [Google Scholar] [CrossRef]
- Hsu, C.P.; Shen, G.H.; Ko, J.L. Matrix metalloproteinase-13 expression is associated with bone marrow microinvolvement and prognosis in non-small cell lung cancer. Lung Cancer 2006, 52, 349–357. [Google Scholar] [CrossRef]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor angiogenesis: Causes, consequences, challenges and opportunities. Cell Mol. Life Sci. 2020, 77, 1745–1770. [Google Scholar] [CrossRef]
- Liu, Z.L.; Chen, H.H.; Zheng, L.L.; Sun, L.P.; Shi, L. Angiogenic signaling pathways and anti-angiogenic therapy for cancer. Signal Transduct. Tar. 2023, 8, 198. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.; Cai, M.; Zhu, J.; Geng, J.Q.; Zhu, K.J.; Jin, X.Y.; Ding, W.J. Matrix Metalloproteinase Activity in Early-Stage Lung Cancer. Onkologie 2013, 36, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.M.; Simon, M.C. The tumor microenvironment. Curr. Biol. 2020, 30, R921–R925. [Google Scholar] [CrossRef]
- Yuan, Z.N.; Li, Y.P.; Zhang, S.F.; Wang, X.Y.; Dou, H.; Yu, X.; Zhang, Z.R.; Yang, S.S.; Xiao, M. Extracellular matrix remodeling in tumor progression and immune escape: From mechanisms to treatments. Mol. Cancer 2023, 22, 48. [Google Scholar] [CrossRef]
- Yu, W.H.; Wu, E.; Li, Y.; Hou, H.H.; Yu, S.C.; Huang, P.T.; Kuo, W.H.; Qi, D.; Yu, C.J. Matrix Metalloprotease-7 Mediates Nucleolar Assembly and Intra-nucleolar Cleaving p53 in Gefitinib-Resistant Cancer Stem Cells. iScience 2020, 23, 101600. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhang, J.; Shi, L.Z.; Ning, Y.Y.; Zhu, Y.; Chen, S.; Yang, M.; Chen, J.Y.; Zhou, G.W.; Li, Q. NOGO-B promotes EMT in lung fibrosis via MMP14 mediates free TGF-beta1 formation. Oncotarget 2017, 8, 71024–71037. [Google Scholar] [CrossRef]
- Neophytou, C.M.; Panagi, M.; Stylianopoulos, T.; Papageorgis, P. The Role of Tumor Microenvironment in Cancer Metastasis: Molecular Mechanisms and Therapeutic Opportunities. Cancers 2021, 13, 2053. [Google Scholar] [CrossRef]
- Shah, K.; Mallik, S.B.; Gupta, P.; Iyer, A. Targeting Tumour-Associated Fibroblasts in Cancers. Front. Oncol. 2022, 12, 908156. [Google Scholar] [CrossRef]
- Gabasa, M.; Radisky, E.S.; Ikemori, R.; Bertolini, G.; Arshakyan, M.; Hockla, A.; Duch, P.; Rondinone, O.; Llorente, A.; Maqueda, M.; et al. MMP1 drives tumor progression in large cell carcinoma of the lung through fibroblast senescence. Cancer Lett. 2021, 507, 1–12. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, K.; Tan, X.; Li, Z.; Wang, H. Immunomodulatory role of metalloproteases in cancers: Current progress and future trends. Front. Immunol. 2022, 13, 1064033. [Google Scholar] [CrossRef]
- Kolesnikoff, N.; Chen, C.H.; Samuel, M.S. Interrelationships between the extracellular matrix and the immune microenvironment that govern epithelial tumour progression. Clin. Sci. 2022, 136, 361–377. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Huo, R.; Fang, G.T.; Ma, T.T.; Shang, Y.H. MMP11 is associated with the immune response and immune microenvironment in EGFR-mutant lung adenocarcinoma. Front. Oncol. 2023, 13, 1055122. [Google Scholar] [CrossRef] [PubMed]
- Juric, V.; O’Sullivan, C.; Stefanutti, E.; Kovalenko, M.; Greenstein, A.; Barry-Hamilton, V.; Mikaelian, I.; Degenhardt, J.; Yue, P.; Smith, V.; et al. MMP-9 inhibition promotes anti-tumor immunity through disruption of biochemical and physical barriers to T-cell trafficking to tumors. PLoS ONE 2018, 13, e0207255. [Google Scholar] [CrossRef] [PubMed]
- De Paiva, C.S.; Yoon, K.C.; Pangelinan, S.B.; Pham, S.; Puthenparambil, L.M.; Chuang, E.Y.; Farley, W.J.; Stern, M.E.; Li, D.Q.; Pflugfelder, S.C. Cleavage of functional IL-2 receptor alpha chain (CD25) from murine corneal and conjunctival epithelia by MMP-9. J. Inflamm. 2009, 6, 31. [Google Scholar] [CrossRef]
- Anichini, A.; Perotti, V.E.; Sgambelluri, F.; Mortarini, R. Immune Escape Mechanisms in Non Small Cell Lung Cancer. Cancers 2020, 12, 3605. [Google Scholar] [CrossRef]
- Gobin, E.; Bagwell, K.; Wagner, J.; Mysona, D.; Sandirasegarane, S.; Smith, N.; Bai, S.; Sharma, A.; Schleifer, R.; She, J.X. A pan-cancer perspective of matrix metalloproteases (MMP) gene expression profile and their diagnostic/prognostic potential. BMC Cancer 2019, 19, 581. [Google Scholar] [CrossRef]
- Haydinger, C.D.; Ashander, L.M.; Tan, A.C.R.; Smith, J.R. Intercellular Adhesion Molecule 1: More than a Leukocyte Adhesion Molecule. Biology 2023, 12, 743. [Google Scholar] [CrossRef]
- Xiao, Y.; Li, Z.Z.; Zhong, N.N.; Cao, L.M.; Liu, B.; Bu, L.L. Charting new frontiers: Co-inhibitory immune checkpoint proteins in therapeutics, biomarkers, and drug delivery systems in cancer care. Transl. Oncol. 2023, 38, 101794. [Google Scholar] [CrossRef]
- Ando, F.; Kashiwada, T.; Kuroda, S.; Fujii, T.; Takano, R.; Miyabe, Y.; Kunugi, S.; Sakatani, T.; Miyanaga, A.; Asatsuma-Okumura, T.; et al. Combination of plasma MMPs and PD-1-binding soluble PD-L1 predicts recurrence in gastric cancer and the efficacy of immune checkpoint inhibitors in non-small cell lung cancer. Front. Pharmacol. 2024, 15, 1384731. [Google Scholar] [CrossRef]
- He, Z.N.; Zhang, C.Y.; Zhao, Y.W.; He, S.L.; Li, Y.; Shi, B.L.; Hu, J.Q.; Qi, R.Z.; Hua, B.J. Regulation of T cells by myeloid-derived suppressor cells: Emerging immunosuppressor in lung cancer. Discov. Oncol. 2023, 14, 185. [Google Scholar] [CrossRef]
- Hao, Z.N.; Li, R.Y.; Wang, Y.Y.; Li, S.Y.; Hong, Z.Y.; Han, Z.Q. Landscape of Myeloid-derived Suppressor Cell in Tumor Immunotherapy. Biomark. Res. 2021, 9, 77. [Google Scholar] [CrossRef] [PubMed]
- Shubayev, V.I.; Angert, M.; Dolkas, J.; Campana, W.M.; Palenscar, K.; Myers, R.R. TNFalpha-induced MMP-9 promotes macrophage recruitment into injured peripheral nerve. Mol. Cell Neurosci. 2006, 31, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Yang, J.; Moses, M.A. Matrix metalloproteinases as novel biomarkers and potential therapeutic targets in human cancer. J. Clin. Oncol. 2009, 27, 5287–5297. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.A.; Spinale, F.G.; Ikonomidis, J.S.; Stroud, R.E.; Chang, E.I.; Reed, C.E. Differential matrix metalloproteinase levels in adenocarcinoma and squamous cell carcinoma of the lung. J. Thorac. Cardiovasc. Surg. 2010, 139, 984–990; discussion 990. [Google Scholar] [CrossRef]
- Koc, M.; Ediger, D.; Budak, F.; Karadag, M.; Oral, H.B.; Uzaslan, E.; Ege, E.; Gozu, R.O. Matrix metalloproteinase-9 (MMP-9) elevated in serum but not in bronchial lavage fluid in patients with lung cancer. Tumori 2006, 92, 149–154. [Google Scholar] [CrossRef]
- Jumper, C.; Cobos, E.; Lox, C. Determination of the serum matrix metalloproteinase-9 (MMP-9) and tissue inhibitor of matrix metalloproteinase-1 (TIMP-1) in patients with either advanced small-cell lung cancer or non-small-cell lung cancer prior to treatment. Resp. Med. 2004, 98, 173–177. [Google Scholar] [CrossRef]
- Sauter, W.; Rosenberger, A.; Beckmann, L.; Kropp, S.; Mittelstrass, K.; Timofeeva, M.; Wölke, G.; Steinwachs, A.; Scheiner, D.; Meese, E.; et al. Matrix metalloproteinase 1 (MMP1) is associated with early-onset lung cancer. Cancer Epidem. Biomar. 2008, 17, 1127–1135. [Google Scholar] [CrossRef]
- Hussain, T.; Nguyen, Q.T. Molecular imaging for cancer diagnosis and surgery. Adv. Drug Deliv. Rev. 2014, 66, 90–100. [Google Scholar] [CrossRef]
- Souza, V.G.P.; Forder, A.; Brockley, L.J.; Pewarchuk, M.E.; Telkar, N.; de Araujo, R.P.; Trejo, J.; Benard, K.; Seneda, A.L.; Minutentag, I.W.; et al. Liquid Biopsy in Lung Cancer: Biomarkers for the Management of Recurrence and Metastasis. Int. J. Mol. Sci. 2023, 24, 8894. [Google Scholar] [CrossRef]
- Metzenmacher, M.; Hegedus, B.; Forster, J.; Schramm, A.; Horn, P.A.A.; Klein, C.A.A.; Bielefeld, N.; Ploenes, T.; Aigner, C.; Theegarten, D.; et al. Combined multimodal ctDNA analysis and radiological imaging for tumor surveillance in Non-small cell lung cancer. Transl. Oncol. 2022, 15, 101279. [Google Scholar] [CrossRef]
- Zheng, Z.; Peng, F.; Zhou, Y. Biomarkers in idiopathic pulmonary fibrosis: Current insight and future direction. Chin. Med. J. Pulm. Crit. Care Med. 2024, 2, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Winkler, K.; Kowalczyk, A.; Bereza, P.; Regulska, K.; Kasprzak, A.; Bamburowicz-Klimkowska, M.; Nowicka, A.M. Levels of active forms of MMP-1, MMP-2, and MMP-9 as independent prognostic factors for differentiating the stage and type of lung cancer (SCLC and NSCLC). Sens. Actuat B-Chem. 2024, 406, 135421. [Google Scholar] [CrossRef]
- Li, M.; Xiao, T.; Zhang, Y.; Feng, L.; Lin, D.M.; Liu, Y.; Mao, Y.S.; Guo, S.P.; Han, N.J.; Di, X.B.; et al. Prognostic significance of matrix metalloproteinase-1 levels in peripheral plasma and tumour tissues of lung cancer patients. Lung Cancer 2010, 69, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Michael, M.; Babic, B.; Khokha, R.; Tsao, M.; Ho, J.; Pintilie, M.; Leco, K.; Chamberlain, D.; Shepherd, F.A. Expression and prognostic significance of metalloproteinases and their tissue inhibitors in patients with small-cell lung cancer. J. Clin. Oncol. 1999, 17, 1802–1808. [Google Scholar] [CrossRef]
- Liu, C.H.; Di, Y.P. Matrix Metallopeptidase-Gene Signature Predicts Stage I Lung Adenocarcinoma Survival Outcomes. Int. J. Mol. Sci. 2023, 24, 2382. [Google Scholar] [CrossRef]
- Kinoshita, T.; Goto, T. Links between Inflammation and Postoperative Cancer Recurrence. J. Clin. Med. 2021, 10, 228. [Google Scholar] [CrossRef]
- Almutairi, S.; Kalloush, H.M.; Manoon, N.A.; Bardaweel, S.K. Matrix Metalloproteinases Inhibitors in Cancer Treatment: An Updated Review (2013–2023). Molecules 2023, 28, 5567. [Google Scholar] [CrossRef]
- Cathcart, J.; Pulkoski-Gross, A.; Cao, J. Targeting Matrix Metalloproteinases in Cancer: Bringing New Life to Old Ideas. Genes. Dis. 2015, 2, 26–34. [Google Scholar] [CrossRef]
- Fields, G.B. New strategies for targeting matrix metalloproteinases. Matrix Biol. 2015, 44–46, 239–246. [Google Scholar] [CrossRef]
- Winer, A.; Adams, S.; Mignatti, P. Matrix Metalloproteinase Inhibitors in Cancer Therapy: Turning Past Failures Into Future Successes. Mol. Cancer Ther. 2018, 17, 1147–1155. [Google Scholar] [CrossRef]
- Ndinguri, M.W.; Bhowmick, M.; Tokmina-Roszyk, D.; Robichaud, T.K.; Fields, G.B. Peptide-based selective inhibitors of matrix metalloproteinase-mediated activities. Molecules 2012, 17, 14230–14248. [Google Scholar] [CrossRef] [PubMed]
- Shoari, A.; Rasaee, M.J.; Kanavi, M.R.; Daraei, B. Functional mimetic peptide discovery isolated by phage display interacts selectively to fibronectin domain and inhibits gelatinase. J. Cell Biochem. 2019, 120, 19699–19711. [Google Scholar] [CrossRef] [PubMed]
- Fields, G.B. The Rebirth of Matrix Metalloproteinase Inhibitors: Moving Beyond the Dogma. Cells 2019, 8, 984. [Google Scholar] [CrossRef]
- Tune, B.X.J.; Sim, M.S.; Poh, C.L.; Guad, R.M.; Woon, C.K.; Hazarika, I.; Das, A.; Gopinath, S.C.B.; Rajan, M.; Sekar, M.; et al. Matrix Metalloproteinases in Chemoresistance: Regulatory Roles, Molecular Interactions, and Potential Inhibitors. J. Oncol. 2022, 25. [Google Scholar] [CrossRef]
- Kasaoka, T.; Nishiyama, H.; Okada, M.; Nakajima, M. Matrix metalloproteinase inhibitor, MMI270 (CGS27023A) inhibited hematogenic metastasis of B16 melanoma cells in both experimental and spontaneous metastasis models. Clin. Exp. Metastasis 2008, 25, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Bissett, D.; O’Byrne, K.J.; von Pawel, J.; Gatzemeier, U.; Price, A.; Nicolson, M.; Mercier, R.; Mazabel, E.; Penning, C.; Zhang, M.H.; et al. Phase III study of matrix metalloproteinase inhibitor prinomastat in non-small-cell lung cancer. J. Clin. Oncol. 2005, 23, 842–849. [Google Scholar] [CrossRef]
- Nguyen, Y.T.; Kim, N.; Lee, H.J. Metal Complexes as Promising Matrix Metalloproteinases Regulators. Int. J. Mol. Sci. 2023, 24, 1258. [Google Scholar] [CrossRef]
- Poulaki, V. BMS-275291. Bristol-Myers Squibb. Curr. Opin. Investig. Drugs 2002, 3, 500–504. [Google Scholar] [PubMed]
- Leighl, N.B.; Paz-Ares, L.; Douillard, J.Y.; Peschel, C.; Arnold, A.; Depierre, A.; Santoro, A.; Betticher, D.C.; Gatzemeier, U.; Jassem, J.; et al. Randomized phase III study of matrix metalloproteinase inhibitor BMS-275291 in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: National Cancer Institute of Canada-Clinical Trials Group Study BR.18. J. Clin. Oncol. 2005, 23, 2831–2839. [Google Scholar] [CrossRef]
- Gatto, C.; Rieppi, M.; Borsotti, P.; Innocenti, S.; Ceruti, R.; Drudis, T.; Scanziani, E.; Casazza, A.M.; Taraboletti, G.; Giavazzi, R. BAY 12-9566, a novel inhibitor of matrix metalloproteinases with antiangiogenic activity. Clin. Cancer Res. 1999, 5, 3603–3607. [Google Scholar]
- Almholt, K.; Juncker-Jensen, A.; Laerum, O.D.; Dano, K.; Johnsen, M.; Lund, L.R.; Romer, J. Metastasis is strongly reduced by the matrix metalloproteinase inhibitor Galardin in the MMTV-PymT transgenic breast cancer model. Mol. Cancer Ther. 2008, 7, 2758–2767. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, B.S.; Egeblad, M.; Rank, F.; Askautrud, H.A.; Pennington, C.J.; Pedersen, T.X.; Christensen, I.J.; Edwards, D.R.; Werb, Z.; Lund, L.R. Matrix metalloproteinase 13 is induced in fibroblasts in polyomavirus middle T antigen-driven mammary carcinoma without influencing tumor progression. PLoS ONE 2008, 3, e2959. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T.; Tanioka, M.; Matsuda, H.; Nishimoto, H.; Yoshioka, T.; Suzuki, R.; Uehira, M. Experimental metastasis is suppressed in MMP-9-deficient mice. Clin. Exp. Metastas. 1999, 17, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.D.; Carter, K.J.; Jean-Philippe, S.R.; Chang, M.; Mobashery, S.; Thiolloy, S.; Lynch, C.C.; Matrisian, L.M.; Fingleton, B. Effect of ablation or inhibition of stromal matrix metalloproteinase-9 on lung metastasis in a breast cancer model is dependent on genetic background. Cancer Res. 2008, 68, 6251–6259. [Google Scholar] [CrossRef]
- Itoh, T.; Tanioka, M.; Yoshida, H.; Yoshioka, T.; Nishimoto, H.; Itohara, S. Reduced angiogenesis and tumor progression in gelatinase A-deficient mice. Cancer Res. 1998, 58, 1048–1051. [Google Scholar]
- Chetty, C.; Bhoopathi, P.; Joseph, P.; Chittivelu, S.; Rao, J.S.; Lakka, S. Adenovirus-mediated small interfering RNA against matrix metalloproteinase-2 suppresses tumor growth and lung metastasis in mice. Mol. Cancer Ther. 2006, 5, 2289–2299. [Google Scholar] [CrossRef]
- Chetty, C.; Lakka, S.S.; Bhoopathi, P.; Kunigal, S.; Geiss, R.; Rao, J.S. Tissue inhibitor of metalloproteinase 3 suppresses tumor angiogenesis in matrix metalloproteinase 2-down-regulated lung cancer. Cancer Res. 2008, 68, 4736–4745. [Google Scholar] [CrossRef]
- Chetty, C.; Lakka, S.S.; Bhoopathi, P.; Rao, J.S. MMP-2 alters VEGF expression via alphaVbeta3 integrin-mediated PI3K/AKT signaling in A549 lung cancer cells. Int. J. Cancer 2010, 127, 1081–1095. [Google Scholar] [CrossRef]
- Song, J.M.; Anandharaj, A.; Upadhyaya, P.; Kirtane, A.R.; Kim, J.H.; Hong, K.H.; Panyam, J.; Kassie, F. Honokiol suppresses lung tumorigenesis by targeting EGFR and its downstream effectors. Oncotarget 2016, 7, 57752–57769. [Google Scholar] [CrossRef]
- Pai, J.T.; Hsu, C.Y.; Hsieh, Y.S.; Tsai, T.Y.; Hua, K.T.; Weng, M.S. Suppressing migration and invasion of H1299 lung cancer cells by honokiol through disrupting expression of an HDAC6-mediated matrix metalloproteinase 9. Food Sci. Nutr. 2020, 8, 1534–1545. [Google Scholar] [CrossRef]
- Halder, A.K.; Mallick, S.; Shikha, D.; Saha, A.; Saha, K.D.; Jha, T. Design of dual MMP-2/HDAC-8 inhibitors by pharmacophore mapping, molecular docking, synthesis and biological activity. Rsc Adv. 2015, 5, 72373–72386. [Google Scholar] [CrossRef]
- Chen, C.; Yang, X.; Fang, H.; Hou, X. Design, synthesis and preliminary bioactivity evaluations of 8-hydroxyquinoline derivatives as matrix metalloproteinase (MMP) inhibitors. Eur. J. Med. Chem. 2019, 181, 111563. [Google Scholar] [CrossRef] [PubMed]
- Qi, P.; Li, Y.; Liu, X.; Jafari, F.A.; Zhang, X.; Sun, Q.; Ma, Z. Cryptotanshinone Suppresses Non-Small Cell Lung Cancer via microRNA-146a-5p/EGFR Axis. Int. J. Biol. Sci. 2019, 15, 1072–1079. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Zhang, Y.; Liu, W.; Wang, J. Cryptotanshinone inhibits lung cancer invasion via microRNA-133a/matrix metalloproteinase 14 regulation. Oncol. Lett. 2019, 18, 2554–2559. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Y.; Xiao, Q.; Yin, L.; Yang, L.; He, W. Potent delivery of an MMP inhibitor to the tumor microenvironment with thermosensitive liposomes for the suppression of metastasis and angiogenesis. Signal Transduct. Target. Ther. 2019, 4, 26. [Google Scholar] [CrossRef] [PubMed]
- Levin, M.; Udi, Y.; Solomonov, I.; Sagi, I. Next generation matrix metalloproteinase inhibitors—Novel strategies bring new prospects. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 1927–1939. [Google Scholar] [CrossRef]
- Cheng, X.; Xie, Q.; Sun, Y. Advances in nanomaterial-based targeted drug delivery systems. Front. Bioeng. Biotechnol. 2023, 11, 1177151. [Google Scholar] [CrossRef]
- Kaur, P.; Singh, S.K.; Mishra, M.K.; Singh, S.; Singh, R. Promising Combinatorial Therapeutic Strategies against Non-Small Cell Lung Cancer. Cancers 2024, 16, 2205. [Google Scholar] [CrossRef]
- Waller, V.; Pruschy, M. Combined Radiochemotherapy: Metalloproteinases Revisited. Front. Oncol. 2021, 11, 676583. [Google Scholar] [CrossRef]
- Ye, Y.Q.; Kuang, X.W.; Xie, Z.Z.; Liang, L.; Zhang, Z.; Zhang, Y.C.; Ma, F.Y.; Gao, Q.; Chang, R.M.; Lee, H.H.; et al. Small-molecule MMP2/MMP9 inhibitor SB-3CT modulates tumor immune surveillance by regulating PD-L1. Genome Med. 2020, 12, 83. [Google Scholar] [CrossRef]
- Al-Sammarraie, N.; Ray, S.K. Applications of CRISPR-Cas9 Technology to Genome Editing in Glioblastoma Multiforme. Cells 2021, 10, 2342. [Google Scholar] [CrossRef] [PubMed]
- Frolova, A.S.; Petushkova, A.I.; Makarov, V.A.; Soond, S.M.; Zamyatnin, A.A., Jr. Unravelling the Network of Nuclear Matrix Metalloproteinases for Targeted Drug Design. Biology 2020, 9, 480. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashja Ardalan, A.; Khalili-Tanha, G.; Shoari, A. Shaping the Landscape of Lung Cancer: The Role and Therapeutic Potential of Matrix Metalloproteinases. Int. J. Transl. Med. 2024, 4, 661-679. https://doi.org/10.3390/ijtm4040046
Ashja Ardalan A, Khalili-Tanha G, Shoari A. Shaping the Landscape of Lung Cancer: The Role and Therapeutic Potential of Matrix Metalloproteinases. International Journal of Translational Medicine. 2024; 4(4):661-679. https://doi.org/10.3390/ijtm4040046
Chicago/Turabian StyleAshja Ardalan, Arghavan, Ghazaleh Khalili-Tanha, and Alireza Shoari. 2024. "Shaping the Landscape of Lung Cancer: The Role and Therapeutic Potential of Matrix Metalloproteinases" International Journal of Translational Medicine 4, no. 4: 661-679. https://doi.org/10.3390/ijtm4040046
APA StyleAshja Ardalan, A., Khalili-Tanha, G., & Shoari, A. (2024). Shaping the Landscape of Lung Cancer: The Role and Therapeutic Potential of Matrix Metalloproteinases. International Journal of Translational Medicine, 4(4), 661-679. https://doi.org/10.3390/ijtm4040046






