Abstract
A novel means of applying radiotherapy in cancer treatment is the application of a radiation dose at a very high intensity for a very short time in FLASH radiotherapy (FLASH-RT). This technique involves the exposure of tumors to >40 Gy/s, usually for less than one second. Studies conducted in cell and preclinical models suggest that FLASH-RT seems less damaging to normal tissues from adverse effects relative to the same overall dose of radiation administered in conventional therapy (CONV-RT), which involves the administration of lower levels of radiation repeated intermittently over a protracted period. In contrast, the susceptibility of tumor tissues to FLASH-RT is not diminished relative to CONV-RT. Within solid tumors, both modes of dispensation of radiation produce an equivalent degree of cell damage. The differential treatment between normal and malignant material has been found in isolated tissues, animal studies and, more recently, in clinical trials. However, the classic radiation concept is that high-energy linear transfer radiation (LET) is more damaging than the equivalent total dose of low LET. Thus, the susceptibility of cells should be greater after short-term exposure to high LET. This article discusses the potential reasons that may account for this discrepancy. While the relative protection given to untransformed tissues by FLASH-RT relative to tumor tissue is a major step forward in radiation therapy for cancer, the processes that lie behind this phenomenon are incompletely understood and are considered here.
1. Introduction
Radiotherapy has long been a major source of treatment for cancer as a means of killing malignant cells. Over 50% of cancer patients currently receive such therapy [1]. Radiotherapy is generally used in conjunction with surgical procedures to minimize tumor burden, and with chemotherapeutic agents also, as a means of eradicating cancer cells. Many refinements in the design of this procedure have been made, always with the intent of minimizing harm to surrounding normal tissues while maintaining an intense destructive focus on tumor tissue. A recent means of addressing this problem is the development of FLASH-RT. Its basic concept is the use of a very short pulse of high radiation (around 40–100 Gy/s), roughly 400-fold more intense than the lower level, longer exposures of radiation used in classical radiotherapy. The origins of this procedure have their roots in a 1959 paper reporting that a short burst of high levels of radiation was less lethal to bacteria than the same dose administered over a longer time span [2]. The reduced toxicity caused by this ultra-high-dose short burst of radiation in comparison with a more extended lower dose rate has been replicated in mammalian cell culture and then in intact animals [3,4]. In several of these reports, the relative sparing effect of FLASH-RT is described as being lost at dose rates below around 5–10 Gy/s [5,6].
A second unexpected discovery was that there was a divergence between the susceptibility of tumor cells to FLASH-RT and that of untransformed normal cells. The reduced lethality of FLASH-RT to normal cells was not replicated in tumor tissue. This is in sharp contrast to conventional radiotherapy where the vulnerability of cells, while being sensitive to the rate of mitosis, does not otherwise differ between normal and malignant cells.
Damage caused by conventional radiation has been thought of as being largely due to reactive oxygen species. A possible limitation has been that the hypoxic state of most tumor tissues may make them less susceptible to oxidative injury than normal cells [7]. In 2019, the first successful clinical application of FLASH-RT led to the complete eradication of a multiresistant cutaneous lymphoma [8]. More recently, a range of studies on the effects of FLASH-RT have ensured that this strategy will have a great impact on the future of the radiotherapeutic treatment of solid tumors. Several groups have reported the utility of this treatment in animal patients by veterinarians [3,9,10].
2. Accounting for the Differences between FLASH-RT and CONV-RT
There are two major unexplained issues concerning the selective advantage of FLASH-RT over CONV-RT that have not yet been fully explained. These are interrelated and overlap in some measure, but are conveniently discussed as separate problems.
3. Why Is FLASH-RT Less Lethal to Normal Cells than the Same Dose of CONV-RT?
A given dose of radiation applied at a higher dose rate should be more toxic than the equivalent dosage applied for a longer period of time. This is an orthodox and well-established view in radiation biology and has led to the foundation of radiotherapy generally involving relatively low doses of radiation applied at intervals. Exposure to extreme levels of gamma radiation over millisecond periods certainly seems especially lethal, as judged by the effects of the atomic bombs on Hiroshima and Nagasaki. In fact, FLASH-RT has been described as a good model to simulate short-duration high-intensity radiation following the detonating of a nuclear weapon [11].
3.1. Anoxia
There are now many reports of FLASH-RT being less damaging to normal cells than the equivalent dose of CONV-RT [12]. The mechanism underlying this is unclear but the dramatically sudden reduced oxygen tension resulting from FLASH-RT has been proposed to play a major role in its overall effect. It has long been known that radiation can lead to an anoxia-like state of the target tissues [13], and that this becomes especially pronounced after high-intensity radiation [14]. It has been posited that the rapid consumption of oxygen caused by FLASH-RT leads to transient hypoxia, and this reduces the extent of the production of harmful reactive oxygen species caused by the radiation relative to CONV-RT. Probably related to this is the fact that FLASH-RT does not induce neuroinflammation in the brain [15]. This is mediated by the lesser cell damage and the lesser induction of inflammatory cytokines provoked by FLASH-RT [16]. Also, in the context of cerebral tissue, FLASH-RT has been reported as permitting the maintenance of synaptic plasticity [17] and sparing of indices of behavioral and cognitive functioning [15]. Further evidence of the key role of sudden and intense anoxia in FLASH-RT is the finding that the induction of cerebral hyperoxia by means of carbogen inhalation prevented the neuroprotective effects of FLASH-RT [15]. A clinical implication of this is that the higher levels of oxygen used during anesthesia can negate the protective consequences of FLASH-RT [18].
3.2. The Role of Reactive Oxygen Species
The sparing effect of FLASH-RT has a biphasic distribution with respect to oxygen concentration. It is maximal at around 4% pO2 and falls off at oxygen concentrations above or below this [19]. A very low presence of oxygen in tissues can be insufficient to allow the formation of reactive oxygen species under either type of application of radiation [20].
As illustrated by oxidative injury following ischemia reperfusion, a rapid re-oxygenation of anoxic cells can lead to the formation of reactive oxygen species. Such an oxygen rebound event has been shown to occur after the sudden anoxia incurred after FLASH-RT [21], and can be a source of the formation of active oxidants existing momentarily. It may be that the different responses to FLASH and CONV radiation are related to the very transient high levels of free radicals caused by FLASH, resulting in rapid detoxifying radical–radical interactions between peroxyl free radicals. This would lead to a reduction in the overall presence of free radicals and could limit damage, including that incurred by the migration of oxidant species to other sites [22]. This idea is supported by physicochemical modeling of reaction kinetics, suggesting that peroxyl radical recombination is a major factor in enabling FLASH-RT protection [23]. As distances between charged particle trajectories are much closer with FLASH, detoxifying interactions between formed reactive oxidizing species such as peroxy radicals become more likely [24,25]. The fraction of damage due to secondary events is then likely to be reduced in FLASH-RT.
In contrast, CONV-RT may lead to a more prolonged presence of harmful short-lived reactive oxygen. The relatively diluted form of free radicals and their precursors generated in this manner can prolong their existence and lead to the diffusion of oxidizing species.
3.3. Damage to DNA
DNA can be harmed by radiation causing both double-stranded or single-stranded breaks in the DNA double helix. This can occur either by radiation energy directly impacting DNA, or by DNA being indirectly attacked by highly reactive and short-lived species formed by reactive electrons in proximity to the DNA. In aqueous media, low-energy radiation contributes more to indirect DNA damage than high-energy X-rays do [26]. At very high doses and dose rates of radiation, where oxygen depletion in the cellular environment plays a major role, there may be a consequent decline in DNA damage due to a lesser ability to form reactive oxygen species [27]. This may be due to a saturation effect caused by the close proximity of the penetrant beams in FLASH [28].
In isolated plasmids, almost all DNA damage seems indirect since no potential scavenging molecules are present. In such defined isolated cell-free media, FLASH reduced the extent of single-stranded breaks (but not double-stranded breaks) in plasmid DNA in comparison to CONV [29,30]. The suspension of plasmids in a protein-free medium containing only Tris buffer may account for the attenuation of the differences between conventional and FLASH radiation.
Non-lethal radiation damage to DNA can lower overall cellular effectiveness and lead to the emergence of cells of a senescence-associated secretory phenotype (SASP). These cells secrete inflammatory cytokines which can also further spread inflammatory events in nearby cells [31].
3.4. Inflammation
A major component of the lessening of the overall radiation damage caused by FLASH may be attributable to reduced inflammation. FLASH-RT is far less toxic to circulating immune cells than CONV-RT [6]. The evidence of FLASH-RT causing less inflammation seems to coincide with the elevated alertness of T-cells, which can inhibit tumor progression [32], and is likely due to a lower presence of reactive oxygen. In many instances, the inhibition of non-productive and unfocused inflammation has been observed to bring about improved immune function [33].
Inflammation represents a response to altered oxidant conditions and to modifications of signaling pathways, and thus generally reflects a secondary consequence of metabolic change. In the case of FLASH, the shortened persistence of ROS production will also lead to a reduction in the pathways of activation of the inflammatory response. This may account for the reduced inflammation and lower DNA damage found following FLASH [28].
There is evidence that the differential effect of FLASH involves its ability to maintain the integrity of mitochondrial function such as mitochondrial membrane potential. In contrast, standard radiation protocols lead to severe adverse morphological and functional changes in mitochondria [34]. The mechanism underlying this relative shielding effect of FLASH involves sustaining the phosphorylated form of Dynamin-related protein 1 (Drp1). Drp1 is dephosphorylated after CONV-RT, resulting in the inactivation of its protective signaling pathway. Significantly, in a parallel study, cell death following CONV-RT was largely by way of necrosis, which led to inflammation, while FLASH-RT led to apoptosis [35]. Since necrosis leads to inflammatory events while apoptosis is a more organized means of dismantling cells, this difference could account for the lesser inflammation found with the FLASH procedure. The ability of FLASH-RT to induce regulated immune reactions rather than provoking uncontrolled inflammation has long term advantages. Thus, the treatment of the lung tissue of rodents with spatially focused microbeam irradiation at FLASH levels does not lead to radiation fibrosis even after one year [36].
FLASH-RT reduced the number of lung-derived fibroblasts that were converted into cells expressing indices of senescence relative to CONV. This is likely to underlie the prevention of radiation-generated fibrosis in the lungs by FLASH-RT [37]. Radiation-induced senescence can also be transferred to cells at remote sites, perhaps by a bystander mechanism involving the circulation of exosomes [38]. Since the damage to DNA affected by FLASH treatment is less pronounced, this can reduce the formation of SASP-type cells [39]. These findings suggest that FLASH-RT is less potent in facilitating the premature aging events that have been ascribed to radiotherapy.
4. Why Are Tumor Cells More Sensitive than Normal Cells to FLASH-RT?
Unlike normal tissues, tumor cells appear to be just as sensitive to FLASH-RT as to CONV-RT. This creates a beneficial disparity between the responses of the two cell types. Since higher dose rates of radiation are generally described as producing greater damage to cells, FLASH-RT should cause a better tumor reaction than CONV-RT, and this is indeed the case. The reasons underlying this divergence remain unresolved, but several separate hypotheses are substantiated by credible evidence.
4.1. Tumor Metabolism Is Unlike That of Normal Tissue
The excess sensitivity of tumor cells to FLASH-RT has some enigmatic aspects, as tumor tissues are often hypoxic and can live under conditions under which normal cells could not survive [40], yet the FLASH effect seems to involve transient but intense anoxia. Most solid tumors have a preferential utilization of anaerobic glycolysis as opposed to the emphasis on aerobic metabolism characteristic of normal cells. The less-efficient energy metabolism of tumor cells is compensated for by enhanced glucose consumption [41,42]. This lack of dependence on high levels of oxygen accounts for the frequently encountered resistance to CONV-RT. In fact, tumor growth is attenuated by hyperoxia. However, tumor cells have a greater intrinsic pro-oxidant environment than normal cells and are more consistently subject to oxidative stress [43]. They are therefore more susceptible to free-radical-induced death than normal cells [44]. While hypoxia induces resistance in normal cells to FLASH-RT [45], tumor cells remain selectively vulnerable to FLASH-RT even under low oxygen tension. The solution to this paradox of the selective susceptibility of tumor cells to FLASH-RT may lie in altered signaling pathways leading to disruption of the cell cycle. It is likely that the metabolism of normal cells may bring about a different response to FLASH-RT independently of oxygen concentration [27].
4.2. Differing Production and Disposition of Reactive Oxygen Species
Since hyperoxia can eliminate the differential response of normal and tumor cells to FLASH-RT in mice [15], oxygen tension is undoubtedly involved in the varying reactions of normal and transformed cells to FLASH-RT. However, other factors are obviously involved, and these remain largely unresolved despite several persuasive suggestions.
The short but powerful hypoxia encountered with FLASH is unlike the continuous hypoxia found with many solid tumors. High dose rates of irradiation elevate the rare local energy deposition leading to the severe depletion of intracellular pO2. The consequences of this may be more critical under the already relatively anoxic conditions characterizing tumor tissue. Thus, already-hypoxic tumor tissues where hypoxia is previously established may be unable to withstand further FLASH-induced hypoxia [46]. Normal cells have a lower oxidant load and a higher catalase content than tumor tissue, and thus can detoxify hydrogen peroxide more rapidly, and this ability leads to a lower concentration of intensely oxidant species which persist much longer in transformed cells [47]. The differing response of normal and malignant tissue to FLASH may involve a combination of reactions to the initial depletion of oxygen and subsequent relative persistence of harmful reactive oxygen species in the tumor tissue [47]. A physicochemical modeling of FLASH-RT also suggests that the relative anoxia of tumor tissue can increase the extent of their exposure to reactive oxygen radicals [48].
Another factor that distinguishes tumors from normal tissues is that iron content in the form of low-molecular-weight complexes is higher in tumor tissues. This redox labile iron catalyzes key Fenton transformations, which instigates a greater formation of reactive oxygen species in tumors. The greater ability of normal tissue, relative to tumor tissues, to sequester labile iron can permit a more rapid clearance of the hydroperoxides produced by FLASH-RT. This will then result in a reduction in the consequent cascade of events furthering lipid peroxidation, accounting for the relative protection conferred by FLASH-RT [49].
There is undoubtedly a range of intrinsic differences between the metabolic events triggered by ROS in normal tissues and tumor tissues, and this initiated differing responses to antioxidants administered after excess oxidant activity. While tumor cells are often harmed by high doses of antioxidants, normal cells remain unaffected [50]. Taking advantage of this difference has been proposed as a means of enhancing the divergence of responses to FLASH-RT relative to CONV-RT [19,51].
4.3. Reduction in Bystander Effects by FLASH
Normal tissues that are near tumor sites are frequently damaged by radiation procedures. These “bystander” effects are not confined to tissues adjacent to the tumor undergoing therapy, but can also manifest at sites that are more distant. For example, radiation therapy for prostate cancer increases the risk of a range of tumors in other tissues [52]. This problem is particularly relevant in the treatment of pediatric cancer where survivors can have a long life expectancy [53]. Excess micronuclei were induced when isolated cells were exposed to serum from survivors of Chernobyl 20 years after their exposure to high levels of radiation. The overall viability of such cells was reduced relative to those exposed to control serum from an unexposed population [54]. This illustrates the persistence of changes incurred after exposure to intense radiation. The mechanisms underlying these indirect consequences are complex, but likely involve lasting genetic changes, the release of inflammatory cytokines such as IL-6 and TNF-alpha from the target irradiated cells and the upregulation of oxidative metabolism. Some of the relatively low impact of FLASH on the induction of inflammation appears to have a genomic basis relating to the differential expressions of pro- and anti-inflammatory genes [16]. Since bystander signals can remain active in tissues for extended periods of time, other genetic and epigenetic factors are also probably involved [55]. The genetic regulatory pathways which may contribute to the protective effect of FLASH-RT are not yet well defined [56]. The very short time interval employed in FLASH-RT will reduce the fraction of cells in the circulation that are exposed to radiation. It has been estimated that 100 times more blood volume is subjected to irradiation by CONV-RT than by FLASH-RT, and this large difference could account for a significant proportion of the reduced damage effected by FLASH-RT [57]. This difference may help to maintain immune surveillance in an effective state following FLASH rather than having it descend into non-targeted inflammation.
4.4. Immune Responses
The extent of T-cell incursion in tumors was increased by FLASH-RT, suggesting a more effective immune attack upon transformed cells [57]. The activation of myosin light chain proteins (MLPs) promotes the invasiveness of tumors [58,59] and is also involved in the activation of double-stranded DNA breaks [58]. CONV-RT, but not FLASH-RT, activates MLP. The pharmacological inhibition of MLP activation enables CONV-RT to mimic some of the advantages of FLASH-RT, including the promotion of the penetrance of active immune cells into tumor tissue and the inhibition of the repair of double stranded DNA [58]. This reveals DNA repair and effective immune responses to comprise a significant component of the distinctive efficacy of FLASH-RT. In addition, the damage to DNA incurred by FLASH-RT is lower than that caused by a comparable dose administered at the conventional rate. This protective effect applies to both as far as single- and double-stranded DNA breaks are concerned [22,60].
The dissimilar response of the immune system to FLASH-RT in comparison to CONV-RT may also account for the selective susceptibility of tumor cells. The improved recruitment of lymphocytes found in FLASH-RT may markedly favor the destruction of tumor cells [61].
5. Conclusions, Problems and Future Directions
Overall, FLASH-RT has a multitude of protective effects on biological tissues that differ from CONV-RT, including limiting inflammatory cell infiltration, decreasing the generation of inflammatory factors and decreasing the extent of radiation-induced fibrosis. The desirable protective effects of FLASH have now been reported in a comprehensive variety of organ systems [62]. Stem cell function is preserved and beneficial immune responses tolerated. In the brain, synaptic connectivity is and measures of cerebral function are maintained.
Both of the two questions posited above remain incompletely answered, and challenge orthodox thinking in this area. Despite the fact that the mechanisms underlying the difference between FLASH-RT and conventional, more prolonged radiotherapy are not fully accounted for, the tactic of delivering radiation to cancer patients at a high dose rate for a short time should have a major effect on improving the line of attack of cancer treatment.
Since there is no distinction between the susceptibility of normal and tumor tissues to conventional radiation, application of CONV-RT relies on directing the radiation beam to a very focused target. The efficacy of this treatment relies solely on precisely distinguishing between the location of healthy and tumor tissues. In the case of the brain, stereotactic surgery, involving bringing many sources of radiation to a focal point, the “gamma knife”, has been employed. However, since brain tumors, while often not metastatic, are very comingled with brain tissue, the 3-month survival rate following this procedure remains under 10 months [63]. In contrast, FLASH-RT does not rely solely on the spatial separation of normal and abnormal tissue regions but takes advantage of their differential metabolic characteristics, with tumor tissues generally relying more on glycolysis [42].
Any explanation of the mechanism of action leading to the advantageous properties of FLASH-RT must consider the remarkable fact that they have been described using several very different means of irradiation, including electrons, protons and X-rays [64]. While many suggestions have been made to account for the FLASH phenomenon, these need not be mutually exclusive but rather may reinforce each other. Figure 1 represents a possible integration of data that is inclusive and incorporates various hypotheses.
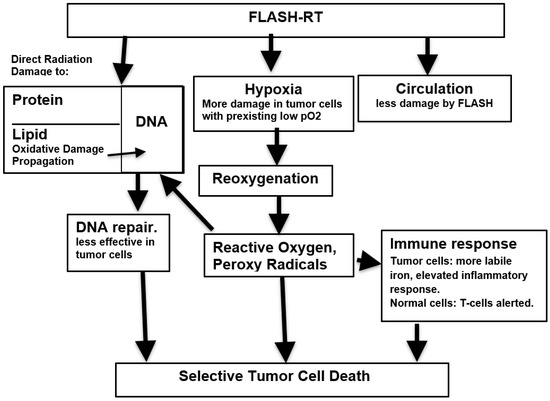
Figure 1.
Putative mechanisms underlying susceptibility of tumor cells to FLASH radiation.
Research in this area is at a relatively early stage and several contradictory results have been reported. For example, FLASH has been found to have a sparing effect on some tumor cell lines under normoxic conditions [19]. There are especially conflicting data surrounding the effects of FLASH-RT on lymphocytes. In animal models of radiation-induced cardiac and splenic lymphopenia, high-dose short-duration exposure irradiation actually worsens the resulting injury when compared with lower-dose more prolonged exposure [65]. A similar absence of a sparing effect has been reported by others [66]. However, other reports describe a sparing effect of FLASH-RT on lymphocytes in both experimental animals and in humans [67,68]. The normal oxygen pO2 within tissues is in the range of 3–7%, considerably below that of ambient atmospheric pO2 levels of 18% [69]. Hyperoxia is known to block the advantageous effects of FLASH. Since many of the studies in isolated systems have been performed under normal concentrations of atmospheric oxygen, they are subject to the limitation that they are not replicating the more hypoxic conditions existing within intact tissues [70]. This could block the ability to observe the distinctive FLASH differential behavior distinguishing it from CONV, and may account for some of the conflicting data reported from isolated systems. Replication of the exact physiological conditions prevailing in vivo is virtually impossible to achieve. Nevertheless, while it is more challenging to unravel mechanistic pathways in intact animals, perhaps this is the only means to fully establish the exact sequence of events that lie behind the FLASH phenomenon. A report using an in vivo preparation found that a single FLASH dose did not cause profound anoxia in intact muscle tissue. As a result, it was proposed that the difference in cellular damage found between FLASH-RT and CONV-RT, rather than being due to acute anoxia, is caused by the difference in the rate of tissue oxygen consumption between the two conditions. This can be higher in the case of CONV-RT [21].
Delayed undesirable effects, such as osteonecrosis following treatment of canine oral cancer, have been reported [10]. The treatment of human tumors requires an improved and more detailed understanding of the optimal parameters for many factors such as radiation spectrum, image guidance and underlying mechanisms. Unanswered considerations include the question of whether a high energy burst using a proton beam may be as effective as an electron beam [71]. The optimal variables for establishing these variables remain to be delineated before this treatment can be clinically applied on a broad scale. Protocols are currently being developed in more detail in order to allow the safe testing and application of this procedure in humans [72].
It has been found that in an animal model of glioblastoma, while FLASH-RT produced fewer unwanted side effects than exposure to a standard radiation pattern, the overall survival rate was nevertheless unaffected [73]. If such results were more widely confirmed, the value of FLASH-RT would be more limited than is hoped. However, findings using experimental animals have generally found more lasting positive effects. It must be borne in mind that the FLASH approach is not necessarily devoid of potential harm to normal tissues if the wrong radiation protocols are chosen.
A further understanding of the events that lead to the distinctive properties of FLASH-RT will allow the refinement of this strategy and enhancement of its efficiency. Many parameters remain to be studied in more detail. A variant of FLASH therapy whereby treatment is divided into two consecutive exposures may have particular value in the protection of the developing juvenile brain, which is particularly sensitive to radiation-induced damage [74]. The relative insensitivity of normal tissue to FLASH suggests that higher overall doses than those currently used might be employed, thus effecting great damage to tumor tissue. Surprisingly, the shielding nature of the FLASH effect has been observed after the exposure of biological tissues to a wide range of particles, including electrons, X-rays and protons [16]. The utilization of proton beams has the advantage over the more widely reported electron beam therapy in that protons have the ability to penetrate deeper into tissues [75,76]. It is important that reports on this subject clearly define the exact physical nature of the beam used, including dose rate and beam width. The importance of the precise description of the parameters used is paramount in this emerging and fluid field. In addition to pinpointing the most suitable conditions for application of the procedure, the relative suitability of various types of radiation remain to be unambiguously defined. However, even in the absence of a more comprehensive expansion of knowledge concerning FLASH, this novel technique is already proving to be of growing usefulness in clinical radiotherapy.
Funding
This research received no external funding.
Acknowledgments
The author would like to thank MeiXia Wu for great help with assembling the structure of this report and for carefully checking the final version of the manuscript.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Begg, A.C.; Stewart, F.A.; Vens, C. Strategies to improve radiotherapy with targeted drugs. Nat. Rev. Cancer 2011, 11, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Dewey, D.L.; Boag, J.W. Modification of the oxygen effect when bacteria are given large pulses of radiation. Nature 1959, 183, 1450–1451. [Google Scholar] [CrossRef] [PubMed]
- Vozenin, M.C.; De Fornel, P.; Petersson, K.; Favaudon, V.; Jaccard, M.; Germond, J.F.; Petit, B.; Burki, M.; Ferrand, G.; Patin, D.; et al. The Advantage of FLASH Radiotherapy Confirmed in Mini-pig and Cat-cancer Patients. Clin. Cancer Res. 2019, 25, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Gao, F.; Yang, Y.; Wu, D.; Zhang, Y.; Feng, G.; Dai, T.; Du, X. FLASH Radiotherapy: History and Future. Front. Oncol. 2021, 11, 644400. [Google Scholar] [CrossRef]
- Jin, J.Y.; Gu, A.; Wang, W.; Oleinick, N.L.; Machtay, M.; Spring Kong, F.M. Ultra-high dose rate effect on circulating immune cells: A potential mechanism for FLASH effect? Radiother. Oncol. 2020, 149, 55–62. [Google Scholar] [CrossRef]
- Favaudon, V.; Labarbe, R.; Limoli, C.L. Model studies of the role of oxygen in the FLASH effect. Med. Phys. 2022, 49, 2068–2081. [Google Scholar] [CrossRef]
- Hubenak, J.R.; Zhang, Q.; Branch, C.D.; Kronowitz, S.J. Mechanisms of injury to normal tissue after radiotherapy: A review. Plast. Reconstr. Surg. 2014, 133, 49e–56e. [Google Scholar] [CrossRef]
- Bourhis, J.; Sozzi, W.J.; Jorge, P.G.; Gaide, O.; Bailat, C.; Duclos, F.; Patin, D.; Ozsahin, M.; Bochud, F.; Germond, J.F.; et al. Treatment of a first patient with FLASH-radiotherapy. Radiother. Oncol. 2019, 139, 18–22. [Google Scholar] [CrossRef]
- Konradsson, E.; Szecsenyi, R.E.; Adrian, G.; Coskun, M.; Børresen, B.; Arendt, M.L.; Erhart, K.; Bäck, S.Å.; Petersson, K.; Ceberg, C. Evaluation of intensity-modulated electron FLASH radiotherapy in a clinical setting using veterinary cases. Med. Phys. 2023, 50, 6569. [Google Scholar] [CrossRef]
- Børresen, B.; Arendt, M.L.; Konradsson, E.; Bastholm Jensen, K.; Bäck, S.Å.; Munck, A.f.; Rosenschöld, P.; Ceberg, C.; Petersson, K. Evaluation of single-fraction high dose FLASH radiotherapy in a cohort of canine oral cancer patients. Front. Oncol. 2023, 13, 256760. [Google Scholar] [CrossRef]
- Swarts, S.G.; Flood, A.B.; Swartz, H.M. Implications of “flash” radiotherapy for biodosimetry. Radiat. Prot. Dosim. 2023, 199, 1450–1459. [Google Scholar] [CrossRef]
- Bourhis, J.; Montay-Gruel, P.; Gonçalves Jorge, P.; Bailat, C.; Petit, B.; Ollivier, J.; Jeanneret-Sozzi, W.; Ozsahin, M.; Bochud, F.; Moeckli, R.; et al. Clinical translation of FLASH radiotherapy: Why and how? Radiother. Oncol. 2019, 139, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Hendry, J.H.; Moore, J.V.; Hodgson, B.W.; Keene, J.P. The constant low oxygen concentration in all the target cells for mouse tail radionecrosis. Radiat. Res. 1982, 92, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Hall, E.J.; Brenner, D.J. The dose-rate effect revisited: Radiobiological considerations of importance in radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 1991, 21, 1403–1414. [Google Scholar] [CrossRef] [PubMed]
- Montay-Gruel, P.; Acharya, M.M.; Petersson, K.; Alikhani, L.; Yakkala, C.; Allen, B.D.; Ollivier, J.; Petit, B.; Jorge, P.G.; Syage, A.R.; et al. Long-term neurocognitive benefits of FLASH radiotherapy driven by reduced reactive oxygen species. Proc. Natl. Acad. Sci. USA 2019, 116, 10943–10951. [Google Scholar] [CrossRef] [PubMed]
- Friedl, A.A.; Prise, K.M.; Butterworth, K.T.; Montay-Gruel, P.; Favaudon, V. Radiobiology of the FLASH effect. Med. Phys. 2022, 49, 1993–2013. [Google Scholar] [CrossRef]
- Limoli, C.L.; Kramár, E.A.; Almeida, A.; Petit, B.; Grilj, V.; Baulch, J.E.; Ballesteros-Zebadua, P.; Loo, B.W.J.; Wood, M.A.; Vozenin, M.C. The sparing effect of FLASH-RT on synaptic plasticity is maintained in mice with standard fractionation. Radiother. Oncol. 2023, 186, 109767. [Google Scholar] [CrossRef]
- Tavakkoli, A.D.; Clark, M.A.; Kheirollah, A.; Sloop, A.M.; Soderholm, H.E.; Daniel, N.J.; Petusseau, A.F.; Huang, Y.H.; Thomas, C.R.J.; Jarvis, L.A.; et al. Anesthetic Oxygen Use and Sex Are Critical Factors in the FLASH Sparing Effect. Adv. Radiat. Oncol. 2024, 9, 101492. [Google Scholar] [CrossRef]
- Adrian, G.; Konradsson, E.; Lempart, M.; Bäck, S.; Ceberg, C.; Petersson, K. The FLASH effect depends on oxygen concentration. Br. J. Radiol. 2020, 93, 20190702. [Google Scholar] [CrossRef]
- Moon, E.J.; Petersson, K.; Olcina, M.M. The importance of hypoxia in radiotherapy for the immune response, metastatic potential and FLASH-RT. Int. J. Radiat. Biol. 2022, 98, 439–451. [Google Scholar] [CrossRef]
- El Khatib, M.; Van Slyke, A.L.; Velalopoulou, A.; Kim, M.M.; Shoniyozov, K.; Allu, S.R.; Diffenderfer, E.E.; Busch, T.M.; Wiersma, R.D.; Koch, C.J.; et al. Ultrafast Tracking of Oxygen Dynamics During Proton FLASH. Int. J. Radiat. Oncol. Biol. Phys. 2022, 113, 624–634. [Google Scholar] [CrossRef] [PubMed]
- Labarbe, R.; Hotoiu, L.; Barbier, J.; Favaudon, V. A physicochemical model of reaction kinetics supports peroxyl radical recombination as the main determinant of the FLASH effect. Radiother. Oncol. 2020, 153, 303–310. [Google Scholar] [CrossRef]
- Hu, A.; Qiu, R.; Li, W.B.; Zhou, W.; Wu, Z.; Zhang, H.; Li, J. Radical recombination and antioxidants: A hypothesis on the FLASH effect mechanism. Int. J. Radiat. Biol. 2023, 9, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Méndez, J.; Dom.ínguez-Kondo, N.; Schuemann, J.; McNamara, A.; Moreno-Barbosa, E.; Faddegon, B. LET-Dependent Intertrack Yields in Proton Irradiation at Ultra-High Dose Rates Relevant for FLASH Therapy. Radiat. Res. 2020, 194, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Perstin, A.; Poirier, Y.; Sawant, A.; Tambasco, M. Quantifying the DNA-damaging Effects of FLASH Irradiation With Plasmid DNA. Int. J. Radiat. Oncol. Biol. Phys. 2022, 113, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, E.; Sanz, A.G.; García, G.; Sanche, L. Radiation Damage to DNA: The Indirect Effect of Low Energy Electrons. J. Phys. Chem. Lett. 2013, 4, 820–825. [Google Scholar] [CrossRef]
- Garty, G.; Obaid, R.; Deoli, N.; Royba, E.; Tan, Y.; Harken, A.D.; Brenner, D.J. Ultra-high dose rate FLASH irradiator at the radiological research accelerator facility. Sci. Rep. 2022, 12, 22149. [Google Scholar] [CrossRef]
- Schüler, E.; Acharya, M.; Montay-Gruel, P.; Loo, B.W.J.; Vozenin, M.C.; Maxim, P.G. Ultra-high dose rate electron beams and the FLASH effect: From preclinical evidence to a new radiotherapy paradigm. Med. Phys. 2022, 49, 2082–2095. [Google Scholar] [CrossRef]
- Small, K.L.; Henthorn, N.T.; Angal-Kalinin, D.; Chadwick, A.L.; Santina, E.; Aitkenhead, A.; Kirkby, K.J.; Smith, R.J.; Surman, M.; Jones, J.; et al. Evaluating very high energy electron RBE from nanodosimetric pBR322 plasmid DNA damage. Sci. Rep. 2021, 11, 3341. [Google Scholar] [CrossRef]
- Ohsawa, D.; Hiroyama, Y.; Kobayashi, A.; Kusumoto, T.; Kitamura, H.; Hojo, S.; Kodaira, S.; Konishi, T. DNA strand break induction of aqueous plasmid DNA exposed to 30 MeV protons at ultra-high dose rate. J. Radiat. Res. 2022, 63, 255–260. [Google Scholar] [CrossRef]
- Kumar, K.; Kumar, S.; Datta, K.; Fornace, A.J.J.; Suman, S. High-LET-Radiation-Induced Persistent DNA Damage Response Signaling and Gastrointestinal Cancer Development. Curr. Oncol. 2023, 30, 5497–5514. [Google Scholar] [CrossRef] [PubMed]
- Iturri, L.; Bertho, A.; Lamirault, C.; Juchaux, M.; Gilbert, C.; Espenon, J.; Sebrie, C.; Jourdain, L.; Pouzoulet, F.; Verrelle, P.; et al. Proton FLASH Radiation Therapy and Immune Infiltration: Evaluation in an Orthotopic Glioma Rat Model. Int. J. Radiat. Oncol. Biol. Phys. 2023, 116, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Bondy, S.C. Mitochondrial Dysfunction as the Major Basis of Brain Aging. Biomolecules 2024, 14, 402. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Li, N.; Wang, Y.; Wang, Y.; Zhang, X.; Zhang, H. Effects of X-irradiation on mitochondrial DNA damage and its supercoiling formation change. Mitochondrion 2011, 11, 886–892. [Google Scholar] [CrossRef]
- Guo, Z.; Buonanno, M.; Harken, A.; Zhou, G.; Hei, T.K. Mitochondrial Damage Response and Fate of Normal Cells Exposed to FLASH Irradiation with Protons. Radiat. Res. 2022, 197, 569–582. [Google Scholar] [CrossRef]
- Trappetti, V.; Fazzari, J.; Fernandez-Palomo, C.; Smyth, L.; Potez, M.; Shintani, N.; de Breuyn Dietler, B.; Martin, O.A.; Djonov, V. Targeted Accumulation of Macrophages Induced by Microbeam Irradiation in a Tissue-Dependent Manner. Biomedicines 2022, 10, 735. [Google Scholar] [CrossRef]
- Buonanno, M.; Grilj, V.; Brenner, D.J. Biological effects in normal cells exposed to FLASH dose rate protons. Radiother. Oncol. 2019, 139, 51–55. [Google Scholar] [CrossRef]
- Elbakrawy, E.; Kaur Bains, S.; Bright, S.; Al-Abedi, R.; Mayah, A.; Goodwin, E.; Kadhim, M. Radiation-Induced Senescence Bystander Effect: The Role of Exosomes. Biology 2020, 9, 191. [Google Scholar] [CrossRef]
- Fouillade, C.; Curras-Alonso, S.; Giuranno, L.; Quelennec, E.; Heinrich, S.; Bonnet-Boissinot, S.; Beddok, A.; Leboucher, S.; Karakurt, H.U.; Bohec, M.; et al. FLASH Irradiation Spares Lung Progenitor Cells and Limits the Incidence of Radio-induced Senescence. Clin. Cancer Res. 2020, 26, 1497–1506. [Google Scholar] [CrossRef]
- Giaccia, A.J. Hypoxic Stress Proteins: Survival of the Fittest. Semin. Radiat. Oncol. 1996, 6, 46–58. [Google Scholar] [CrossRef]
- Adekola, K.; Rosen, S.T.; Shanmugam, M. Glucose transporters in cancer metabolism. Curr. Opin. Oncol. 2012, 24, 650–654. [Google Scholar] [CrossRef] [PubMed]
- Ganapathy-Kanniappan, S.; Geschwind, J.F. Tumor glycolysis as a target for cancer therapy: Progress and prospects. Mol. Cancer 2013, 12, 152. [Google Scholar] [CrossRef] [PubMed]
- Aboelella, N.S.; Brandle, C.; Kim, T.; Ding, Z.C.; Zhou, G. Oxidative Stress in the Tumor Microenvironment and Its Relevance to Cancer Immunotherapy. Cancers 2021, 13, 986. [Google Scholar] [CrossRef] [PubMed]
- Alanazi, A.; Jay-Gerin, J.P.; Blázquez-Castro, A. Is singlet oxygen involved in FLASH-RT? J. Appl. Clin. Med. Phys. 2023, 24, e13974. [Google Scholar] [CrossRef]
- Leavitt, R.J.; Almeida, A.; Grilj, V.; Montay-Gruel, P.; Godfroid, C.; Petit, B.; Bailat, C.; Limoli, C.L.; Vozenin, M.C. Acute Hypoxia Does Not Alter Tumor Sensitivity to FLASH Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2024, 119, 1493–1505. [Google Scholar] [CrossRef]
- Abolfath, R.; Grosshans, D.; Mohan, R. Oxygen depletion in FLASH ultra-high-dose-rate radiotherapy: A molecular dynamics simulation. Med. Phys. 2020, 47, 6551–6561. [Google Scholar] [CrossRef]
- Ma, J.; Gao, H.; Shen, X.; Bai, X.; Tang, M. A FLASH model of radiolytic oxygen depletion and reactive oxygen species for differential tumor and normal-tissue response. medRxiv 2023, medRxiv:10.20.23297337. [Google Scholar] [CrossRef]
- Spitz, D.R.; Buettner, G.R.; Petronek, M.S.; St-Aubin, J.J.; Flynn, R.T.; Waldron, T.J.; Limoli, C.L. An integrated physico-chemical approach for explaining the differential impact of FLASH versus conventional dose rate irradiation on cancer and normal tissue responses. Radiother. Oncol. 2019, 139, 23–27. [Google Scholar] [CrossRef]
- Tan, H.S.; Teo, K.B.K.; Dong, L.; Friberg, A.; Koumenis, C.; Diffenderfer, E.; Zou, J.W. Modeling ultra-high dose rate electron and proton FLASH effect with the physicochemical approach. Phys. Med. Biol. 2023, 68, 145013. [Google Scholar] [CrossRef]
- Prasad, K.N.; Sinha, P.K.; Ramanujam, M.; Sakamoto, A. Sodium ascorbate potentiates the growth inhibitory effect of certain agents on neuroblastoma cells in culture. Proc. Natl. Acad. Sci. USA 1979, 76, 829–832. [Google Scholar] [CrossRef]
- Prasad, K.N.; Bondy, S.C. FLASH Radiation vs. Conventional Radiation: Basis of Varying Effects and Potential Improvement of Their Differential Effects on Normal and Malignant Cells. J. Adv. Med. Sci. 2024; in press. [Google Scholar]
- Wallis, C.J.; Mahar, A.L.; Choo, R.; Herschorn, S.; Kodama, R.T.; Shah, P.S.; Danjoux, C.; Narod, S.A.; Nam, R.K. Second malignancies after radiotherapy for prostate cancer: Systematic review and meta-analysis. BMJ 2016, 352, i851. [Google Scholar] [CrossRef]
- Casey, D.L.; Vogelius, I.R.; Brodin, N.P.; Roberts, K.B.; Avanzo, M.; Moni, J.; Owens, C.; Ronckers, C.M.; Constine, L.S.; Bentzen, S.M.; et al. Risk of Subsequent Neoplasms in Childhood Cancer Survivors After Radiation Therapy: A PENTEC Comprehensive Review. Int. J. Radiat. Oncol. Biol. Phys. 2024, 119, 640–654. [Google Scholar] [CrossRef] [PubMed]
- Marozik, P.; Mothersill, C.; Seymour, C.B.; Mosse, I.; Melnov, S. Bystander effects induced by serum from survivors of the Chernobyl accident. Exp. Hematol. 2007, 35, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Cai, L.; He, X.; Niu, Z.; Huang, H.; Hu, W.; Bian, H.; Huang, H. Radiation-induced bystander effect and its clinical implications. Front. Oncol. 2023, 13, 1124412. [Google Scholar] [CrossRef] [PubMed]
- Rudigkeit, S.; Schmid, T.E.; Dombrowsky, A.C.; Stolz, J.; Bartzsch, S.; Chen, C.B.; Matejka, N.; Sammer, M.; Bergmaier, A.; Dollinger, G.; et al. Proton-FLASH: Effects of ultra-high dose rate irradiation on an in-vivo mouse ear model. Sci. Rep. 2024, 14, 1418. [Google Scholar] [CrossRef]
- Chow, J.C.L.; Ruda, H.E. Mechanisms of Action in FLASH Radiotherapy: A Comprehensive Review of Physicochemical and Biological Processes on Cancerous and Normal Cells. Cells 2024, 13, 835. [Google Scholar] [CrossRef]
- Kim, Y.E.; Gwak, S.H.; Hong, B.J.; Oh, J.M.; Choi, H.S.; Kim, M.S.; Oh, D.; Lartey, F.M.; Rafat, M.; Schüler, E.; et al. Effects of Ultra-high dose rate FLASH Irradiation on the Tumor Microenvironment in Lewis Lung Carcinoma: Role of Myosin Light Chain. Int. J. Radiat. Oncol. Biol. Phys. 2021, 109, 1440–1453. [Google Scholar] [CrossRef] [PubMed]
- Foroumadi, R.; Rashedi, S.; Asgarian, S.; Mardani, M.; Keykhaei, M.; Farrokhpour, H.; Javanshir, S.; Sarallah, R.; Rezaei, N. Circular RNA MYLK as a prognostic biomarker in patients with cancers: A systematic review and meta-analysis. Cancer Rep. 2022, 5, e1653. [Google Scholar] [CrossRef] [PubMed]
- Borghini, A.; Labate, L.; Piccinini, S.; Panaino, C.M.V.; Andreassi, M.G.; Gizzi, L.A. FLASH Radiotherapy: Expectations, Challenges, and Current Knowledge. Int. J. Mol. Sci. 2024, 25, 2546. [Google Scholar] [CrossRef]
- Zhou, R.; Chen, J.; Xu, Y.; Ye, Y.; Zhong, G.; Chen, T.; Qiu, L. PRPF19 facilitates colorectal cancer liver metastasis through activation of the Src-YAP1 pathway via K63-linked ubiquitination of MYL9. Cell Death Dis. 2023, 14, 258. [Google Scholar] [CrossRef]
- Bogaerts, E.; Macaeva, E.; Isebaert, S.; Haustermans, K. Potential Molecular Mechanisms behind the Ultra-High Dose Rate “FLASH” Effect. Int. J. Mol. Sci. 2022, 23, 12109. [Google Scholar] [CrossRef]
- Tang, R.; Yin, J.; Liu, Y.; Xue, J. FLASH radiotherapy: A new milestone in the field of cancer radiotherapy. Cancer Lett. 2024, 587, 216651. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Chung, H.T.; Kim, J.W.; Dho, Y.S.; Lee, E.J. A 3-month survival model after Gamma Knife surgery in patients with brain metastasis from lung cancer with Karnofsky performance status ≤70. Sci. Rep. 2023, 13, 13159. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Liu, R.; Chang, C.W.; Charyyev, S.; Zhou, J.; Bradley, J.D.; Liu, T.; Yang, X. A potential revolution in cancer treatment: A topical review of FLASH radiotherapy. J. Appl. Clin. Med. Phys. 2022, 23, e13790. [Google Scholar] [CrossRef] [PubMed]
- Venkatesulu, B.P.; Sharma, A.; Pollard-Larkin, J.M.; Sadagopan, R.; Symons, J.; Neri, S.; Singh, P.K.; Tailor, R.; Lin, S.H.; Krishnan, S. Ultra high dose rate (35 Gy/sec) radiation does not spare the normal tissue in cardiac and splenic models of lymphopenia and gastrointestinal syndrome. Sci. Rep. 2019, 9, 17180. [Google Scholar] [CrossRef]
- Zhang, Q.; Gerweck, L.E.; Cascio, E.; Gu, L.; Yang, Q.; Dong, X.; Huang, P.; Bertolet, A.; Nesteruk, K.P.; Sung, W.; et al. Absence of Tissue-Sparing Effects in Partial Proton FLASH Irradiation in Murine Intestine. Cancers 2023, 15, 2269. [Google Scholar] [CrossRef]
- Cucinotta, F.A.; Smirnova, O.A. Effects of Flash Radiotherapy on Blood Lymphocytes in Humans and Small Laboratory Animals. Radiat. Res. 2023, 199, 240–251. [Google Scholar] [CrossRef]
- Galts, A.; Hammi, A. FLASH radiotherapy sparing effect on the circulating lymphocytes in pencil beam scanning proton therapy: Impact of hypofractionation and dose rate. Phys. Med. Biol. 2024, 69, 025006. [Google Scholar] [CrossRef]
- Wilson, J.D.; Hammond, E.M.; Higgins, G.S.; Petersson, K. Ultra-High Dose Rate (FLASH) Radiotherapy: Silver Bullet or Fool’s Gold? Front. Oncol. 2020, 9, 1563. [Google Scholar] [CrossRef]
- Melia, E.; Parsons, J.L. DNA damage and repair dependencies of ionising radiation modalities. Biosci. Rep. 2023, 43, BSR20222586. [Google Scholar] [CrossRef]
- Böhlen, T.T.; Germond, J.F.; Desorgher, L.; Veres, I.; Bratel, A.; Landström, E.; Engwall, E.; Herrera, F.G.; Ozsahin, E.M.; Bourhis, J.; et al. Very high-energy electron therapy as light-particle alternative to transmission proton FLASH therapy—An evaluation of dosimetric performances. Radiother. Oncol. 2024, 194, 110177. [Google Scholar] [CrossRef]
- Moeckli, R.; Gonçalves Jorge, P.; Grilj, V.; Oesterle, R.; Cherbuin, N.; Bourhis, J.; Vozenin, M.C.; Germond, J.F.; Bochud, F.; Bailat, C. Commissioning of an ultra-high dose rate pulsed electron beam medical LINAC for FLASH RT preclinical animal experiments and future clinical human protocols. Med. Phys. 2021, 48, 3134–3142. [Google Scholar] [CrossRef] [PubMed]
- Liljedahl, E.; Konradsson, E.; Linderfalk, K.; Gustafsson, E.; Petersson, K.; Ceberg, C.; Redebrandt, H.N. Comparable survival in rats with intracranial glioblastoma irradiated with single-fraction conventional radiotherapy or FLASH radiotherapy. Front. Oncol. 2024, 13, 1309174. [Google Scholar] [CrossRef] [PubMed]
- Allen, B.D.; Alaghband, Y.; Kramár, E.A.; Ru, N.; Petit, B.; Grilj, V.; Petronek, M.S.; Pulliam, C.F.; Kim, R.Y.; Doan, N.L.; et al. Elucidating the neurological mechanism of the FLASH effect in juvenile mice exposed to hypofractionated radiotherapy. Neuro Oncol 2023, 25, 927–939. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.R.; Parsons, J.L. FLASH Radiotherapy: Current Knowledge and Future Insights Using Proton-Beam Therapy. Int. J. Mol. Sci. 2020, 21, 6492. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).