Photodynamic Therapy of Atherosclerotic Plaque Monitored by T1 and T2 Relaxation Times of Magnetic Resonance Imaging
Abstract
1. Introduction
Atherosclerosis Characteristics
2. Materials and Methods
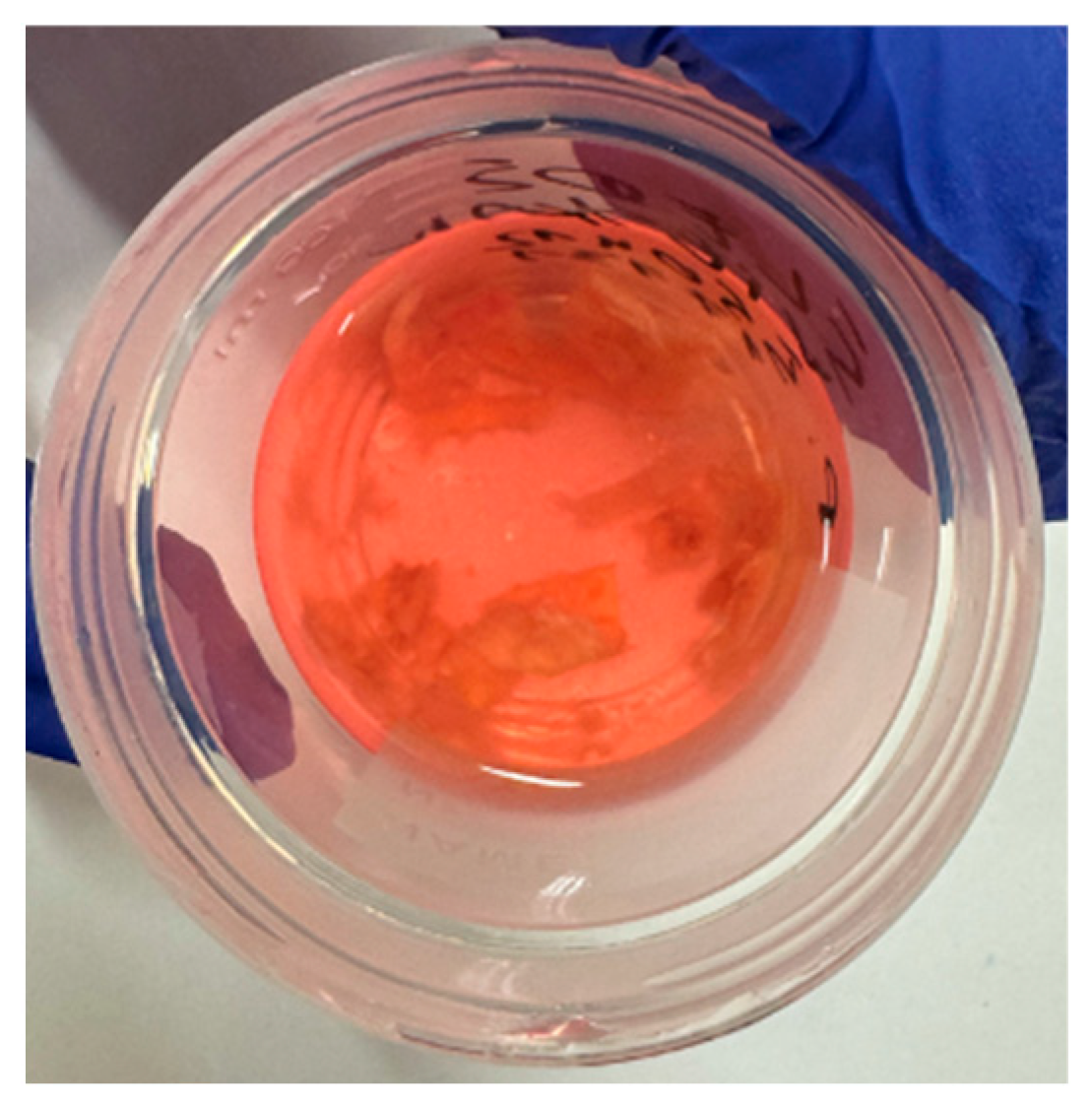
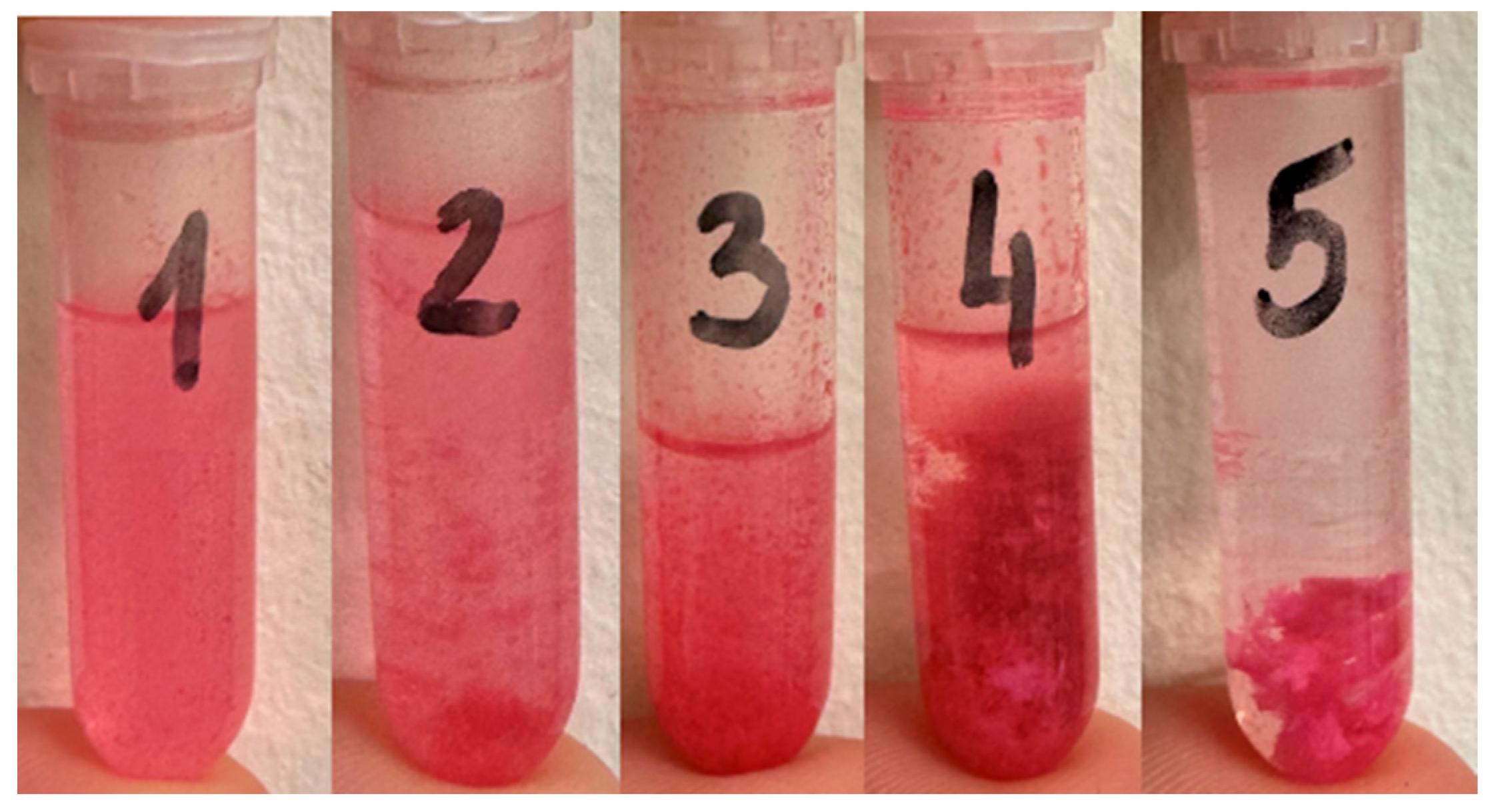
2.1. Carotid Artery Samples
2.2. Rose Bengal Concentration
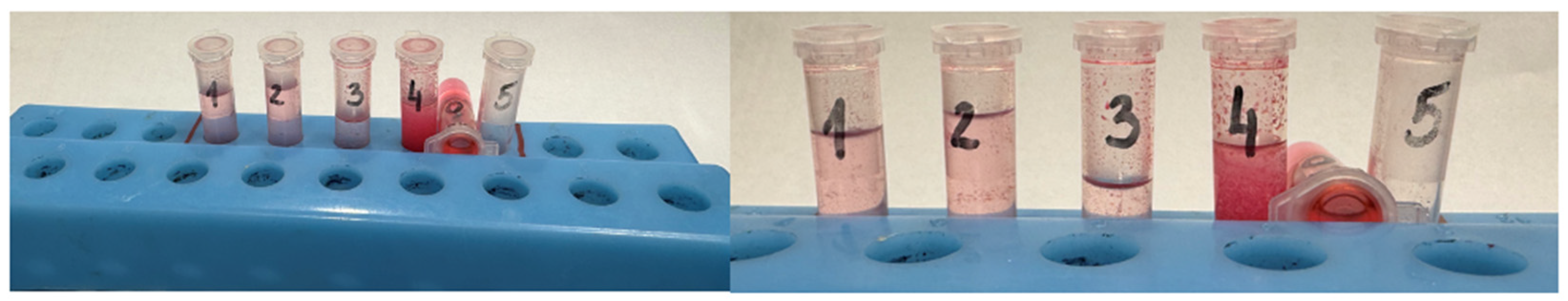
2.3. PDT Procedure
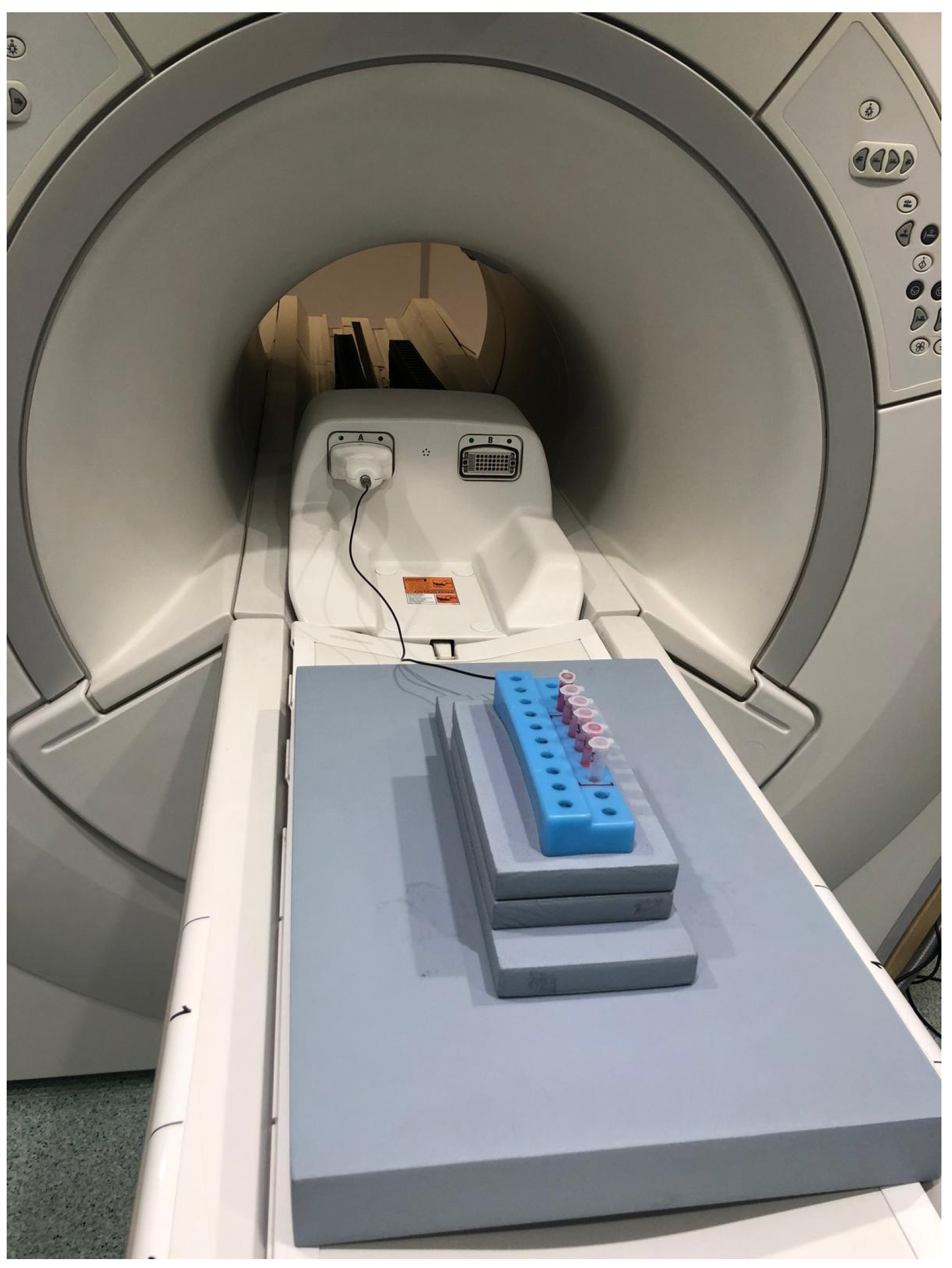
2.4. MRI Analysis
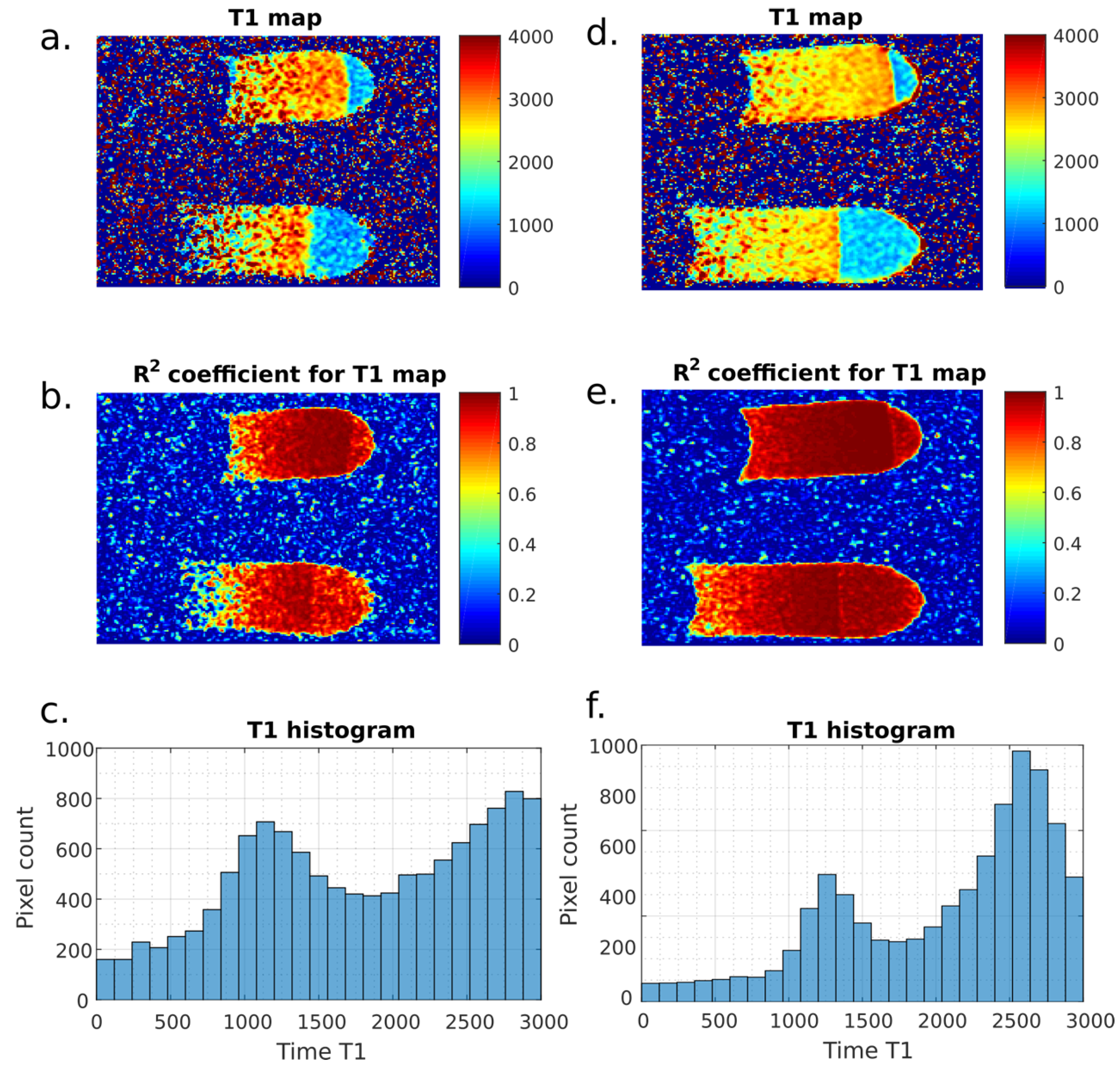
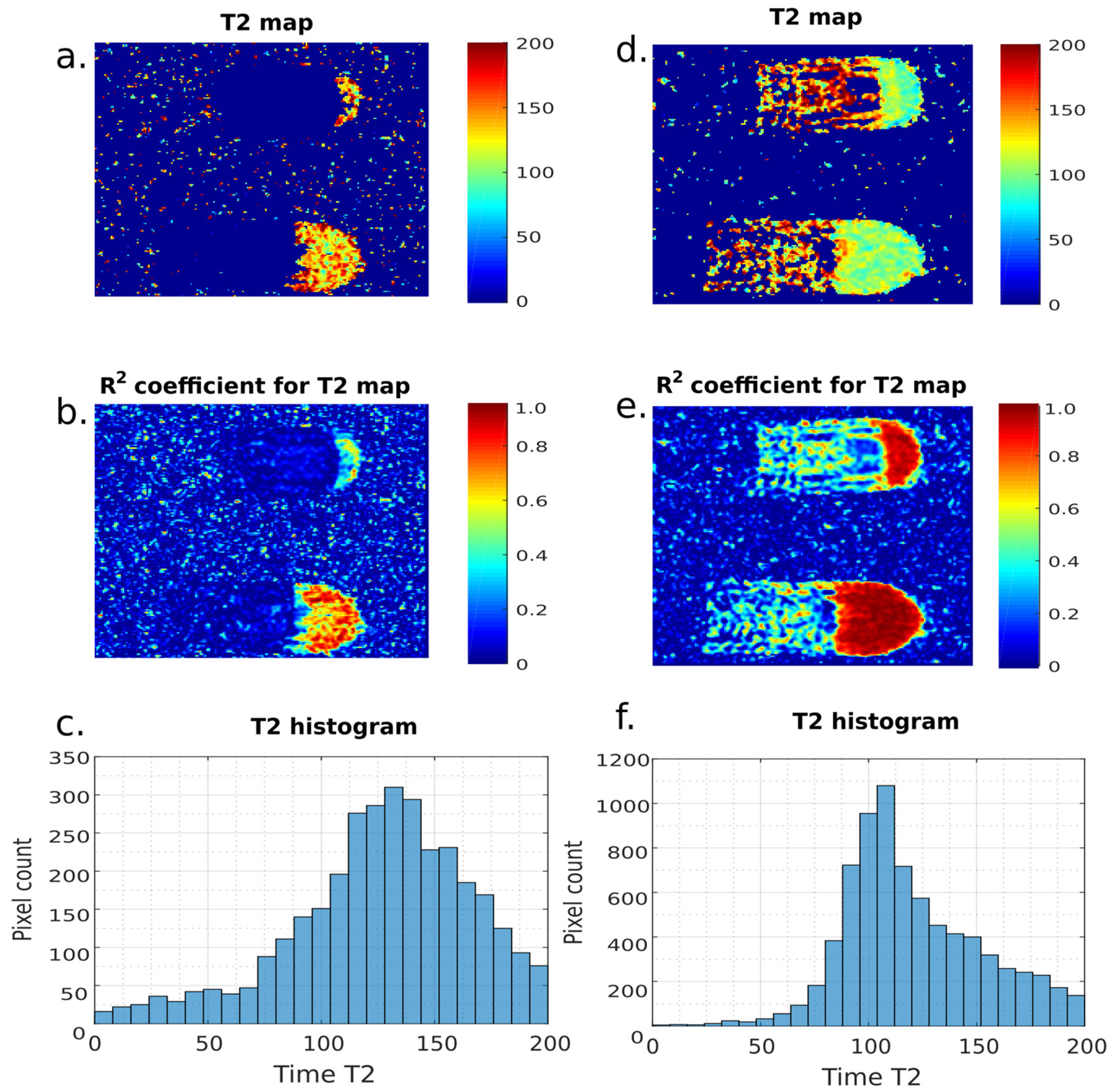
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Münzel, T.; Sørensen, M.; Hahad, O.; Nieuwenhuijsen, M.; Daiber, A. The contribution of the exposome to the burden of cardiovascular disease. Nat. Rev. Cardiol. 2023, 20, 651–669. [Google Scholar] [CrossRef] [PubMed]
- Kouhpeikar, H.; Delbari, Z.; Sathyapalan, T.; Simental-Mendía, L.E.; Jamialahmadi, T.; Sahebkar, A. The Effect of Statins through Mast Cells in the Pathophysiology of Atherosclerosis: A Review. Curr. Atheroscler. Rep. 2020, 22, 19. [Google Scholar] [CrossRef] [PubMed]
- Gawaz, M.; Geisler, T.; Borst, O. Current concepts and novel targets for antiplatelet therapy. Nat. Rev. Cardiol. 2023, 20, 583–599. [Google Scholar] [CrossRef] [PubMed]
- Lawton, J.S.; Tamis-Holland, J.E.; Bangalore, S.; Bates, E.R.; Beckie, T.M.; Bischoff, J.M.; Bittl, J.A.; Cohen, M.G.; DiMaio, J.M.; Don, C.W.; et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e4–e17. [Google Scholar] [CrossRef] [PubMed]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef] [PubMed]
- Safian, R.D. Carotid Artery Revascularization: The Known Knowns and the Known Unknowns. J. Am. Coll. Cardiol. 2017, 69, 2276–2278. [Google Scholar] [CrossRef] [PubMed]
- Shamaki, G.R.; Markson, F.; Soji-Ayoade, D.; Agwuegbo, C.C.; Bamgbose, M.O.; Tamunoinemi, B.-M. Peripheral Artery Disease: A Comprehensive Updated Review. Curr. Probl. Cardiol. 2022, 47, 101082. [Google Scholar] [CrossRef] [PubMed]
- Shibutani, H.; Fujii, K.; Matsumura, K.; Otagaki, M.; Morishita, S.; Bando, K.; Motohiro, M.; Umemura, S.; Shiojima, I. Differential influence of lesion length on fractional flow reserve in intermediate coronary lesions between each coronary artery. Catheter. Cardiovasc. Interv. 2020, 95, E168–E174. [Google Scholar] [CrossRef] [PubMed]
- Leone, A.M.; Arioti, M.; Cialdella, P.; Vergallo, R.; Zimbardo, G.; Migliaro, S.; Anastasia, G.; Di Giusto, F.; Galante, D.; Basile, E.; et al. Prognostic impact of FFR/contrast FFR discordance. Int. J. Cardiol. 2021, 327, 40–44. [Google Scholar] [CrossRef]
- Nørgaard, B.L.; Leipsic, J.; Gaur, S.; Seneviratne, S.; Ko, B.S.; Ito, H.; Jensen, J.M.; Mauri, L.; De Bruyne, B.; Bezerra, H.; et al. Diagnostic performance of noninvasive fractional flow reserve derived from coronary computed tomography angiography in suspected coronary artery disease: The NXT trial (Analysis of Coronary Blood Flow Using CT Angi-ography: Next Steps). J. Am. Coll. Cardiol. 2014, 63, 1145–1155. [Google Scholar] [CrossRef] [PubMed]
- Cortese, B.; Piraino, D.; Gentile, D.; Onea, H.; Lazar, L. Intravascular imaging for left main stem assessment: An update on the most recent clinical data. Catheter. Cardiovasc. Interv. 2022, 100, 1220–1228. [Google Scholar] [CrossRef]
- A Byrne, R.; Fremes, S.; Capodanno, D.; Czerny, M.; Doenst, T.; Emberson, J.R.; Falk, V.; Gaudino, M.; McMurray, J.J.V.; Mehran, R.; et al. 2022 Joint ESC/EACTS review of the 2018 guideline recommendations on the revascularization of left main coronary artery disease in patients at low surgical risk and anatomy suitable for PCI or CABG. Eur. J. Cardio-Thoracic Surg. 2023, 64, ezad286. [Google Scholar] [CrossRef] [PubMed]
- Davidson, L.J.; Cleveland, J.C.; Welt, F.G.; Anwaruddin, S.; Bonow, R.O.; Firstenberg, M.S.; Gaudino, M.F.; Gersh, B.J.; Grubb, K.J.; Kirtane, A.J.; et al. A Practical Approach to Left Main Coronary Artery Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022, 80, 2119–2134. [Google Scholar] [CrossRef] [PubMed]
- Lamanna, A.; Maingard, J.; Barras, C.D.; Kok, H.K.; Handelman, G.; Chandra, R.V.; Thijs, V.; Brooks, D.M.; Asadi, H. Carotid artery stenting: Current state of evidence and future directions. Acta Neurol. Scand. 2019, 139, 318–333. [Google Scholar] [CrossRef] [PubMed]
- Meads, C.; Cummins, C.; Jolly, K.; Stevens, A.; Burls, A.; Hyde, C. Coronary artery stents in the treatment of ischaemic heart disease: A rapid and systematic review. Health Technol Assess. 2000, 4, 1–153. [Google Scholar] [CrossRef] [PubMed]
- Sawada, T.; Shite, J.; Garcia-Garcia, H.M.; Shinke, T.; Watanabe, S.; Otake, H.; Matsumoto, D.; Tanino, Y.; Ogasawara, D.; Kawamori, H.; et al. Feasibility of combined use of intravascular ultrasound radiofrequency data analysis and optical coherence tomography for detecting thin-cap fibroatheroma. Eur. Heart J. 2008, 29, 1136–1146. [Google Scholar] [CrossRef] [PubMed]
- Hirai, T.; Chen, Z.; Zhang, L.; Baaj, S.; Kovarnik, T.; Porcaro, K.; Kaminski, J.; Hawn, S.; Agrawal, A.; Makki, N.; et al. Evaluation of Variable Thin-Cap Fibroatheroma Definitions and Association of Virtual Histology-Intravascular Ultrasound Findings with Cavity Rupture Size. Am. J. Cardiol. 2016, 118, 162–169. [Google Scholar] [CrossRef]
- Libby, P. The changing landscape of atherosclerosis. Nature 2021, 592, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Geovanini, G.R.; Libby, P. Atherosclerosis and inflammation: Overview and updates. Clin. Sci. 2018, 132, 1243–1252. [Google Scholar] [CrossRef]
- Bentzon, J.F.; Otsuka, F.; Virmani, R.; Falk, E. Mechanisms of Plaque Formation and Rupture. Circ. Res. 2014, 114, 1852–1866. [Google Scholar] [CrossRef]
- Libby, P.; Pasterkamp, G.; Crea, F.; Jang, I.-K. Reassessing the Mechanisms of Acute Coronary Syndromes. Circ. Res. 2019, 124, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Wohlschlaeger, J.; Bertram, S.; Theegarten, D.; Hager, T.; Baba, H. Coronary atherosclerosis and progression to unstable plaques: Histomorphological and molecular aspects. Herz 2015, 40, 837–844. [Google Scholar] [CrossRef]
- Desai, K.P.; Sidhu, M.S.; Boden, W.E. Evaluation of the stable coronary artery disease patient: Anatomy trumps physiology. Trends Cardiovasc. Med. 2014, 24, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Badimon, L.; Vilahur, G. Thrombosis formation on atherosclerotic lesions and plaque rupture. J. Intern. Med. 2014, 276, 618–632. [Google Scholar] [CrossRef] [PubMed]
- Narula, J.; Kovacic, J.C. Putting TCFA in Clinical Perspective. J. Am. Coll. Cardiol. 2014, 64, 681–683. [Google Scholar] [CrossRef] [PubMed]
- Virmani, R. Are our tools for the identification of TCFA ready and do we know them? JACC Cardiovasc. Imaging 2011, 4, 656–658. [Google Scholar] [CrossRef][Green Version]
- Salem, A.M.; Davis, J.; Gopalan, D.; Rudd, J.H.; Clarke, S.C.; Schofield, P.M.; Bennett, M.R.; Brown, A.J.; Obaid, D.R. Characteristics of conventional high-risk coronary plaques and a novel CT defined thin-cap fibroatheroma in patients undergoing CCTA with stable chest pain. Clin. Imaging 2023, 101, 69–76. [Google Scholar] [CrossRef]
- Kunadian, V.; Chieffo, A.; Camici, P.G.; Berry, C.; Escaned, J.; Maas, A.H.E.M.; Prescott, E.; Karam, N.; Appelman, Y.; Fraccaro, C.; et al. Consensus Document on Ischaemia with Non-Obstructive Coronary Arteries in Collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology & Microcirculation Endorsed by Coronary Vasomotor Disorders International Study Group. Eur. Heart J. 2020, 41, 3504–3520. [Google Scholar] [CrossRef] [PubMed]
- Hellings, W.E.; Peeters, W.; Moll, F.L.; Piers, S.R.; van Setten, J.; Van der Spek, P.J.; de Vries, J.-P.P.; Seldenrijk, K.A.; De Bruin, P.C.; Vink, A.; et al. Composition of carotid atherosclerotic plaque is associated with cardiovascular outcome: A prog-nostic study. Circulation 2010, 121, 1941–1950. [Google Scholar] [CrossRef]
- Wu, G.; Yu, G.; Zheng, M.; Peng, W.; Li, L. Recent Advances for Dynamic-Based Therapy of Atherosclerosis. Int. J. Nanomed. 2023, 18, 3851–3878. [Google Scholar] [CrossRef]
- Wagnières, G.; van den Bergh, H.; Cook, S.; Giraud, M.; Jain, M.; Zellweger, M. Photodynamic therapy for the treatment of atherosclerotic plaque: Lost in translation? Cardiovasc. Ther. 2017, 35, e12238. [Google Scholar] [CrossRef]
- Mroz, P.; Yaroslavsky, A.; Kharkwal, G.B.; Hamblin, M.R. Cell death pathways in photodynamic therapy of cancer. Cancers 2011, 3, 2516–2539. [Google Scholar] [CrossRef] [PubMed]
- Glick, D.; Barth, S.; MacLeod, K.F. Autophagy: Cellular and molecular mechanisms. J. Pathol. 2010, 221, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.K.; Lutzke, L.; Borkenhagen, L.; Westra, W.; Song, M.W.K.; Prasad, G.; Buttar, N.S. Photodynamic therapy for Barrett’s esophagus: Does light still have a role? Endoscopy 2008, 40, 1021–1025. [Google Scholar] [CrossRef] [PubMed]
- Fei, B.; Wang, H.; Meyers, J.D.; Feyes, D.K.; Oleinick, N.L.; Duerk, J.L. High-field magnetic resonance imaging of the response of human prostate cancer to Pc 4-based photodynamic therapy in an animal model. Lasers Surg. Med. 2007, 39, 723–730. [Google Scholar] [CrossRef]
- Allison, B.A.; Crespo, M.T.; Jain, A.K.; Richter, A.M.; Hsiang, Y.N.; Levy, J.G. Delivery of benzoporphyrin derivative, a photosensitizer, into atherosclerotic plaque of Watanabe heritable hyperlipidemic rabbits and balloon-injured New Zealand rabbits. Photochem. Photobiol. 1997, 65, 877–883. [Google Scholar] [CrossRef]
- Usui, M.; Asahara, T.; Naitoh, Y.; Katoh, T.; Ibukiyama, C. Photodynamic therapy for the prevention of intimal hyperplasia in balloon-injured rabbit arteries. Jpn. Circ. J. 1999, 63, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Granville, D.J.; Cassidy, B.A.; Ruehlmann, D.O.; Choy, J.C.; Brenner, C.; Kroemer, G.; van Breemen, C.; Margaron, P.; Hunt, D.W.; McManus, B.M. Mitochondrial release of apoptosis-inducing factor and cytochrome c during smooth muscle cell apoptosis. Am. J. Pathol. 2001, 159, 305–311. [Google Scholar] [CrossRef]
- Hsiang, Y.N.; Crespo, M.T.; Richter, A.M.; Jain, A.K.; Fragoso, M.; Levy, J.G. In vitro and in vivo uptake of benzoporphyrin derivative into human and miniswine atherosclerotic plaque. Photochem. Photobiol. 1993, 57, 670–674. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Woodburn, K.W.; Hayase, M.; Hoyt, G.; Robbins, R.C. Photodynamic therapy with motexafin lutetium (Lu-Tex) reduces experimental graft coronary artery disease. Transplantation 2001, 71, 1526–1532. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Woodburn, K.W.; Hayase, M.; Robbins, R.C. Reduction of vein graft disease using photodynamic therapy with motexafin lutetium in a rodent isograft model. Circulation 2000, 102 (Suppl. S3), III275–III280. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Xie, R.; Yu, T. Photodynamic Therapy for Atherosclerosis: Past, Present, and Future. Pharmaceutics 2024, 16, 729. [Google Scholar] [CrossRef]
- Jenkins, M.P.; Buonaccorsi, G.A.; Mansfield, R.; Bishop, C.C.; Bown, S.G.; McEwan, J.R. Reduction in the response to coronary and iliac artery injury with pho-todynamic therapy using 5-aminolaevulinic acid. Cardiovasc. Res. 2000, 45, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.; Yoon, H.; Kim, K.; Kim, H.; Yoon, Y.; Kim, J. Fluorescence kinetics of protoporphyrin-IX induced from 5-ALA compounds in rabbit postballoon injury model for ALA-photoangioplasty. Photochem. Photobiol. 2008, 84, 1209–1214. [Google Scholar] [CrossRef] [PubMed]
- Van Eps, R.G.S.; ChandraSekar, N.R.; Hasan, T.; LaMuraglia, G.M. Importance of the treatment field for the application of vascular photodynamic therapy to inhibit intimal hyperplasia. Photochem Photobiol. 1998, 67, 337–342. [Google Scholar] [CrossRef]
- Waksman, R.; McEwan, P.E.; Moore, T.I.; Pakala, R.; Kolodgie, F.D.; Hellinga, D.G.; Seabron, R.C.; Rychnovsky, S.J.; Vasek, J.; Scott, R.W.; et al. PhotoPoint photodynamic therapy promotes stabilization of atherosclerotic plaques and inhibits plaque progression. J. Am. Coll. Cardiol. 2008, 52, 1024–1032. [Google Scholar] [CrossRef] [PubMed]
- Van Eps, R.G.S.; Adili, F.; Watkins, M.T.; Anderson, R.R.; Lamuraglia, G.M. Photodynamic therapy of extracellular matrix stimulates endothelial cell growth by inactivation of matrix-associated transforming growth factor-beta. Lab. Investig. A J. Tech. Methods Pathol. 1997, 76, 257–266. [Google Scholar]
- Giustino, G.; Colombo, A.; Camaj, A.; Yasumura, K.; Mehran, R.; Stone, G.W.; Kini, A.; Sharma, S.K. Coronary In-Stent Restenosis: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022, 80, 348–372. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, Q.M.; Zhao, K.M.; Bian, Y.-J.M.; Liu, Y.M.; Xue, Y.-T. Risk factors for in-stent restenosis after coronary stent implantation in patients with coronary artery disease: A retrospective observational study. Medicine 2022, 101, e31707. [Google Scholar] [CrossRef] [PubMed]
- Habib, R.H.; Dimitrova, K.R.; Badour, S.A.; Yammine, M.B.; El-Hage-Sleiman, A.-K.M.; Hoffman, D.M.; Geller, C.M.; Schwann, T.A.; Tranbaugh, R.F. CABG Versus PCI: Greater Benefit in Long-Term Outcomes with Multiple Arterial Bypass Grafting. J. Am. Coll. Cardiol. 2015, 66, 1417–1427. [Google Scholar] [CrossRef]
- Peng, C.; Li, Y.; Liang, H.; Cheng, J.; Li, Q.; Sun, X.; Li, Z.; Wang, F.; Guo, Y.; Tian, Z.; et al. Detection and photodynamic therapy of inflamed atherosclerotic plaques in the carotid artery of rabbits. J. Photochem. Photobiol. B Biol. 2011, 102, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Borshch, V.N.; Andreeva, E.R.; Kuz’min, S.G.; Vozovikov, I.N. New medicines and approaches to treatment of atherosclerosis. Russ. J. Gen. Chem. 2012, 82, 554–563. [Google Scholar] [CrossRef]
- Xie, Y.; Mintz, G.S.; Yang, J.; Doi, H.; Iñiguez, A.; Dangas, G.D.; Serruys, P.W.; McPherson, J.A.; Wennerblom, B.; Xu, K.; et al. Clinical outcome of nonculprit plaque ruptures in patients with acute coronary syndrome in the PROSPECT study. JACC Cardiovasc. Imaging 2014, 7, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Komiyama, H.; Takano, M.; Hata, N.; Seino, Y.; Shimizu, W.; Mizuno, K. Neoatherosclerosis: Coronary stents seal atherosclerotic lesions but result in making a new problem of atherosclerosis. World J. Cardiol. 2015, 7, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Cortese, B.; Orrego, P.S.; Agostoni, P.; Buccheri, D.; Piraino, D.; Andolina, G.; Seregni, R.G. Effect of Drug-Coated Balloons in Native Coronary Artery Disease Left with a Dissection. JACC Cardiovasc. Interv. 2015, 8, 2003–2009. [Google Scholar] [CrossRef]
- Kalra, A.; Rehman, H.; Khera, S.; Thyagarajan, B.; Bhatt, D.L.; Kleiman, N.S.; Yeh, R.W. New-Generation Coronary Stents: Current Data and Future Directions. Curr. Atheroscler. Rep. 2017, 19, 14. [Google Scholar] [CrossRef]
- Gonzalo, N.; Ryan, N.; Escaned, J. New light on second-generation drug-eluting stent restenosis. EuroIntervention 2017, 13, 265–266. [Google Scholar] [CrossRef]
- Yerasi, C.; Case, B.C.; Forrestal, B.J.; Torguson, R.; Weintraub, W.S.; Garcia-Garcia, H.M.; Waksman, R. Drug-Coated Balloon for De Novo Coronary Artery Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 1061–1073. [Google Scholar] [CrossRef]
- Saam, T.; Ferguson, M.; Yarnykh, V.; Takaya, N.; Xu, D.; Polissar, N.; Hatsukami, T.; Yuan, C. Quantitative evaluation of carotid plaque composition by in vivo MRI. Arter. Thromb. Vasc. Biol. 2007, 27, 2342–2348. [Google Scholar] [CrossRef]
- Underhill, H.R.; Hatsukami, T.S.; Fayad, Z.A.; Fuster, V.; Yuan, C. MRI of carotid atherosclerosis: Clinical impli-cations and future directions. Nat. Rev. Cardiol. 2010, 7, 165–173. [Google Scholar] [CrossRef]
- Corti, R.; Fuster, V. Imaging of atherosclerosis: Magnetic resonance imaging. Eur. Heart J. 2011, 32, 1709–1719. [Google Scholar] [CrossRef] [PubMed]
- Fuster, V.; Badimon, L. Mechanisms of disease: The pathogenesis of coronary disease and the acute coronary syndromes. N. Engl. J. Med. 2010, 326, 242–250. [Google Scholar]
- Yuan, C.; Mitsumori, L.M.; Ferguson, M.S.; Polissar, N.L.; Echelard, D.; Ortiz, G.; Small, R.; Davies, J.W.; Kerwin, W.S.; Hatsukami, T.S. In vivo accuracy of multispectral magnetic resonance imaging for identifying lipid-rich necrotic cores and intraplaque hemorrhage in advanced human carotid plaques. Circulation 2001, 104, 2051–2056. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.Q.; Yuan, C.; Hatsukami, T.S.; Frechette, E.H.; Kang, X.J.; Maravilla, K.R.; Brown, B.G. Effects of prolonged intensive lipid-lowering therapy on the characteristics of carotid atherosclerotic plaques in vivo by MRI: A case-control study. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1623–1629. [Google Scholar] [CrossRef]
- Cai, J.; Hatsukami, T.S.; Ferguson, M.S.; Kerwin, W.S.; Saam, T.; Chu, B.; Takaya, N.; Polissar, N.L.; Yuan, C. In vivo quantitative measurement of intact fibrous cap and lipid-rich necrotic core size in atherosclerotic carotid plaque: Comparison of high-resolution, contrast-enhanced magnetic resonance imaging and histology. Circulation 2005, 112, 3437–3444. [Google Scholar] [CrossRef]
- Yuan, C.; Mitsumori, L.M.; Beach, K.W.; Maravilla, K.R. Carotid atherosclerotic plaque: Noninvasive MR characterization and identification of vulnerable lesions. Radiology 2001, 221, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Cappendijk, V.C.; Cleutjens, K.B.; Heeneman, S.; Schurink, G.W.H.; Welten, R.J.T.J.; Kessels, A.G.; van Suylen, R.J.; Daemen, M.J.; van Engelshoven, J.M.; Kooi, M.E. In vivo detection of hemorrhage in human atherosclerotic plaques with magnetic resonance imaging. J. Magn. Reson. Imaging 2004, 20, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, R.A.; U-King-Im, J.M.; Graves, M.J.; Cross, J.J.; Horsley, J.; Goddard, M.J.; Skepper, J.N.; Quartey, G.; Warburton, E.; Joubert, I.; et al. In vivo detection of macrophages in human carotid atheroma: Temporal dependence of ultrasmall superparamagnetic particles of iron oxide-enhanced MRI. Stroke 2004, 35, 1631–1635. [Google Scholar] [CrossRef] [PubMed]
- Fayad, Z.A.; Fallon, J.T.; Shinnar, M.; Wehrli, S.; Dansky, H.M.; Poon, M.; Badimon, J.J.; Charlton, S.A.; Fisher, E.A.; Breslow, J.L.; et al. Noninvasive in vivo high-resolution magnetic resonance imaging of atherosclerotic lesions in genetically engineered mice. Circulation 1998, 98, 1541–1547. [Google Scholar] [CrossRef]
- Edelman, R.R.; Chien, D.; Kim, D. Fast selective black blood MR imaging. Radiology 1991, 181, 655–660. [Google Scholar] [CrossRef]
- Edelman, R.R.; Mattle, H.P.; Wallner, B.; Bajakian, R.; Kleefield, J.; Kent, C.; Skillman, J.J.; Mendel, J.B.; Atkinson, D.J. Extracranial carotid arteries: Evaluation with “black blood” MR angiography. Radiology 1990, 177, 45–50. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, J.H.; Lee, J.H.; Bell, D.S.; Holick, M.F. The ATHEROMA (Atorvastatin Therapy: Effects on Reduction of Macrophage Activity) Study. Evaluation using ultrasmall superparamagnetic iron oxide-enhanced magnetic resonance imaging in carotid disease. J. Am. Coll. Cardiol. 2009, 53, 2039–2050. [Google Scholar] [CrossRef]
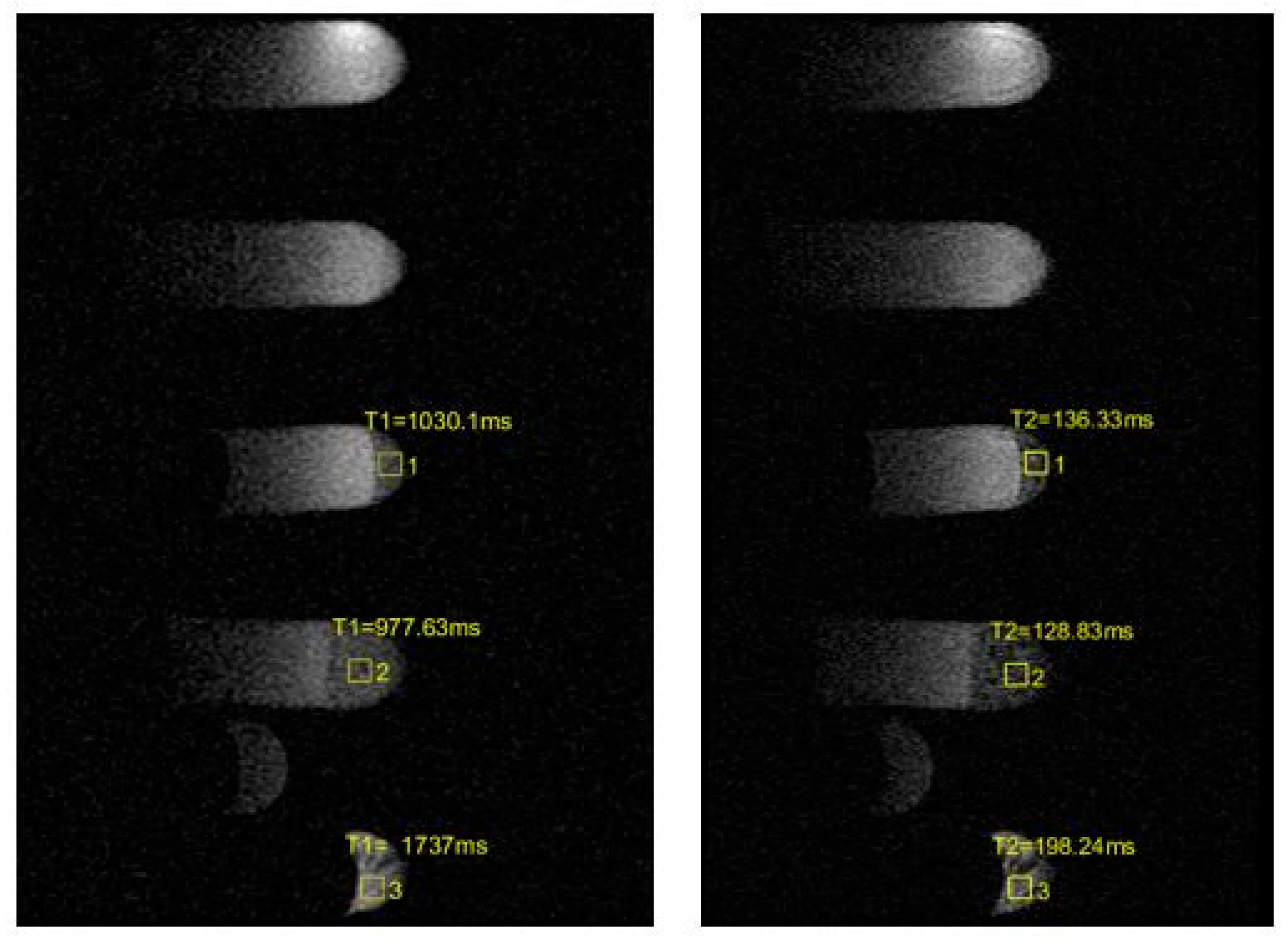
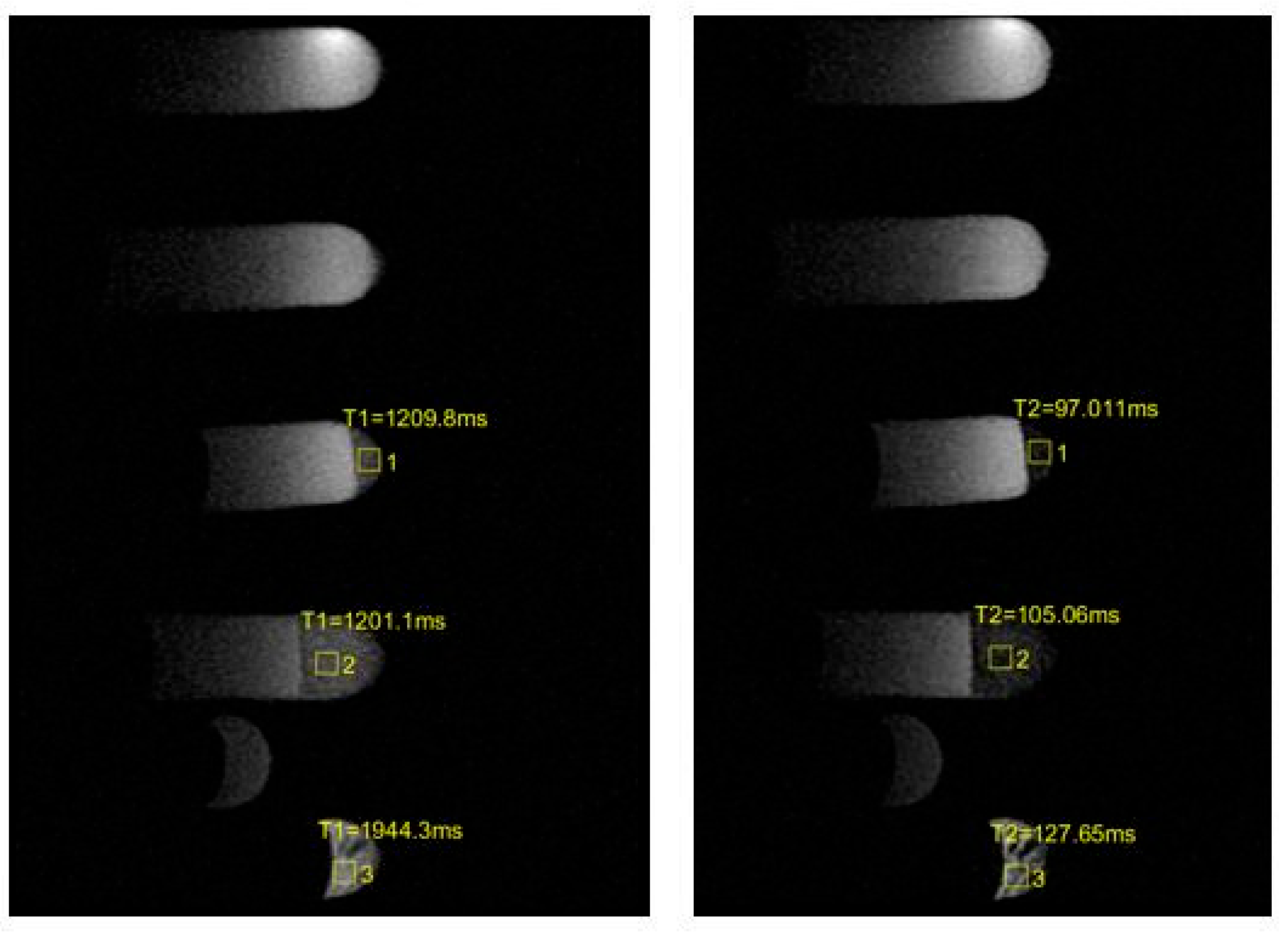
| Sample | Before | After |
|---|---|---|
| 1 | 0 | 0 |
| 2 | 0 | 0 |
| 3 | T1 = 1030.1 ms ± 154.52 T2 = 136.33 ms ± 20.45 | T1 = 1209.8 ms ± 181.47 T2 = 97.011 ms ± 14.55 |
| 4 | T1 = 977.63 ms ± 146.64 T2 = 128.83 ms ± 19.32 | T1 = 1201.1 ms ± 180.17 T2 = 105.06 ms ± 15.76 |
| 5 | T1 = 1737 ms ± 260.55 T2 = 198.24 ms ± 29.74 | T1 = 1944.3 ms ± 291.60 T2 = 127.65 ms ± 19.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wańczura, P.; Aebisher, D.; Leksa, D.; Mytych, W.; Dynarowicz, K.; Myśliwiec, A.; Leksa, N.; Truszkiewicz, A.; Bartusik-Aebisher, D. Photodynamic Therapy of Atherosclerotic Plaque Monitored by T1 and T2 Relaxation Times of Magnetic Resonance Imaging. Int. J. Transl. Med. 2024, 4, 505-518. https://doi.org/10.3390/ijtm4030034
Wańczura P, Aebisher D, Leksa D, Mytych W, Dynarowicz K, Myśliwiec A, Leksa N, Truszkiewicz A, Bartusik-Aebisher D. Photodynamic Therapy of Atherosclerotic Plaque Monitored by T1 and T2 Relaxation Times of Magnetic Resonance Imaging. International Journal of Translational Medicine. 2024; 4(3):505-518. https://doi.org/10.3390/ijtm4030034
Chicago/Turabian StyleWańczura, Piotr, David Aebisher, Dawid Leksa, Wiktoria Mytych, Klaudia Dynarowicz, Angelika Myśliwiec, Natalia Leksa, Adrian Truszkiewicz, and Dorota Bartusik-Aebisher. 2024. "Photodynamic Therapy of Atherosclerotic Plaque Monitored by T1 and T2 Relaxation Times of Magnetic Resonance Imaging" International Journal of Translational Medicine 4, no. 3: 505-518. https://doi.org/10.3390/ijtm4030034
APA StyleWańczura, P., Aebisher, D., Leksa, D., Mytych, W., Dynarowicz, K., Myśliwiec, A., Leksa, N., Truszkiewicz, A., & Bartusik-Aebisher, D. (2024). Photodynamic Therapy of Atherosclerotic Plaque Monitored by T1 and T2 Relaxation Times of Magnetic Resonance Imaging. International Journal of Translational Medicine, 4(3), 505-518. https://doi.org/10.3390/ijtm4030034








