Overlapping Receptor-Based Pathogenic Cascades in Degenerative Disease: Implications Ranging from Tumor Targeting to Aging and Dementia Therapeutics
Abstract
1. Background
2. Cardiovascular Risk Factors, Inflammation, Oxidative Stress, and Serum Amyloid A (SAA) Inflammatory Effects
3. Gut–Brain Axis, ApoA-I, Amyloidosis, Aging, SAA versus SR-BI Targeting, and Dementia
4. Analysis of Nanocarrier: Formulation, Biophysical Structure, Particle Stability, and Bio-Safety
4.1. Formulation
4.2. Biophysical Structure
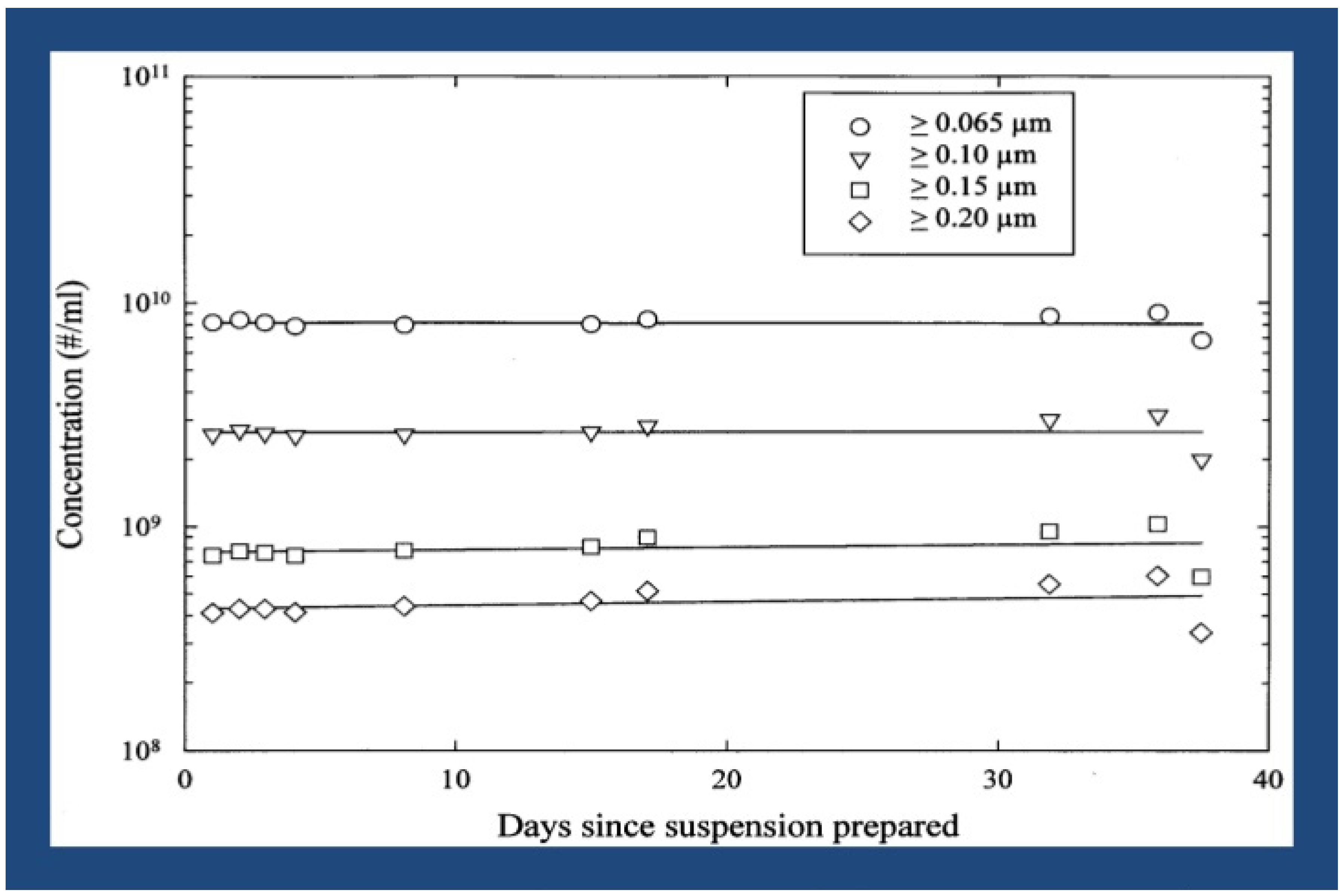
4.3. Particle Stability
4.4. Bio-Safety
5. Concluding Remarks
Funding
Acknowledgments
Conflicts of Interest
References
- D’Arrigo, J.S. Stable Nanoemulsions: Self-Assembly in Nature and Nanomedicine; Elsevier: Amsterdam, The Netherlands, 2011; p. 415. ISBN 978-0-444-53798-0. [Google Scholar]
- D’Arrigo, J.S. Nanotargeting of drug(s) for delaying dementia: Relevance of COVID-19 impact on dementia. Am. J. Alzheimer’s Dis. Other Demen. 2020, 35, 1533317520976761. [Google Scholar] [CrossRef]
- Wong, J.; Brugger, A.; Khare, A.; Chaubal, M.; Papadopoulos, P.; Rabinow, B.; Kipp, J.; Ning, J. Suspensions for intravenous (I.V.) injection: A review of development, preclinical and clinical aspects. Adv. Drug Deliv. Rev. 2008, 60, 939–954. [Google Scholar] [CrossRef]
- Solans, C.; Esquena, J.; Forgiarini, A.M.; Uson, N.; Morales, D.; Izquierdo, P.P.; Azemar, N.; Celma, M.J.G. Nanoemulsions: Formation, properties and applications. Surfactant Sci. Ser. 2003, 109, 525–554. [Google Scholar]
- Petri, B.; Bootz, A.; Khalansky, A.; Hekmatara, T.; Muller, R.; Uhl, R.; Kreuter, J.; Gelperina, S. Chemotherapy of brain tumour using doxorubicin bound to surfactant-coated poly(butyl cyanoacrylate) nanoparticles: Revisiting the role of surfactants. J. Control Release 2007, 117, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Treguier, M.; Moreau, M.; Sposito, A.; Chapman, M.J.; Huby, T. LDL particle subspecies are distinct in their capacity to mediate free cholesterol efflux via the SR-BI/Cla-1 receptor. Biochim. Biophys. Acta 2007, 1771, 129–138. [Google Scholar] [CrossRef]
- Baranova, I.N.; Vishnyakova, T.G.; Bocharov, A.V.; Kurlander, R.; Chen, Z.; Kimelman, M.L.; Remaley, A.T.; Csako, G.; Thomas, F.; Eggerman, T.L.; et al. Serum amyloid A binding to CLA-1 (CD36 and LIMPII Analogous-1) mediates serum amyloid A protein-induced activation of ERK1/2 and p38 mitogen-activated protein kinases. J. Biol. Chem. 2005, 280, 8031–8040. [Google Scholar] [CrossRef] [PubMed]
- Wasan, K.M.; Brocks, D.R.; Lee, S.D.; Sachs-Barrable, K.; Thornton, S.J. Impact of lipoproteins on the biological activity and disposition of hydrophobic drugs: Implications for drug discovery. Nat. Rev. Drug Discov. 2008, 7, 84–99. [Google Scholar] [CrossRef] [PubMed]
- Out, R.; Kruijt, J.K.; Rensen, P.C.; Hildebrand, R.B.; de Vos, P.; van Eck, M.; Van Berkel, T.J. Scavenger receptor BI plays a role in facilitating chylomicron metabolism. J. Biol. Chem. 2004, 279, 18401–18406. [Google Scholar] [CrossRef]
- Greaves, D.R.; Gordon, S. Recent insights into the biology of macrophage scavenger receptors. J. Lipid Res. 2005, 46, 11–20. [Google Scholar] [CrossRef]
- D’Arrigo, J.S. Biomimetic nanocarrier targeting drug(s) to upstream-receptor mechanisms in dementia: Focusing on linking pathogenic cascades. Biomimetics 2020, 5, 11. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, P.; Feng, J.; Wu, M. Dysfunction of NMDA receptors in Alzheimer’s disease. Neurol. Sci. 2016, 37, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, V.; Giordano, J.; Signorile, A.; Ontario, M.L.; Castorina, S.; De Pasquale, C.; Eckert, G.; Calabrese, E.J. Major pathogenic mechanisms in vascular dementia: Roles of cellular stress response and hormesis in neuroprotection. J. Neurosci. Res. 2016, 94, 1588–1603. [Google Scholar] [CrossRef]
- Daulatzai, M.A. Cerebral hypoperfusion and glucose hypometabolism: Key pathophysiological modulators promote neurodegeneration, cognitive impairment, and Alzheimer’s disease. J. Neurosci. Res. 2016, 95, 943–972. [Google Scholar] [CrossRef]
- Gambini, J. Oxidative stress and inflammation: From mechanisms to therapeutic approaches. Biomedicines 2020, 10, 753. [Google Scholar] [CrossRef]
- D’Arrigo, J.S. Arterial elasticity: Linking of cardiovascular risks, pulse pressure, dementia, aging, and drug targeting. OBM Neurobiol. 2022, 6, 117. [Google Scholar] [CrossRef]
- Khalil, A.; Berrougui, H.; Pawelec, G.; Fulop, T. Impairment of the ABCA1 and SR-BI-mediated cholesterol efflux pathways and HDL anti-inflammatory activity in Alzheimer’s disease. Mech. Ageing Dev. 2012, 133, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Thanopoulou, K.; Fragkouli, A.; Stylianopoulou, F.; Georgopoulos, S. Scavenger receptor class B type I (SR-BI) regulates perivascular macrophages and modifies amyloid pathology in an Alzheimer mouse model. Proc. Natl. Acad. Sci. USA 2010, 107, 20816–20821. [Google Scholar] [CrossRef] [PubMed]
- Fung, K.Y.; Wang, C.; Nyegaard, S.; Heit, B.; Fairn, G.D.; Lee, W.L. SR-BI mediated transcytosis of HDL in brain microvascular endothelial cells is independent of caveolin, clathrin, and PDZK1. Front. Physiol. 2017, 8, 841. [Google Scholar] [CrossRef]
- Lacko, A.G.; Nair, M.; Prokai, L.; McConathy, W.J. Prospects and challenges of the development of lipoprotein-based formulations for anti-cancer drugs. Expert Opin. Drug Deliv. 2007, 4, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Lacko, A.G.; Nair, M.; Paranjape, S.; Mooberry, L.; McConathy, W.J. Trojan horse meets magic bullet to spawn a novel, highly effective drug delivery model. Chemotherapy 2006, 52, 171–173. [Google Scholar] [CrossRef]
- McConathy, W.J.; Nair, M.P.; Paranjape, S.; Mooberry, L.; Lacko, A.G. Evaluation of synthetic/reconstituted high-density lipoproteins as delivery vehicles for paclitaxel. Anticancer Drugs 2008, 19, 183–188. [Google Scholar] [CrossRef]
- Lacko, A.G.; Nair, M.; Paranjape, S.; Johnson, S.; McConathy, W.J. High density lipoprotein complexes as delivery vehicles for anticancer drugs. Anticancer Res. 2002, 22, 2045–2050. [Google Scholar]
- Williams, K.J.; Scanu, A.M. Uptake of endogenous cholesterol by a synthetic lipoprotein. Biochim. Biophys. Acta 1986, 875, 183–194. [Google Scholar] [CrossRef]
- Levine, D.M.; Gordon, B.R.; Parker, T.S.; Rubin, A.L.; Saal, S.D.; Simon, S.R. Reconstituted HDL Particles and Uses Thereof. U.S. Patent 5,128,318, 7 July 1992. [Google Scholar]
- Wang, Y.; Feng, X.; Shen, B.; Ma, J.; Zhao, W. Is vascular amyloidosis intertwined with arterial aging, hypertension and atherosclerosis? Front. Genet. 2017, 8, 126. [Google Scholar]
- D’Arrigo, J.S. Vascular risks, aging, and late-onset dementia: Overlapping etiologies point to ‘scavenger receptor’-mediated therapeutics. OBM Geriatr. 2023, 7, 244. [Google Scholar] [CrossRef]
- D’Arrigo, J.S. Biobased nanoemulsions for targeted drug delivery to treat dementia and aging. Aging Pathobiol. Ther. 2023, 5, 107–111. [Google Scholar] [CrossRef]
- Talwar, P.; Kushwaha, S.; Gupta, R.; Agarwal, R. Systemic immune dyshomeostasis model and pathways in Alzheimer’s disease. Front. Aging Neurosci. 2019, 11, 290. [Google Scholar] [CrossRef] [PubMed]
- Osorio, C.; Kanukuntla, T.; Diaz, E.; Jafri, N.; Cummings, M.; Sfera, A. The post-amyloid era in Alzheimer’s disease: Trust your gut feeling. Front. Aging Neurosci. 2019, 11, 143. [Google Scholar] [CrossRef]
- Guo, J.T.; Yu, J.; Grass, D.; de Beer, F.C.; Kindy, M.S. Inflammation-dependent cerebral deposition of serum amyloid A protein in a mouse model of amyloidosis. J. Neurosci. 2002, 22, 5900–5909. [Google Scholar] [CrossRef] [PubMed]
- Birch, A.M.; Katsouri, L.; Sastre, M. Modulation of inflammation in transgenic models of Alzheimer’s disease. J. Inflamm. 2014, 11, 25. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, D.; Mao, Q.; Xia, H. Role of neuroinflammation in neurodegeneration development. Nature Sig. Trans. Targ. Ther. 2023, 8, 267. [Google Scholar] [CrossRef] [PubMed]
- Mullan, R.H.; McCormick, J.; Connolly, M.; Bresnihan, B.; Veale, D.J.; Fearon, U. A role for the high-density lipoprotein receptor SR-BI in synovial inflammation via serum amyloid-A. Am. J. Pathol. 2010, 176, 1999–2008. [Google Scholar] [CrossRef]
- Erickson, M.A.; Jude, J.; Zhao, H.; Rhea, E.M.; Salameh, T.S.; Jester, W.; Pu, S.; Harrowitz, J.; Nguyen, N.; Banks, W.A.; et al. Serum amyloid A: An ozone induced circulating factor with potentially important functions in the lung-brain axis. FASEB J. 2017, 31, 3950–3965. [Google Scholar] [CrossRef]
- Robert, J.; Stukas, S.; Button, E.; Cheng, W.H.; Lee, M.; Fan, J.; Wilkinson, A.; Kulic, I.; Wright, S.D.; Wellington, C.L. Reconstituted high-density lipoproteins acutely reduce soluble brain Aβ levels in symptomatic APP/PS1 mice. Biochim. Biophys. Acta 2016, 1862, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Barbarese, E.; Ho, S.Y.; D’Arrigo, J.S.; Simon, R.H. Internalization of microbubbles by tumor cells in vivo and in vitro. J. Neurooncol. 1995, 26, 25–34. [Google Scholar] [CrossRef]
- Salentinig, S.; Yaghmur, A.; Guillot, S.; Glatter, O. Preparation of highly concentrated nanostructured dispersions of controlled size. J. Colloid Interface Sci. 2008, 326, 211–220. [Google Scholar] [CrossRef]
- Amar-Yuli, I.; Libster, D.; Aserin, A.; Garti, N. Solubilization of food bioactives within lyotropic liquid crystalline mesophases. Curr. Opin. Colloid Interface Sci. 2009, 14, 21–32. [Google Scholar] [CrossRef]
- Dong, Y.D.; Dong, A.W.; Larson, I.; Rappolt, M.; Amenitsch, H.; Hanley, T.; Boyd, B.J. Impurities in commercial phytantriol significantly alter its lyotropic liquid-crystalline phase behavior. Langmuir 2008, 24, 6998–7003. [Google Scholar] [CrossRef] [PubMed]
- Efrat, R.; Kesselman, E.; Aserin, A.; Garti, N.; Danino, D. Solubilization of hydrophobic guest molecules in the monoolein discontinuous QL cubic mesophase and its soft nanoparticles. Langmuir 2009, 25, 1316–1326. [Google Scholar] [CrossRef]
- Fong, W.K.; Hanley, T.; Boyd, B.J. Stimuli responsive liquid crystals provide ‘on-demand’ drug delivery in vitro and in vivo. J. Control Release 2009, 135, 218–226. [Google Scholar] [CrossRef]
- Dong, A.W.; Pascual-Izarra, C.; Pas, S.J.; Hill, A.J.; Boyd, B.J.; Drummond, C.J. Positron annihilation lifetime spectroscopy (PALS) as a characterization technique for nanostructured self-assembled amphiphile systems. J. Phys. Chem. B 2009, 113, 84–91. [Google Scholar] [CrossRef]
- Yaghmur, A.; Glatter, O. Characterization and potential applications of nanostructured aqueous dispersions. Adv. Colloid Interface Sci. 2009, 147–148, 333–342. [Google Scholar] [CrossRef]
- Boyd, B.J.; Dong, Y.D.; Rades, T. Nonlamellar liquid crystalline nanostructured particles: Advances in materials and structure determination. J. Liposome Res. 2009, 19, 12–28. [Google Scholar] [CrossRef]
- Fong, C.; Wells, D.; Krodkiewska, I.; Booth, J.; Hartley, P.G. Synthesis and mesophases of glycerate surfactants. J. Phys. Chem. B 2007, 111, 1384–1392. [Google Scholar] [CrossRef] [PubMed]
- Moitzi, C.; Guillot, S.; Fritz, G.; Salentinig, S.; Glatter, O. Phase reorganization in self-assembled systems through interparticle material transfer. Adv. Mater. 2007, 19, 1352–1358. [Google Scholar] [CrossRef]
- Boyd, B.J.; Khoo, S.M.; Whittaker, D.V.; Davey, G.; Porter, C.J.H. A lipid-based liquid crystalline matrix that provides sustained release and enhanced oral bioavailability for a model poorly water soluble drug in rats. Int. J. Pharm. 2007, 340, 52–60. [Google Scholar] [CrossRef]
- Amselem, S.; Friedman, D. Solid Fat Nanoemulsions. United States Patent No. 5,662,932, 2 September 1997. [Google Scholar]
- D’Arrigo, J.S. Surfactant Mixtures, Stable Gas-in-Liquid Emulsions, and Methods for the Production of Such Emulsions from Said Mixtures. United States Patent No. 4,684,479, 4 August 1987. [Google Scholar]
- D’Arrigo, J.S. Method for the Production of Medical-Grade Lipid-Coated Microbubbles, Paramagnetic Labeling of Such Microbubbles and Therapeutic Uses of Microbubbles. United States Patent No. 5,215,680, 30 November 1993. [Google Scholar]
- Garg, G.; Saraf, S.; Saraf, S. Cubosomes: An overview. Biol. Pharm. Bull. 2007, 30, 350–353. [Google Scholar] [CrossRef]
- Pouton, C.W. Properties and uses of common formulation lipids, surfactants and cosurfactants. In Proceedings of the AAPSWorkshop—Effective Utilization of Lipid-Based Systems to Enhance the Delivery of Poorly Soluble Drugs: Physicochemical, Biopharmaceutical and Product Development Considerations, North Bethesda, MD, USA, 5–6 March 2007; Constantinides, P.P., Porter, C.J.H., Eds.; AAPS: Arlington, VA, USA, 2007. [Google Scholar]
- Kaasgaard, T.; Drummond, C.J. Ordered 2-D and 3-D nano-structured amphiphile self-assembly materials stable in excess solvent. Phys. Chem. Chem. Phys. 2006, 8, 4957–4975. [Google Scholar] [CrossRef] [PubMed]
- Small, D.M. The behavior of biological lipids. Pure Appl. Chem. 1981, 53, 2095–2103. [Google Scholar] [CrossRef]
- Seddon, J.M.; Robins, J.; Gulik-Krzywicki, T.; Delacroix, H. Inverse micellar phases of phospholipids and glycolipids. Phys. Chem. Chem. Phys. 2000, 2, 4485–4493. [Google Scholar] [CrossRef]
- Luzzati, V.; Vargas, R.; Mariani, P.; Gulik, A.; Delacroix, H. Cubic phases of lipid-containing systems: Elements of a theory and biological connotations. J. Mol. Biol. 1993, 229, 540–551. [Google Scholar] [CrossRef]
- Luzzati, V.; Vargas, R.; Gulik, A.; Mariani, P.; Seddon, J.M.; Rivas, E. Lipid polymorphism: A correction. The structure of the cubic phase of extinction symbol Fd—Consists of two types of disjointed reverse micelles embedded in a three-dimensional hydrocarbon matrix. Biochemistry 1992, 31, 279–285. [Google Scholar] [CrossRef]
- D’Arrigo, J.S. Treating dementia early: Limiting cellular damage in brain tissue. OBM Geriatr. 2019, 3, 19. [Google Scholar] [CrossRef]
- D’Arrigo, J.S. Biomaterial to improve drug delivery in Alzheimer’s disease: Linking major pathogenic pathways. OBM Geriatr. 2020, 4, 10. [Google Scholar] [CrossRef]
- Schwarz, U.S.; Gompper, G. Bending frustration of lipid-water mesophases based on cubic minimal surfaces. Langmuir 2001, 17, 2084–2096. [Google Scholar] [CrossRef]
- Ces, O.; Mulet, X. Physical coupling between lipids and proteins: A paradigm for cellular control. Signal Transduct. 2006, 6, 112–132. [Google Scholar] [CrossRef]
- Pouzot, M.; Mezzenga, R.; Leser, M.; Sagalowicz, L.; Guillot, S.; Glatter, O. Structural and rheological investigation of Fd3m inverse micellar cubic phases. Langmuir 2007, 23, 9618–9628. [Google Scholar] [CrossRef]
- Seddon, J.M.; Zeb, N.; Templer, R.H.; McElhaney, R.N.; Mannock, D.A. An Fd3m lyotropic cubic phase in a binary glycolipid/water system. Langmuir 1996, 12, 5250–5253. [Google Scholar] [CrossRef]
- D’Arrigo, J.S. Targeting early dementia: Using lipid cubic phase nanocarriers to cross the blood-brain barrier. Biomimetics 2018, 3, 4. [Google Scholar] [CrossRef] [PubMed]
- Seddon, J.M.; Templer, R.H.; Warrender, N.A.; Huang, G.; Cevc, G.; Marsh, D. Phosphatidylcholine-fatty acid membranes: Effects of headgroup hydration on the phase behaviour and structural parameters of the gel and inverse hexagonal (HII) phases. Biochim. Biophys. Acta 1997, 1327, 131–147. [Google Scholar] [CrossRef]
- Gonzales, M.M.; Garbarino, V.R.; Pollet, E.; Palavicini, J.P.; Kellogg, D.L.; Kraig, E.; Orr, M.E. Biological aging processes underlying cognitive decline and neurodegenerative disease. J. Clin. Investig. 2022, 132, e158453. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, H.J. Normal aging induces changes in the brain and neurodegeneration progress: Review of the structural, biochemical, metabolic, cellular, and molecular changes. Front. Aging Neurosci. 2022, 14, 931536. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, H.G.; Hendrix, C.J.; Murcia, J.D.G.; Haynie, C.; Weber, K.S. The role of atypical chemokine receptors in neuroinflammation and neurodegenerative disorders. Int. J. Mol. Sci. 2023, 24, 16493. [Google Scholar] [CrossRef] [PubMed]
- Hartigh, L.J.D.; May, K.S.; Zhang, X.-S.; Chait, A.; Blaser, M.J. Serum amyloid A and metabolic disease: Evidence for a critical role in chronic inflammatory conditions. Front. Cardiovasc. Med. 2023, 10, 1197432. [Google Scholar] [CrossRef] [PubMed]
- Weaver, D.F. Thirty risk factors for Alzheimer’s disease unified by a common neuroimmune-neuroinflammation mechanism. Brain Sci. 2024, 14, 41. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Arrigo, J.S. Overlapping Receptor-Based Pathogenic Cascades in Degenerative Disease: Implications Ranging from Tumor Targeting to Aging and Dementia Therapeutics. Int. J. Transl. Med. 2024, 4, 152-162. https://doi.org/10.3390/ijtm4010008
D’Arrigo JS. Overlapping Receptor-Based Pathogenic Cascades in Degenerative Disease: Implications Ranging from Tumor Targeting to Aging and Dementia Therapeutics. International Journal of Translational Medicine. 2024; 4(1):152-162. https://doi.org/10.3390/ijtm4010008
Chicago/Turabian StyleD’Arrigo, Joseph S. 2024. "Overlapping Receptor-Based Pathogenic Cascades in Degenerative Disease: Implications Ranging from Tumor Targeting to Aging and Dementia Therapeutics" International Journal of Translational Medicine 4, no. 1: 152-162. https://doi.org/10.3390/ijtm4010008
APA StyleD’Arrigo, J. S. (2024). Overlapping Receptor-Based Pathogenic Cascades in Degenerative Disease: Implications Ranging from Tumor Targeting to Aging and Dementia Therapeutics. International Journal of Translational Medicine, 4(1), 152-162. https://doi.org/10.3390/ijtm4010008





