Advancements Exploring Major Depressive Disorder: Insights on Oxidative Stress, Serotonin Metabolism, BDNF, HPA Axis Dysfunction, and Pharmacotherapy Advances
Abstract
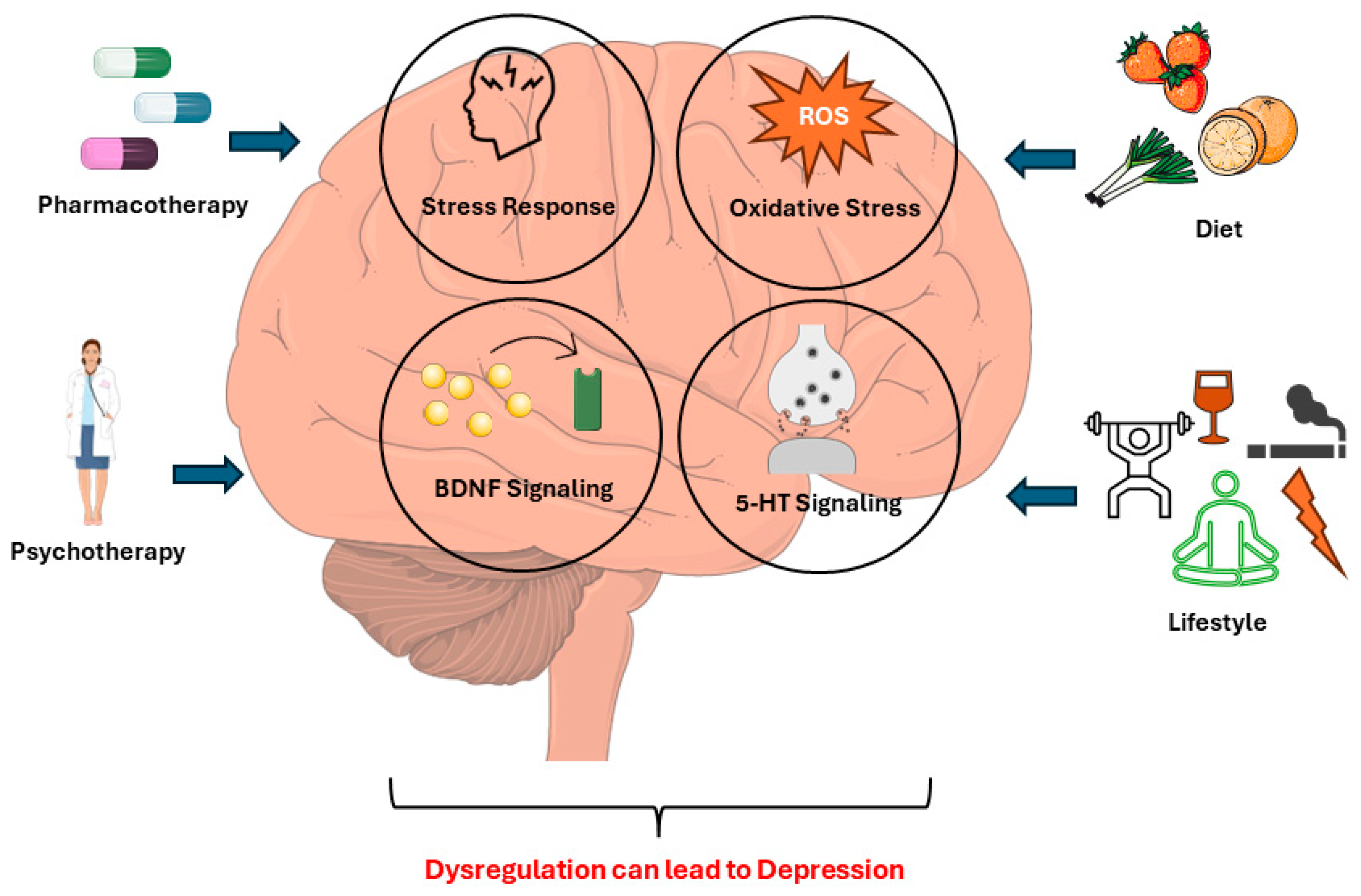
1. Background
2. Exploring the Role of Oxidative Stress, Tryptophan-Serotonin Metabolism, Brain-Derived Neurotrophic Factor, and Hypothalamic–Pituitary–Adrenal Axis Dysfunction in Major Depressive Disorder
2.1. Oxidative Stress as a Key Contributor to Major Depressive Disorder Pathogenesis
2.2. Unraveling the Hypothalamic–Pituitary–Adrenal Axis Dysregulation in Major Depressive Disorder
2.3. Exploring Tryptophan/Serotonin’s Role in Major Depressive Disorder
2.4. Exploring Brain-Derived Neurotrophic Factor in Major Depressive Disorder
| Feature | Explanation |
|---|---|
| Levels of BDNF | Reduced BDNF levels have been observed in individuals with MDD [118]. |
| Structure and function | Deficiencies or imbalances in BDNF levels contribute to depression by promoting structural and functioning changes [131]. |
| 5-HT | BDNF is affected by 5-HT, and 5-HT stimulation can increase BDNF production and release. 5-HT receptors can also control BDNF production, which influences neuronal function and, consequently, mood modulation [132]. |
| Neuroplasticity | BDNF has a role in neuroplasticity, which is essential for synaptic connections and structural changes in the brain connected to MDD [133]. |
| Oxidative stress | Oxidative stress can impair BDNF production and signaling pathways. The link between oxidative stress and BDNF levels is critical in the development and progression of depression [15]. |
| HPA axis dysregulation | Stress-induced HPA axis hyperactivity and the subsequent increase in glucocorticoid levels diminish BDNF expression, which plays an important role in the development of depression [134]. |
3. Pharmacological Interventions in the Management of Major Depressive Disorder
3.1. An Overlook of Pharmacotherapy for Major Depressive Disorder
3.2. Antidepressant Breakthroughs: Advancements in Pharmacotherapy for Depression
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Depressive Disorder (Depression). Available online: https://www.who.int/news-room/fact-sheets/detail/depression (accessed on 28 June 2023).
- Otte, C.; Gold, S.M.; Penninx, B.W.; Pariante, C.M.; Etkin, A.; Fava, M.; Mohr, D.C.; Schatzberg, A.F. Major depressive disorder. Nat. Rev. Dis. Prim. 2016, 2, 16065. [Google Scholar] [CrossRef]
- Kennedy, S.H. Core symptoms of major depressive disorder: Relevance to diagnosis and treatment. Dialogues Clin. Neurosci. 2008, 10, 271–277. [Google Scholar] [CrossRef]
- Major depressive disorder. Nat. Rev. Dis. Prim. 2023, 9, 45. [CrossRef]
- Cuijpers, P. The Challenges of Improving Treatments for Depression. JAMA 2018, 320, 2529. [Google Scholar] [CrossRef] [PubMed]
- Maj, M.; Stein, D.J.; Parker, G.; Zimmerman, M.; Fava, G.A.; De Hert, M.; Demyttenaere, K.; McIntyre, R.S.; Widiger, T.; Wittchen, H. The clinical characterization of the adult patient with depression aimed at personalization of management. World Psychiatry 2020, 19, 269–293. [Google Scholar] [CrossRef]
- Jesulola, E.; Micalos, P.; Baguley, I.J. Understanding the pathophysiology of depression: From monoamines to the neurogenesis hypothesis model—Are we there yet? Behav. Brain Res. 2018, 341, 79–90. [Google Scholar] [CrossRef]
- Burtscher, J.; Niedermeier, M.; Hüfner, K.; van den Burg, E.; Kopp, M.; Stoop, R.; Burtscher, M.; Gatterer, H.; Millet, G.P. The interplay of hypoxic and mental stress: Implications for anxiety and depressive disorders. Neurosci. Biobehav. Rev. 2022, 138, 104718. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Peng, W.; Sweeney, J.A.; Jia, Z.; Gong, Q. Brain structure alterations in depression: Psychoradiological evidence. CNS Neurosci. Ther. 2018, 24, 994–1003. [Google Scholar] [CrossRef]
- Gradin, V.B.; Pomi, A. The Role of Hippocampal Atrophy in Depression: A Neurocomputational Approach. J. Biol. Phys. 2008, 34, 107–120. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Videbech, P. Hippocampal Volume and Depression: A Meta-Analysis of MRI Studies. Am. J. Psychiatry 2004, 161, 1957–1966. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Carraro, E.; Schilirò, T.; Biorci, F.; Romanazzi, V.; Degan, R.; Buonocore, D.; Verri, M.; Dossena, M.; Bonetta, S.; Gilli, G. Physical Activity, Lifestyle Factors and Oxidative Stress in Middle Age Healthy Subjects. Int. J. Environ. Res. Public Health 2018, 15, 1152. [Google Scholar] [CrossRef] [PubMed]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef]
- Correia, A.S.; Cardoso, A.; Vale, N. Oxidative Stress in Depression: The Link with the Stress Response, Neuroinflammation, Serotonin, Neurogenesis and Synaptic Plasticity. Antioxidants 2023, 12, 470. [Google Scholar] [CrossRef]
- Sies, H. Oxidative Stress: Concept and Some Practical Aspects. Antioxidants 2020, 9, 852. [Google Scholar] [CrossRef]
- Deponte, M. Glutathione catalysis and the reaction mechanisms of glutathione-dependent enzymes. Biochim. Biophys. Acta-Gen. Subj. 2013, 1830, 3217–3266. [Google Scholar] [CrossRef]
- Sindhu, R.K.; Kaur, P.; Kaur, P.; Singh, H.; Batiha, G.E.S.; Verma, I. Exploring multifunctional antioxidants as potential agents for management of neurological disorders. Environ. Sci. Pollut. Res. Int. 2022, 29, 24458–24477. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Wang, Z.-Z.; Zhao, M.; Zhang, Y.; Chen, N.-H. Role of non-coding RNA in the pathogenesis of depression. Gene 2020, 735, 144276. [Google Scholar] [CrossRef]
- Bhatt, S.; Nagappa, A.N.; Patil, C.R. Role of oxidative stress in depression. Drug Discov. Today 2020, 25, 1270–1276. [Google Scholar] [CrossRef]
- Ji, N.; Lei, M.; Chen, Y.; Tian, S.; Li, C.; Zhang, B. How Oxidative Stress Induces Depression? ASN Neuro 2023, 15. [Google Scholar] [CrossRef]
- Ferriani, L.O.; Silva, D.A.; Molina, M.d.C.B.; Mill, J.G.; Brunoni, A.R.; da Fonseca, M.d.J.M.; Moreno, A.B.; Benseñor, I.M.; de Aguiar, O.B.; Barreto, S.M.; et al. Associations of depression and intake of antioxidants and vitamin B complex: Results of the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). J. Affect. Disord. 2022, 297, 259–268. [Google Scholar] [CrossRef]
- Lanctot, K.L.; Mazereeuw, G.; Herrmann, N.; Andreazza, A.; Khan, M. A meta-analysis of lipid peroxidation markers in major depression. Neuropsychiatr. Dis. Treat. 2015, 11, 2479–2491. [Google Scholar] [CrossRef]
- Yager, S.; Forlenza, M.J.; Miller, G.E. Depression and oxidative damage to lipids. Psychoneuroendocrinology 2010, 35, 1356–1362. [Google Scholar] [CrossRef]
- Camkurt, M.A.; Fındıklı, E.; İzci, F.; Kurutaş, E.B.; Tuman, T.C. Evaluation of malondialdehyde, superoxide dismutase and catalase activity and their diagnostic value in drug naïve, first episode, non-smoker major depression patients and healthy controls. Psychiatry Res. 2016, 238, 81–85. [Google Scholar] [CrossRef] [PubMed]
- McGarry, T.; Biniecka, M.; Veale, D.J.; Fearon, U. Hypoxia, oxidative stress and inflammation. Free Radic. Biol. Med. 2018, 125, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.; Rocha, R.; Correia, A.S.; Mota, B.; Madeira, M.D.; Vale, N.; Cardoso, A. Repurposed Edaravone, Metformin, and Perampanel as a Potential Treatment for Hypoxia–Ischemia Encephalopathy: An In Vitro Study. Biomedicines 2022, 10, 3043. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhong, S.; Liao, X.; Chen, J.; He, T.; Lai, S.; Jia, Y. A Meta-Analysis of Oxidative Stress Markers in Depression. PLoS ONE 2015, 10, e0138904. [Google Scholar] [CrossRef] [PubMed]
- Khanzode, S.D.; Dakhale, G.N.; Khanzode, S.S.; Saoji, A.; Palasodkar, R. Oxidative damage and major depression: The potential antioxidant action of selective serotonin re-uptake inhibitors. Redox Rep. 2003, 8, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Correia, A.S.; Fraga, S.; Teixeira, J.P.; Vale, N. Cell Model of Depression: Reduction of Cell Stress with Mirtazapine. Int. J. Mol. Sci. 2022, 23, 4942. [Google Scholar] [CrossRef] [PubMed]
- Correia, A.S.; Cardoso, A.; Vale, N. Significant Differences in the Reversal of Cellular Stress Induced by Hydrogen Peroxide and Corticosterone by the Application of Mirtazapine or L-Tryptophan. Int. J. Transl. Med. 2022, 2, 482–505. [Google Scholar] [CrossRef]
- DeMorrow, S. Role of the Hypothalamic–Pituitary–Adrenal Axis in Health and Disease. Int. J. Mol. Sci. 2018, 19, 986. [Google Scholar] [CrossRef]
- Frigerio, F. The HPA Axis and the Regulation of Energy Balance. In Cellular Physiology and Metabolism of Physical Exercise; Luzi, L., Ed.; Springer: Milan, Italy, 2012; pp. 109–121. ISBN 978-88-470-2418-2. [Google Scholar]
- Smith, S.M.; Vale, W.W. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin. Neurosci. 2006, 8, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Hemmerle, A.M.; Herman, J.P.; Seroogy, K.B. Stress, depression and Parkinson’s disease. Exp. Neurol. 2012, 233, 79–86. [Google Scholar] [CrossRef]
- Su, Y.A.; Lin, J.Y.; Liu, Q.; Lv, X.Z.; Wang, G.; Wei, J.; Zhu, G.; Chen, Q.L.; Tian, H.J.; Zhang, K.R.; et al. Associations among serum markers of inflammation, life stress and suicide risk in patients with major depressive disorder. J. Psychiatr. Res. 2020, 129, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Dziurkowska, E.; Wesolowski, M. Cortisol as a Biomarker of Mental Disorder Severity. J. Clin. Med. 2021, 10, 5204. [Google Scholar] [CrossRef]
- Russell, G.; Lightman, S. The human stress response. Nat. Rev. Endocrinol. 2019, 15, 525–534. [Google Scholar] [CrossRef]
- Vaz, R.P.; Cardoso, A.; Serrão, P.; Pereira, P.A.; Madeira, M.D. Chronic stress leads to long-lasting deficits in olfactory-guided behaviors, and to neuroplastic changes in the nucleus of the lateral olfactory tract. Horm. Behav. 2018, 98, 130–144. [Google Scholar] [CrossRef] [PubMed]
- Law, R.; Clow, A. Stress, the cortisol awakening response and cognitive function. Int. Rev. Neurobiol. 2020, 150, 187–217. [Google Scholar]
- McEwen, B.S. Physiology and Neurobiology of Stress and Adaptation: Central Role of the Brain. Physiol. Rev. 2007, 87, 873–904. [Google Scholar] [CrossRef]
- de Kloet, E.R.; Vreugdenhil, E.; Oitzl, M.S.; Joëls, M. Brain Corticosteroid Receptor Balance in Health and Disease. Endocr. Rev. 1998, 19, 269–301. [Google Scholar] [CrossRef]
- Dallman, M.F. Fast glucocorticoid actions on brain: Back to the future. Front. Neuroendocrinol. 2005, 26, 103–108. [Google Scholar] [CrossRef]
- Groeneweg, F.L.; Karst, H.; de Kloet, E.R.; Joëls, M. Rapid non-genomic effects of corticosteroids and their role in the central stress response. J. Endocrinol. 2011, 209, 153–167. [Google Scholar] [CrossRef]
- Pariante, C.M.; Lightman, S.L. The HPA axis in major depression: Classical theories and new developments. Trends Neurosci. 2008, 31, 464–468. [Google Scholar] [CrossRef]
- Pariante, C.M. Why are depressed patients inflamed? A reflection on 20 years of research on depression, glucocorticoid resistance and inflammation. Eur. Neuropsychopharmacol. 2017, 27, 554–559. [Google Scholar] [CrossRef]
- Menke, A. Is the HPA axis as target for depression outdated, or is there a new hope? Front. Psychiatry 2019, 10, 101. [Google Scholar] [CrossRef]
- Anacker, C.; Zunszain, P.A.; Carvalho, L.A.; Pariante, C.M. The glucocorticoid receptor: Pivot of depression and of antidepressant treatment? Psychoneuroendocrinology 2011, 36, 415–425. [Google Scholar] [CrossRef]
- Leonard, B.E. The concept of depression as a dysfunction of the immune system. In Depression: From Psychopathology to Pharmacotherapy; S. Karger AG: Basel, Switzerland, 2010; Volume 27, pp. 53–71. ISBN 9783805596060. [Google Scholar]
- Belleau, E.L.; Treadway, M.T.; Pizzagalli, D.A. The Impact of Stress and Major Depressive Disorder on Hippocampal and Medial Prefrontal Cortex Morphology. Biol. Psychiatry 2019, 85, 443–453. [Google Scholar] [CrossRef]
- Song, Y.; Miyaki, K.; Suzuki, T.; Sasaki, Y.; Tsutsumi, A.; Kawakami, N.; Shimazu, A.; Takahashi, M.; Inoue, A.; Kan, C.; et al. Altered DNA methylation status of human brain derived neurotrophis factor gene could be useful as biomarker of depression. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2014, 165, 357–364. [Google Scholar] [CrossRef]
- Sapolsky, R.; Krey, L.; McEwen, B. Prolonged glucocorticoid exposure reduces hippocampal neuron number: Implications for aging. J. Neurosci. 1985, 5, 1222–1227. [Google Scholar] [CrossRef]
- Tian, L.; Hui, C.W.; Bisht, K.; Tan, Y.; Sharma, K.; Chen, S.; Zhang, X.; Tremblay, M.-E. Microglia under psychosocial stressors along the aging trajectory: Consequences on neuronal circuits, behavior, and brain diseases. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2017, 79, 27–39. [Google Scholar] [CrossRef]
- Banasr, M.; Dwyer, J.M.; Duman, R.S. Cell atrophy and loss in depression: Reversal by antidepressant treatment. Curr. Opin. Cell Biol. 2011, 23, 730–737. [Google Scholar] [CrossRef]
- Ait Tayeb, A.E.K.; Poinsignon, V.; Chappell, K.; Bouligand, J.; Becquemont, L.; Verstuyft, C. Major Depressive Disorder and Oxidative Stress: A Review of Peripheral and Genetic Biomarkers According to Clinical Characteristics and Disease Stages. Antioxidants 2023, 12, 942. [Google Scholar] [CrossRef]
- Trifunovic, S.; Stevanovic, I.; Milosevic, A.; Ristic, N.; Janjic, M.; Bjelobaba, I.; Savic, D.; Bozic, I.; Jakovljevic, M.; Tesovic, K.; et al. The Function of the Hypothalamic–Pituitary–Adrenal Axis during Experimental Autoimmune Encephalomyelitis: Involvement of Oxidative Stress Mediators. Front. Neurosci. 2021, 15, 649485. [Google Scholar] [CrossRef]
- Du, J.; Wang, Y.; Hunter, R.; Wei, Y.; Blumenthal, R.; Falke, C.; Khairova, R.; Zhou, R.; Yuan, P.; Machado-Vieira, R.; et al. Dynamic regulation of mitochondrial function by glucocorticoids. Proc. Natl. Acad. Sci. USA 2009, 106, 3543–3548. [Google Scholar] [CrossRef]
- Spiers, J.G.; Chen, H.-J.C.; Sernia, C.; Lavidis, N.A. Activation of the hypothalamic-pituitary-adrenal stress axis induces cellular oxidative stress. Front. Neurosci. 2015, 8, 456. [Google Scholar] [CrossRef]
- Correia, A.S.; Cardoso, A.; Vale, N. Highlighting Immune System and Stress in Major Depressive Disorder, Parkinson’s, and Alzheimer’s Diseases, with a Connection with Serotonin. Int. J. Mol. Sci. 2021, 22, 8525. [Google Scholar] [CrossRef]
- Amasi-Hartoonian, N.; Sforzini, L.; Cattaneo, A.; Pariante, C.M. Cause or consequence? Understanding the role of cortisol in the increased inflammation observed in depression. Curr. Opin. Endocr. Metab. Res. 2022, 24, 100356. [Google Scholar] [CrossRef]
- Weber, M.D.; Godbout, J.P.; Sheridan, J.F. Repeated Social Defeat, Neuroinflammation, and Behavior: Monocytes Carry the Signal. Neuropsychopharmacology 2017, 42, 46–61. [Google Scholar] [CrossRef]
- Horowitz, M.A.; Zunszain, P.A.; Anacker, C.; Musaelyan, K.; Pariante, C.M. Glucocorticoids and Inflammation: A Double-Headed Sword in Depression? In Inflammation in Psychiatry; Halaris, A., Leonard, B.E., Eds.; S. Karger AG: Basel, Switzerland, 2013; Volume 28, pp. 127–143. ISBN 978-3-318-02310-7. [Google Scholar]
- Houwing, D.J.; Buwalda, B.; van der Zee, E.A.; de Boer, S.F.; Olivier, J.D.A. The Serotonin Transporter and Early Life Stress: Translational Perspectives. Front. Cell. Neurosci. 2017, 11, 117. [Google Scholar] [CrossRef]
- Dantzer, R. Cytokine, Sickness Behavior, and Depression. Immunol. Allergy Clin. N. Am. 2009, 29, 247–264. [Google Scholar] [CrossRef]
- Qing, H.; Desrouleaux, R.; Israni-Winger, K.; Mineur, Y.S.; Fogelman, N.; Zhang, C.; Rashed, S.; Palm, N.W.; Sinha, R.; Picciotto, M.R.; et al. Origin and Function of Stress-Induced IL-6 in Murine Models. Cell 2020, 182, 372–387.e14. [Google Scholar] [CrossRef]
- Goshen, I.; Yirmiya, R. Interleukin-1 (IL-1): A central regulator of stress responses. Front. Neuroendocrinol. 2009, 30, 30–45. [Google Scholar] [CrossRef]
- Ting, E.Y.-C.; Yang, A.C.; Tsai, S.-J. Role of Interleukin-6 in Depressive Disorder. Int. J. Mol. Sci. 2020, 21, 2194. [Google Scholar] [CrossRef]
- Platten, M.; Nollen, E.A.A.; Röhrig, U.F.; Fallarino, F.; Opitz, C.A. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat. Rev. Drug Discov. 2019, 18, 379–401. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.; Gray, J.A.; Roth, B.L. The Expanded Biology of Serotonin. Annu. Rev. Med. 2009, 60, 355–366. [Google Scholar] [CrossRef]
- Comai, S.; Bertazzo, A.; Brughera, M.; Crotti, S. Tryptophan in health and disease. Adv. Clin. Chem. 2020, 95, 165–218. [Google Scholar]
- Hsu, C.-N.; Tain, Y.-L. Developmental Programming and Reprogramming of Hypertension and Kidney Disease: Impact of Tryptophan Metabolism. Int. J. Mol. Sci. 2020, 21, 8705. [Google Scholar] [CrossRef]
- Höglund, E.; Øverli, Ø.; Winberg, S. Tryptophan Metabolic Pathways and Brain Serotonergic Activity: A Comparative Review. Front. Endocrinol. 2019, 10, 158. [Google Scholar] [CrossRef]
- Correia, A.S.; Vale, N. Tryptophan Metabolism in Depression: A Narrative Review with a Focus on Serotonin and Kynurenine Pathways. Int. J. Mol. Sci. 2022, 23, 8493. [Google Scholar] [CrossRef] [PubMed]
- Correia, A.S.; Silva, I.; Reguengo, H.; Oliveira, J.C.; Vasques-Nóvoa, F.; Cardoso, A.; Vale, N. The Effect of the Stress Induced by Hydrogen Peroxide and Corticosterone on Tryptophan Metabolism, Using Human Neuroblastoma Cell Line (SH-SY5Y). Int. J. Mol. Sci. 2023, 24, 4389. [Google Scholar] [CrossRef]
- Mohammad-Zadeh, L.F.; Moses, L.; Gwaltney-Brant, S.M. Serotonin: A review. J. Vet. Pharmacol. Ther. 2008, 31, 187–199. [Google Scholar] [CrossRef]
- Marin, P.; Bécamel, C.; Chaumont-Dubel, S.; Vandermoere, F.; Bockaert, J.; Claeysen, S. Chapter 5—Classification and signaling characteristics of 5-HT receptors: Toward the concept of 5-HT receptosomes. Handb. Behav. Neurosci. 2020, 31, 91–120. [Google Scholar]
- Pytliak, M.; Vargová, V.; Mechírová, V.; Felšöci, M. Serotonin Receptors—From Molecular Biology to Clinical Applications. Physiol. Res. 2011, 60, 15–25. [Google Scholar] [CrossRef]
- Overview—Antidepressants—NHS. Available online: https://www.nhs.uk/mental-health/talking-therapies-medicine-treatments/medicines-and-psychiatry/antidepressants/overview/ (accessed on 11 October 2023).
- Cowen, P.J.; Browning, M. What has serotonin to do with depression? World Psychiatry 2015, 14, 158–160. [Google Scholar] [CrossRef]
- Amidfar, M.; Colic, L.; Walter, M.; Kim, Y.-K. Complex Role of the Serotonin Receptors in Depression: Implications for Treatment BT. In Understanding Depression: Volume 1. Biomedical and Neurobiological Background; Kim, Y.-K., Ed.; Springer: Singapore, 2018; pp. 83–95. ISBN 978-981-10-6580-4. [Google Scholar]
- Nautiyal, K.M.; Hen, R. Serotonin receptors in depression: From A to B. F1000Research 2017, 6, 123. [Google Scholar] [CrossRef]
- Correia, A.S.; Silva, I.; Oliveira, J.C.; Reguengo, H.; Vale, N. Serotonin Type 3 Receptor Is Potentially Involved in Cellular Stress Induced by Hydrogen Peroxide. Life 2022, 12, 1645. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.L.W.; Harmer, C.J.; Cowen, P.J.; Murphy, S.E. The Serotonin 1A (5-HT1A) Receptor as a Pharmacological Target in Depression. CNS Drugs 2023, 37, 571–585. [Google Scholar] [CrossRef] [PubMed]
- Yohn, C.N.; Gergues, M.M.; Samuels, B.A. The role of 5-HT receptors in depression. Mol. Brain 2017, 10, 28. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Stackman, R.W., Jr. The role of serotonin 5-HT 2A receptors in memory and cognition. Front. Pharmacol. 2015, 6, 225. [Google Scholar] [CrossRef]
- Bhatt, S.; Devadoss, T.; Manjula, S.N.; Rajangam, J. 5-HT3 Receptor Antagonism: A Potential Therapeutic Approach for the Treatment of Depression and other Disorders. Curr. Neuropharmacol. 2021, 19, 1545–1559. [Google Scholar] [CrossRef]
- Albert, P.; Le François, B. Modifying 5-HT1A Receptor Gene Expression as a New Target for Antidepressant Therapy. Front. Neurosci. 2010, 4, 35. [Google Scholar] [CrossRef]
- Kaufman, J.; DeLorenzo, C.; Choudhury, S.; Parsey, R. V The 5-HT1A receptor in Major Depressive Disorder. Eur. Neuropsychopharmacol. 2016, 26, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Guiard, B.P.; Giovanni, G. Di Central serotonin-2A (5-HT2A) receptor dysfunction in depression and epilepsy: The missing link? Front. Pharmacol. 2015, 6, 46. [Google Scholar] [CrossRef]
- Göőz, M.; Göőz, P.; Luttrell, L.M.; Raymond, J.R. 5-HT2A Receptor Induces ERK Phosphorylation and Proliferation through ADAM-17 Tumor Necrosis Factor-α-converting Enzyme (TACE) Activation and Heparin-bound Epidermal Growth Factor-like Growth Factor (HB-EGF) Shedding in Mesangial Cells. J. Biol. Chem. 2006, 281, 21004–21012. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Q.; Mao, L. The ERK Pathway: Molecular Mechanisms and Treatment of Depression. Mol. Neurobiol. 2019, 56, 6197–6205. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.K.; Dwivedi-Agnihotri, H. Structure and function of β-arrestins, their emerging role in breast cancer, and potential opportunities for therapeutic manipulation. Adv. Cancer Res. 2020, 145, 139–156. [Google Scholar] [PubMed]
- Schmid, C.L.; Raehal, K.M.; Bohn, L.M. Agonist-directed signaling of the serotonin 2A receptor depends on β-arrestin-2 interactions in vivo. Proc. Natl. Acad. Sci. USA 2008, 105, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.; Roth, B.L. Arresting serotonin. Proc. Natl. Acad. Sci. USA 2008, 105, 831–832. [Google Scholar] [CrossRef] [PubMed]
- Felsing, D.E.; Nilson, A.; Jain, M.; Raval, S.; Inoue, A.; Allen, J. β-arrestins mediate rapid 5-HT2A receptor endocytosis to regulate intensity and duration of signaling. FASEB J. 2019, 33, 502.3. [Google Scholar] [CrossRef]
- Peters, J.A.; Lambert, J.J. Electrophysiology of 5-HT3 receptors in neuronal cell lines. Trends Pharmacol. Sci. 1989, 10, 172–175. [Google Scholar] [CrossRef]
- Bétry, C.; Etiévant, A.; Oosterhof, C.; Ebert, B.; Sanchez, C.; Haddjeri, N. Role of 5-HT 3 Receptors in the Antidepressant Response. Pharmaceuticals 2011, 4, 603–629. [Google Scholar] [CrossRef]
- van Hooft, J. 5-HT3 receptors and neurotransmitter release in the CNS: A nerve ending story? Trends Neurosci. 2000, 23, 605–610. [Google Scholar] [CrossRef]
- Kawamoto, E.M.; Vivar, C.; Camandola, S. Physiology and Pathology of Calcium Signaling in the Brain. Front. Pharmacol. 2012, 3, 61. [Google Scholar] [CrossRef]
- Moncrieff, J.; Cooper, R.E.; Stockmann, T.; Amendola, S.; Hengartner, M.P.; Horowitz, M.A. The serotonin theory of depression: A systematic umbrella review of the evidence. Mol. Psychiatry 2022, 28, 3243–3256. [Google Scholar] [CrossRef]
- Jauhar, S.; Arnone, D.; Baldwin, D.S.; Bloomfield, M.; Browning, M.; Cleare, A.J.; Corlett, P.; Deakin, J.F.W.; Erritzoe, D.; Fu, C.; et al. A leaky umbrella has little value: Evidence clearly indicates the serotonin system is implicated in depression. Mol. Psychiatry 2023, 28, 3149–3152. [Google Scholar] [CrossRef] [PubMed]
- Jauhar, S.; Cowen, P.J.; Browning, M. Fifty years on: Serotonin and depression. J. Psychopharmacol. 2023, 37, 237–241. [Google Scholar] [CrossRef]
- Ruhé, H.G.; Mason, N.S.; Schene, A.H. Mood is indirectly related to serotonin, norepinephrine and dopamine levels in humans: A meta-analysis of monoamine depletion studies. Mol. Psychiatry 2007, 12, 331–359. [Google Scholar] [CrossRef] [PubMed]
- Schirò, G.; Iacono, S.; Ragonese, P.; Aridon, P.; Salemi, G.; Balistreri, C.R. A Brief Overview on BDNF-Trk Pathway in the Nervous System: A Potential Biomarker or Possible Target in Treatment of Multiple Sclerosis? Front. Neurol. 2022, 13, 917527. [Google Scholar] [CrossRef]
- Correia, A.S.; Cardoso, A.; Vale, N. BDNF Unveiled: Exploring Its Role in Major Depression Disorder Serotonergic Imbalance and Associated Stress Conditions. Pharmaceutics 2023, 15, 2081. [Google Scholar] [CrossRef]
- Rentería, I.; García-Suárez, P.C.; Fry, A.C.; Moncada-Jiménez, J.; Machado-Parra, J.P.; Antunes, B.M.; Jiménez-Maldonado, A. The Molecular Effects of BDNF Synthesis on Skeletal Muscle: A Mini-Review. Front. Physiol. 2022, 13, 1345. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Ren, Q.; Zhang, J.C.; Chen, Q.X.; Hashimoto, K. Altered expression of BDNF, BDNF pro-peptide and their precursor proBDNF in brain and liver tissues from psychiatric disorders: Rethinking the brain–liver axis. Transl. Psychiatry 2017, 7, e1128. [Google Scholar] [CrossRef]
- Brigadski, T.; Leßmann, V. BDNF: A regulator of learning and memory processes with clinical potential. e-Neuroforum 2014, 5, 1–11. [Google Scholar] [CrossRef]
- Wang, C.S.; Kavalali, E.T.; Monteggia, L.M. BDNF signaling in context: From synaptic regulation to psychiatric disorders. Cell 2022, 185, 62–76. [Google Scholar] [CrossRef]
- Pruunsild, P.; Kazantseva, A.; Aid, T.; Palm, K.; Timmusk, T. Dissecting the human BDNF locus: Bidirectional transcription, complex splicing, and multiple promoters. Genomics 2007, 90, 397–406. [Google Scholar] [CrossRef]
- Nair, B.; Wong-Riley, M.T.T. Transcriptional Regulation of Brain-derived Neurotrophic Factor Coding Exon IX. J. Biol. Chem. 2016, 291, 22583–22593. [Google Scholar] [CrossRef]
- Pathak, H.; Borchert, A.; Garaali, S.; Burkert, A.; Frieling, H. BDNF exon IV promoter methylation and antidepressant action: A complex interplay. Clin. Epigenetics 2022, 14, 187. [Google Scholar] [CrossRef]
- Oh, H.; Lewis, D.A.; Sibille, E. The Role of BDNF in Age-Dependent Changes of Excitatory and Inhibitory Synaptic Markers in the Human Prefrontal Cortex. Neuropsychopharmacology 2016, 41, 3080–3091. [Google Scholar] [CrossRef]
- Bathina, S.; Das, U.N. Brain-derived neurotrophic factor and its clinical implications. Arch. Med. Sci. 2015, 11, 1164–1178. [Google Scholar] [CrossRef]
- Cohen-Cory, S.; Kidane, A.H.; Shirkey, N.J.; Marshak, S. Brain-derived neurotrophic factor and the development of structural neuronal connectivity. Dev. Neurobiol. 2010, 70, 271–288. [Google Scholar] [CrossRef]
- Miranda, M.; Morici, J.F.; Zanoni, M.B.; Bekinschtein, P. Brain-Derived Neurotrophic Factor: A Key Molecule for Memory in the Healthy and the Pathological Brain. Front. Cell. Neurosci. 2019, 13, 363. [Google Scholar] [CrossRef]
- Paduchová, Z.; Katrenčíková, B.; Vaváková, M.; Laubertová, L.; Nagyová, Z.; Garaiova, I.; Zdenkǎduračková, Z.Z.; Trebatická, J. The Effect of Omega-3 Fatty Acids on Thromboxane, Brain-Derived Neurotrophic Factor, Homocysteine, and Vitamin D in Depressive Children and Adolescents: Randomized Controlled Trial. Nutrients 2021, 13, 1095. [Google Scholar] [CrossRef]
- Martinowich, K.; Manji, H.; Lu, B. New insights into BDNF function in depression and anxiety. Nat. Neurosci. 2007, 10, 1089–1093. [Google Scholar] [CrossRef]
- Azman, K.F.; Zakaria, R. Recent Advances on the Role of Brain-Derived Neurotrophic Factor (BDNF) in Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 6827. [Google Scholar] [CrossRef]
- Erickson, K.I.; Voss, M.W.; Prakash, R.S.; Basak, C.; Szabo, A.; Chaddock, L.; Kim, J.S.; Heo, S.; Alves, H.; White, S.M.; et al. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. USA 2011, 108, 3017–3022. [Google Scholar] [CrossRef] [PubMed]
- You, H.; Lu, B. Diverse Functions of Multiple Bdnf Transcripts Driven by Distinct Bdnf Promoters. Biomolecules 2023, 13, 655. [Google Scholar] [CrossRef]
- Yoshimura, R.; Okamoto, N.; Chibaatar, E.; Natsuyama, T.; Ikenouchi, A. The Serum Brain-Derived Neurotrophic Factor Increases in Serotonin Reuptake Inhibitor Responders Patients with First-Episode, Drug-Naïve Major Depression. Biomedicines 2023, 11, 584. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Dowlatshahi, D.; MacQueen, G.M.; Wang, J.F.; Young, L.T. Increased hippocampal bdnf immunoreactivity in subjects treated with antidepressant medication. Biol. Psychiatry 2001, 50, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, Y. Involvement of Brain-Derived Neurotrophic Factor in Late-Life Depression. Am. J. Geriatr. Psychiatry 2013, 21, 433–449. [Google Scholar] [CrossRef]
- Youssef, M.M.; Underwood, M.D.; Huang, Y.-Y.; Hsiung, S.; Liu, Y.; Simpson, N.R.; Bakalian, M.J.; Rosoklija, G.B.; Dwork, A.J.; Arango, V.; et al. Association of BDNF Val66Met Polymorphism and Brain BDNF Levels with Major Depression and Suicide. Int. J. Neuropsychopharmacol. 2018, 21, 528–538. [Google Scholar] [CrossRef]
- Kubera, M.; Obuchowicz, E.; Goehler, L.; Brzeszcz, J.; Maes, M. In animal models, psychosocial stress-induced (neuro) inflammation, apoptosis and reduced neurogenesis are associated to the onset of depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 744–759. [Google Scholar] [CrossRef]
- Jesse, C.R.; Donato, F.; Giacomeli, R.; Del Fabbro, L.; da Silva Antunes, M.; De Gomes, M.G.; Goes, A.T.R.; Boeira, S.P.; Prigol, M.; Souza, L.C. Chronic unpredictable mild stress decreases BDNF and NGF levels and Na+, K+-ATPase activity in the hippocampus and prefrontal cortex of mice: Antidepressant effect of chrysin. Neuroscience 2015, 289, 367–380. [Google Scholar] [CrossRef]
- Park, C.; Kim, J.; Namgung, E.; Lee, D.-W.; Kim, G.H.; Kim, M.; Kim, N.; Kim, T.D.; Kim, S.; Lyoo, I.K. The BDNF Val66Met polymorphism affects the vulnerability of the brain structural network. Front. Hum. Neurosci. 2017, 11, 400. [Google Scholar] [CrossRef]
- Pathak, P.; Mehra, A.; Ram, S.; Pal, A.; Grover, S. Association of serum BDNF level and Val66Met polymorphism with response to treatment in patients of major depressive disease: A step towards personalized therapy. Behav. Brain Res. 2022, 430, 113931. [Google Scholar] [CrossRef]
- Sleiman, S.F.; Henry, J.; Al-Haddad, R.; El Hayek, L.; Abou Haidar, E.; Stringer, T.; Ulja, D.; Karuppagounder, S.S.; Holson, E.B.; Ratan, R.R.; et al. Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body β-hydroxybutyrate. Elife 2016, 5, e15092. [Google Scholar] [CrossRef]
- van Praag, H.; Castren, E.; Ieraci, A.; Aprahamian, I.; Arosio, B.; Rosa Guerini, F.; Oude Voshaar, R.C.; Fondazione Don Carlo Gnocchi, I. Blood Brain-Derived Neurotrophic Factor (BDNF) and Major Depression: Do We Have a Translational Perspective? Front. Behav. Neurosci. 2021, 15, 626906. [Google Scholar] [CrossRef]
- Homberg, J.R.; Molteni, R.; Calabrese, F.; Riva, M.A. The serotonin–BDNF duo: Developmental implications for the vulnerability to psychopathology. Neurosci. Biobehav. Rev. 2014, 43, 35–47. [Google Scholar] [CrossRef]
- Yang, T.; Nie, Z.; Shu, H.; Kuang, Y.; Chen, X.; Cheng, J.; Yu, S.; Liu, H. The Role of BDNF on Neural Plasticity in Depression. Front. Cell. Neurosci. 2020, 14, 82. [Google Scholar] [CrossRef] [PubMed]
- Kunugi, H.; Hori, H.; Adachi, N.; Numakawa, T. Interface between hypothalamic-pituitary-adrenal axis and brain-derived neurotrophic factor in depression. Psychiatry Clin. Neurosci. 2010, 64, 447–459. [Google Scholar] [CrossRef]
- Li, Z.; Ruan, M.; Chen, J.; Fang, Y. Major Depressive Disorder: Advances in Neuroscience Research and Translational Applications. Neurosci. Bull. 2021, 37, 863–880. [Google Scholar] [CrossRef]
- Lopez-Munoz, F.; Alamo, C. Monoaminergic Neurotransmission: The History of the Discovery of Antidepressants from 1950s Until Today. Curr. Pharm. Des. 2009, 15, 1563–1586. [Google Scholar] [CrossRef] [PubMed]
- Hillhouse, T.M.; Porter, J.H.; Clin, E.; Author, P. A brief history of the development of antidepressant drugs: From monoamines to glutamate HHS Public Access Author manuscript. Exp. Clin. Psychopharmacol. 2015, 23, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Stahl, S.M. Mechanism of action of serotonin selective reuptake inhibitors. J. Affect. Disord. 1998, 51, 215–235. [Google Scholar] [CrossRef]
- Sheffler, Z.M.; Patel, P.; Abdijadid, S. Antidepressants; StatPearls: St. Petersburg, FL, USA, 26 May 2023. [Google Scholar]
- Kolovos, S.; Kleiboer, A.; Cuijpers, P. Effect of psychotherapy for depression on quality of life: Meta-analysis. Br. J. Psychiatry 2016, 209, 460–468. [Google Scholar] [CrossRef]
- Stachowicz, K.; Sowa-Kućma, M. The treatment of depression—Searching for new ideas. Front. Pharmacol. 2022, 13, 988648. [Google Scholar] [CrossRef]
- Thase, M.E. Have Effective Antidepressants Finally Arrived? Developments in Major Depressive Disorder Therapy. J. Clin. Psychiatry 2023, 84, 48388. [Google Scholar] [CrossRef]
- Khoodoruth, M.A.S.; Estudillo-Guerra, M.A.; Pacheco-Barrios, K.; Nyundo, A.; Chapa-Koloffon, G.; Ouanes, S. Glutamatergic System in Depression and Its Role in Neuromodulatory Techniques Optimization. Front. Psychiatry 2022, 13, 886918. [Google Scholar] [CrossRef]
- Sarawagi, A.; Soni, N.D.; Patel, A.B. Glutamate and GABA Homeostasis and Neurometabolism in Major Depressive Disorder. Front. Psychiatry 2021, 12, 637863. [Google Scholar] [CrossRef]
- Ghosal, S.; Hare, B.D.; Duman, R.S. Prefrontal cortex GABAergic deficits and circuit dysfunction in the pathophysiology and treatment of chronic stress and depression. Curr. Opin. Behav. Sci. 2017, 14, 1–8. [Google Scholar] [CrossRef]
- Lin, J.; Ling, F.; Huang, P.; Chen, M.; Song, M.; Lu, K.; Wang, W. The Development of GABAergic Network in Depression in Recent 17 Years: A Visual Analysis Based on CiteSpace and VOSviewer. Front. Psychiatry 2022, 13, 874137. [Google Scholar] [CrossRef]
- Aleksandrova, L.R.; Phillips, A.G.; Wang, Y.T. Antidepressant effects of ketamine and the roles of AMPA glutamate receptors and other mechanisms beyond NMDA receptor antagonism. J. Psychiatry Neurosci. 2017, 42, 222–229. [Google Scholar] [CrossRef]
- Berman, R.M.; Cappiello, A.; Anand, A.; Oren, D.A.; Heninger, G.R.; Charney, D.S.; Krystal, J.H. Antidepressant effects of ketamine in depressed patients. Biol. Psychiatry 2000, 47, 351–354. [Google Scholar] [CrossRef]
- Popova, V.; Daly, E.J.; Trivedi, M.; Cooper, K.; Lane, R.; Lim, P.; Mazzucco, C.; Hough, D.; Thase, M.E.; Shelton, R.C.; et al. Efficacy and Safety of Flexibly Dosed Esketamine Nasal Spray Combined with a Newly Initiated Oral Antidepressant in Treatment-Resistant Depression: A Randomized Double-Blind Active-Controlled Study. Am. J. Psychiatry 2019, 176, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Kavakbasi, E.; Hassan, A.; Baune, B.T. Combination of Electroconvulsive Therapy Alternating with Intravenous Esketamine Can Lead to Rapid Remission of Treatment Resistant Depression. J. ECT 2021, 37, e20–e21. [Google Scholar] [CrossRef]
- Ryskamp, D.A.; Korban, S.; Zhemkov, V.; Kraskovskaya, N.; Bezprozvanny, I. Neuronal Sigma-1 Receptors: Signaling Functions and Protective Roles in Neurodegenerative Diseases. Front. Neurosci. 2019, 13, 862. [Google Scholar] [CrossRef]
- Pedraz-Petrozzi, B.; Deuschle, M.; Gilles, M. Improvement of depressive symptoms, after a suicide attempt, with dextromethorphan/bupropion combination treatment in a patient with treatment-resistant depression and psychiatric comorbidities. Clin. Case Rep. 2023, 11, e7045. [Google Scholar] [CrossRef] [PubMed]
- Blair, H.A. Dextromethorphan/bupropion in major depressive disorder: A profile of its use. Drugs Ther. Perspect. 2023, 39, 270–278. [Google Scholar] [CrossRef]
- Fava, M.; Stahl, S.M.; De Martin, S.; Mattarei, A.; Bettini, E.; Comai, S.; Alimonti, A.; Bifari, F.; Pani, L.; Folli, F.; et al. Esmethadone-HCl (REL-1017): A promising rapid antidepressant. Eur. Arch. Psychiatry Clin. Neurosci. 2023, 273, 1463–1476. [Google Scholar] [CrossRef] [PubMed]
- Fava, M.; Stahl, S.; Pani, L.; De Martin, S.; Pappagallo, M.; Guidetti, C.; Alimonti, A.; Bettini, E.; Mangano, R.M.; Wessel, T.; et al. REL-1017 (Esmethadone) as Adjunctive Treatment in Patients with Major Depressive Disorder: A Phase 2a Randomized Double-Blind Trial. Am. J. Psychiatry 2022, 179, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Cornett, E.M.; Rando, L.; Labbé, A.M.; Perkins, W.; Kaye, A.M.; Kaye, A.D.; Viswanath, O.; Urits, I. Brexanolone to Treat Postpartum Depression in Adult Women. Psychopharmacol. Bull. 2021, 51, 115. [Google Scholar]
- Clayton, A.H.; Lasser, R.; Parikh, S.V.; Iosifescu, D.V.; Jung, J.; Kotecha, M.; Forrestal, F.; Jonas, J.; Kanes, S.J.; Doherty, J. Zuranolone for the Treatment of Adults with Major Depressive Disorder: A Randomized, Placebo-Controlled Phase 3 Trial. Am. J. Psychiatry 2023, 180, 676–684. [Google Scholar] [CrossRef]
- Fagan, H.; Jones, E.; Baldwin, D.S. Orexin Receptor Antagonists in the Treatment of Depression: A Leading Article Summarising Pre-clinical and Clinical Studies. CNS Drugs 2023, 37, 1–12. [Google Scholar] [CrossRef]
- Mi, W.; Di, X.; Wang, Y.; Li, H.; Xu, X.; Li, L.; Wang, H.; Wang, G.; Zhang, K.; Tian, F.; et al. A phase 3, multicenter, double-blind, randomized, placebo-controlled clinical trial to verify the efficacy and safety of ansofaxine (LY03005) for major depressive disorder. Transl. Psychiatry 2023, 13, 163. [Google Scholar] [CrossRef]
- Bansal, Y.; Bhandari, R.; Kaur, S.; Kaur, J.; Singh, R.; Kuhad, A. Gepirone hydrochloride: A novel antidepressant with 5-HT1A agonistic properties. Drugs Today 2019, 55, 423–437. [Google Scholar] [CrossRef]
- Goodwin, G.M.; Croal, M.; Feifel, D.; Kelly, J.R.; Marwood, L.; Mistry, S.; O’Keane, V.; Peck, S.K.; Simmons, H.; Sisa, C.; et al. Psilocybin for treatment resistant depression in patients taking a concomitant SSRI medication. Neuropsychopharmacology 2023, 48, 1492–1499. [Google Scholar] [CrossRef]
- Von Rotz, R.; Schindowski, E.M.; Jungwirth, J.; Schuldt, A.; Rieser, N.M.; Zahoranszky, K.; Seifritz, E.; Nowak, A.; Nowak, P.; Jäncke, L.; et al. Single-dose psilocybin-assisted therapy in major depressive disorder: A placebo-controlled, double-blind, randomised clinical trial. EClinicalMedicine 2022, 56, 101809. [Google Scholar] [CrossRef] [PubMed]
- Dulawa, S.C.; Janowsky, D.S. Cholinergic regulation of mood: From basic and clinical studies to emerging therapeutics. Mol. Psychiatry 2019, 24, 694–709. [Google Scholar] [CrossRef]
- Fitzgerald, P.J.; Hale, P.J.; Ghimire, A.; Watson, B.O. Repurposing Cholinesterase Inhibitors as Antidepressants? Dose and Stress-Sensitivity May Be Critical to Opening Possibilities. Front. Behav. Neurosci. 2021, 14, 620119. [Google Scholar] [CrossRef]
- Sun, J.; Qiu, L.; Zhang, H.; Zhou, Z.; Ju, L.; Yang, J. CRHR1 antagonist alleviates LPS-induced depression-like behaviour in mice. BMC Psychiatry 2023, 23, 17. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.J.; Huang, X.-F.; Newell, K.A. The kynurenine pathway in major depression: What we know and where to next. Neurosci. Biobehav. Rev. 2021, 127, 917–927. [Google Scholar] [CrossRef]
- Mohammad Sadeghi, H.; Adeli, I.; Mousavi, T.; Daniali, M.; Nikfar, S.; Abdollahi, M. Drug Repurposing for the Management of Depression: Where Do We Stand Currently? Life 2021, 11, 774. [Google Scholar] [CrossRef]
- Sizar, O.; Khare, S.; Jamil, R.T.; Talati, R. Statin Medications; StatPearls: St. Petersburg, FL, USA, 2023. [Google Scholar]
- Gutlapalli, S.D.; Farhat, H.; Irfan, H.; Muthiah, K.; Pallipamu, N.; Taheri, S.; Thiagaraj, S.S.; Shukla, T.S.; Giva, S.; Penumetcha, S.S. The Anti-Depressant Effects of Statins in Patients with Major Depression Post-Myocardial Infarction: An Updated Review 2022. Cureus 2022, 14, e32323. [Google Scholar] [CrossRef] [PubMed]
- De Giorgi, R.; Pesci, N.R.; Rosso, G.; Maina, G.; Cowen, P.J.; Harmer, C.J. The pharmacological bases for repurposing statins in depression: A review of mechanistic studies. Transl. Psychiatry 2023, 13, 253. [Google Scholar] [CrossRef]
- Lochner, M.; Thompson, A.J. The muscarinic antagonists scopolamine and atropine are competitive antagonists at 5-HT 3 receptors. Neuropharmacology 2016, 108, 220–228. [Google Scholar] [CrossRef]
- Fang, Y.; Guo, P.; Lv, L.; Feng, M.; Wang, H.; Sun, G.; Wang, S.; Qian, M.; Chen, H. Scopolamine augmentation for depressive symptoms and cognitive functions in treatment-resistant depression: A case series. Asian J. Psychiatr. 2023, 82, 103484. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, X.; Ji, X.; Yang, J.; Zhou, J. Group-Based Symptom Trajectory of Intramuscular Administration of Scopolamine Augmentation in Moderate to Severe Major Depressive Disorder: A Post-Hoc Analysis. Neuropsychiatr. Dis. Treat. 2023, 19, 1043–1053. [Google Scholar] [CrossRef] [PubMed]
- Home|ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ (accessed on 18 October 2023).
- Moćko, P.; Śladowska, K.; Kawalec, P.; Babii, Y.; Pilc, A. The Potential of Scopolamine as an Antidepressant in Major Depressive Disorder: A Systematic Review of Randomized Controlled Trials. Biomedicines 2023, 11, 2636. [Google Scholar] [CrossRef]
- Chateauvieux, S.; Morceau, F.; Diederich, M. Valproic Acid. In Encyclopedia of Toxicology, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 905–908. [Google Scholar] [CrossRef]
- Brian Chen, Y.-C.; Liang, C.-S.; Wang, L.-J.; Hung, K.-C.; Carvalho, A.F.; Solmi, M.; Vieta, E.; Tseng, P.-T.; Lin, P.-Y.; Tu, Y.-K.; et al. Comparative effectiveness of valproic acid in different serum concentrations for maintenance treatment of bipolar disorder: A retrospective cohort study using target trial emulation framework. eClinicalMedicine 2022, 54, 101678. [Google Scholar] [CrossRef] [PubMed]
- Ghabrash, M.F.; Comai, S.; Tabaka, J.; Saint-Laurent, M.; Booij, L.; Gobbi, G. Valproate augmentation in a subgroup of patients with treatment-resistant unipolar depression. World J. Biol. Psychiatry 2016, 17, 165–170. [Google Scholar] [CrossRef]
- Yonemoto, L. Lamotrigine. In The Essence of Analgesia and Analgesics; Cambridge University Press: Cambridge, UK, 2010; pp. 306–309. ISBN 9780511841378. [Google Scholar]
- Matsuzaka, Y.; Urashima, K.; Sakai, S.; Morimoto, Y.; Kanegae, S.; Kinoshita, H.; Imamura, A.; Ozawa, H. The effectiveness of lamotrigine for persistent depressive disorder: A case report. Neuropsychopharmacol. Rep. 2022, 42, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Can, A.S.; Correa, R. Pioglitazone; StatPearls: St. Petersburg, FL, USA, 2023. [Google Scholar]
- Colle, R.; de Larminat, D.; Rotenberg, S.; Hozer, F.; Hardy, P.; Verstuyft, C.; Fève, B.; Corruble, E. Pioglitazone could induce remission in major depression: A meta-analysis. Neuropsychiatr. Dis. Treat. 2016, 13, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Watson, K.; Akil, H.; Rasgon, N. Toward a Precision Treatment Approach for Metabolic Depression: Integrating Epidemiology, Neuroscience, and Psychiatry. Biol. Psychiatry Glob. Open Sci. 2023, 3, 623–631. [Google Scholar] [CrossRef]
- Zhao, Q.; Wu, X.; Yan, S.; Xie, X.; Fan, Y.; Zhang, J.; Peng, C.; You, Z. The antidepressant-like effects of pioglitazone in a chronic mild stress mouse model are associated with PPARγ-mediated alteration of microglial activation phenotypes. J. Neuroinflamm. 2016, 13, 259. [Google Scholar] [CrossRef]
- Beheshti, F.; Hosseini, M.; Hashemzehi, M.; Soukhtanloo, M.; Asghari, A. The effects of PPAR-γ agonist pioglitazone on anxiety and depression-like behaviors in lipopolysaccharide injected rats. Toxin Rev. 2021, 40, 1223–1232. [Google Scholar] [CrossRef]
- Rague, J. N-Acetylcysteine. In History of Modern Clinical Toxicology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 201–212. ISBN 9780128222188. [Google Scholar]
- Hans, D.; Rengel, A.; Hans, J.; Bassett, D.; Hood, S. N-Acetylcysteine as a novel rapidly acting anti-suicidal agent: A pilot naturalistic study in the emergency setting. PLoS ONE 2022, 17, e0263149. [Google Scholar] [CrossRef]
- Brivio, P.; Gallo, M.T.; Gruca, P.; Lason, M.; Litwa, E.; Fumagalli, F.; Papp, M.; Calabrese, F. Chronic N-Acetyl-Cysteine Treatment Enhances the Expression of the Immediate Early Gene Nr4a1 in Response to an Acute Challenge in Male Rats: Comparison with the Antidepressant Venlafaxine. Int. J. Mol. Sci. 2023, 24, 7321. [Google Scholar] [CrossRef]
- Nazarian, S.; Akhondi, H. Minocycline; StatPearls: St. Petersburg, FL, USA, 2022. [Google Scholar]
- Nettis, M.A. Minocycline in Major Depressive Disorder: And overview with considerations on treatment-resistance and comparisons with other psychiatric disorders. Brain Behav. Immun.-Health 2021, 17, 100335. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Duan, A.; Yin, Z.; Xie, M.; Chen, Z.; Sun, X.; Wang, Z.; Zhang, X. Efficacy and tolerability of minocycline in depressive patients with or without treatment-resistant: A meta-analysis of randomized controlled trials. Front. Psychiatry 2023, 14, 1139273. [Google Scholar] [CrossRef]
- Rojewska, E.; Ciapała, K.; Piotrowska, A.; Makuch, W.; Mika, J. Pharmacological Inhibition of Indoleamine 2,3-Dioxygenase-2 and Kynurenine 3-Monooxygenase, Enzymes of the Kynurenine Pathway, Significantly Diminishes Neuropathic Pain in a Rat Model. Front. Pharmacol. 2018, 9, 724. [Google Scholar] [CrossRef] [PubMed]
- Tippens, A.S. Nimodipine. In xPharm: The Comprehensive Pharmacology Reference; Elsevier: Amsterdam, The Netherlands, 2007; pp. 1–7. ISBN 9780080552323. [Google Scholar]
- Zink, C.F.; Giegerich, M.; Prettyman, G.E.; Carta, K.E.; van Ginkel, M.; O’Rourke, M.P.; Singh, E.; Fuchs, E.J.; Hendrix, C.W.; Zimmerman, E.; et al. Nimodipine improves cortical efficiency during working memory in healthy subjects. Transl. Psychiatry 2020, 10, 372. [Google Scholar] [CrossRef] [PubMed]
- Taragano, F.E.; Allegri, R.; Vicario, A.; Bagnatti, P.; Lyketsos, C.G. A double blind, randomized clinical trial assessing the efficacy and safety of augmenting standard antidepressant therapy with nimodipine in the treatment of ‘vascular depression’. Int. J. Geriatr. Psychiatry 2001, 16, 254–260. [Google Scholar] [CrossRef]
- Maan, J.S.; Ershadi, M.; Khan, I.; Saadabadi, A. Quetiapine. StatPearls: St. Petersburg, FL, USA, 2023. [Google Scholar]
- Tran, K.; Argáez, C. Quetiapine for Major Depressive Disorder: A Review of Clinical Effectiveness, Cost-Effectiveness, and Guidelines; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, Canada, 2020. [Google Scholar]
- Ravindran, N.; McKay, M.; Paric, A.; Johnson, S.; Chandrasena, R.; Abraham, G.; Ravindran, A.V. Randomized, Placebo-Controlled Effectiveness Study of Quetiapine XR in Comorbid Depressive and Anxiety Disorders. J. Clin. Psychiatry 2022, 83, 40248. [Google Scholar] [CrossRef]
- Bauer, M.; Pretorius, H.W.; Constant, E.L.; Earley, W.R.; Szamosi, J.; Brecher, M. Extended-Release Quetiapine as Adjunct to an Antidepressant in Patients with Major Depressive Disorder: Results of a Randomized, Placebo-Controlled, Double-Blind Study. J. Clin. Psychiatry 2009, 70, 7032. [Google Scholar] [CrossRef]
- Marino, J. Celecoxib. In The Essence of Analgesia and Analgesics; Cambridge University Press: Cambridge, UK, 2010; pp. 238–242. ISBN 9780511841378. [Google Scholar]
- Wang, Z.; Wu, Q.; Wang, Q. Effect of celecoxib on improving depression: A systematic review and meta-analysis. World J. Clin. Cases 2022, 10, 7872–7882. [Google Scholar] [CrossRef] [PubMed]
- Gędek, A.; Szular, Z.; Antosik, A.Z.; Mierzejewski, P.; Dominiak, M. Celecoxib for Mood Disorders: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Clin. Med. 2023, 12, 3497. [Google Scholar] [CrossRef] [PubMed]
| Receptor | Type/and Mechanism | Potential |
|---|---|---|
| 5-HT1A-F | Gi/o-protein coupled; decrease cellular levels of cAMP | Inhibitory |
| 5-HT2A, 5-HT2B, 5-HT2C | Gq/11-protein coupled; increase cellular levels of inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG) | Excitatory |
| 5-HT3 | Ligand-gated Na + and K + cation channel; depolarize plasma membrane | Excitatory |
| 5-HT4 | Gs-protein coupled; increase cellular levels of cAMP | Excitatory |
| 5-HT5A-B | Gi/o-protein coupled; decrease cellular levels of cAMP | Inhibitory |
| 5-HT6 | Gs-protein coupled; increase cellular levels of cAMP | Excitatory |
| 5-HT7 | Gs-protein coupled; increase cellular levels of cAMP | Excitatory |
| 5-HT Receptor | Function in Depression |
|---|---|
| 5-HT1A | Agonists of 5-HT1A receptors are frequently used in the treatment of depression because they can enhance 5-HT signaling. Stimulation of the 5-HT1A receptor is an existing therapeutic target for treating depression and anxiety, using drugs such as buspirone [83]. |
| 5-HT2A | This receptor can control neuronal excitability in most networks involved in depression through interactions with the monoaminergic, GABAergic, and glutamatergic neurotransmissions [84]. Preclinical studies show that 5-HT2A receptor antagonists have antipsychotic and antidepressant properties, whereas agonist ligands possess cognition-enhancing and hallucinogenic properties [85]. |
| 5-HT3 | This is an area of ongoing research. 5-HT3 receptor antagonists inhibit the binding of 5-HT to postsynaptic 5-HT3 receptor and might increase its availability to other receptors like 5-HT1A, 1B and 1D as well as 5-HT2 receptors, producing an antidepressant-like effect [86]. |
| Drug | Main Indication and Mechanism of Action | Relevance in Depression |
|---|---|---|
| Statins | Management and treatment of hypercholesteremia. Selective, competitive inhibitor of hydroxymethylglutaryl-CoA (HMG-CoA) reductase [168]. | Demonstrated antidepressant effects, useful as add-on therapy in patients with cardiovascular disease, with MDD [169]. Beneficial effect through positive actions on 5-HT neurotransmission, neurogenesis, and neuroplasticity, HPA axis regulation and modulation of inflammation [170]. |
| Scopolamine | Postoperative nausea and vomiting and motion sickness. Competitive antagonist of 5-HT3 receptors and nonselective muscarinic antagonist [171]. | Evidence of antidepressant effects in patients with MDD and bipolar depression [172]. Added to antidepressants can effectively relieve the symptoms of patients with severe depression [173]. Currently, 2 clinical trials in MDD and bipolar disorder are ongoing (NCT04719663 and NCT04211961 [174]). Studies in rodents have revealed that the antidepressant-like effects are connected to mTORC1 signaling in the PFC. This activation of mTORC1 seems to be initiated by a glutamate surge in the PFC, resulting from the disinhibition of glutamatergic neurons. This increased glutamate transmission leads to the activation of AMPA receptors, that raises the levels of BDNF, which then stimulates mTORC1 signaling and promotes synaptogenesis processes [175]. |
| Valproic Acid | Treatment/management of epilepsy. Mechanism of action not fully understood: Inhibits voltage-gated sodium channels, GABA transaminase, increases the expression and activity of glutamic acid decarboxylase (GAD), inhibits the action of histone deacetylases (HDAC) enzymes, notably HDAC1, modulates the activity of various calcium channels [176]. | Demonstrated efficacy in preventing mood recurrence and enhancing the quality of life for individuals with bipolar disorder when used as a maintenance therapy [177]. Supplementary use of this drug resulted in significant and sustained clinical enhancement over a prolonged duration in individuals with severe treatment-resistant depression [178]. |
| Lamotrigine | Treatment/management of epilepsy. Mechanism of action for lamotrigine is not entirely understood; selectively binds and inhibits voltage-gated sodium channels, stabilizing presynaptic membranes and inhibiting presynaptic glutamate release [179]. | Used off-label for bipolar disorder [179]. Could potentially offer an effective option in addressing individuals with treatment-resistant persistent depressive disorder, being a viable substitute for the combination of antidepressant and benzodiazepine therapies in this disorder [180]. |
| Pioglitazone | Treatment of type 2 diabetes mellitus. Peroxisome proliferator-activated receptor (PPAR)-gamma and PPAR-alpha agonist [181]. | Pioglitazone, alone or as add-on therapy to conventional treatments, could induce remission of depressive episodes [182]. Evidence of enhancing antidepressant response among people with comorbid MDD and type 2 diabetes [183]. Induced the neuroprotective phenotype of microglia in chronic mild stress-treated mice, mediated by PPARγ [184], and antidepressant effect in LPS injected rats [185]. |
| N-acetyl cysteine | Therapy for acetaminophen toxicity. Serves as a prodrug to L-cysteine, a precursor to glutathione [186]. | Evidence as an adjunctive therapy to reduce symptoms of Bipolar Affective Disorder, MDD, and Schizophrenia [187]. Enhanced coping mechanisms, not only for addressing acute stressors but possibly also for mitigating the impact of persistent stress-inducing factors [188]. |
| Minocycline | Tetracycline antibiotic, anti-infectious activity against both Gram-positive and Gram-negative bacteria. Bind to the 30S ribosomal subunit of bacteria, preventing protein synthesis [189]. | Potential novel treatment for treatment-resistant depression [190]. May improve depressive symptoms and augment response to treatment in patients with treatment-resistant depression [191]. Inhibits both the IDO and the p-38 components of inflammation-induced depression [192]. |
| Nimodipine | Prevent vasospasm secondary to subarachnoid hemorrhage. Blocks voltage-gated L-type calcium channels [193]. | This drug has been shown to be effective in treating mood symptoms for bipolar and unipolar depression [194]. An old clinical trial revealed that this drug in the context of vascular depression, augmentation of fluoxetine with nimodipine led to better treatment results and lower rates of recurrence [195]. |
| Quetiapine | Schizophrenia and acute manic episodes. Antagonist for D2 receptors and 5-HT2A receptors [196]. | Quetiapine monotherapy in older adults with MDD was found to be effective [197]. Quetiapine augmentation may be a useful intervention for MDD with comorbid anxiety [198]. Adjunctive quetiapine was effective in patients with MDD who had shown an inadequate response to antidepressant treatment [199]. |
| Celecoxib | Analgesic for patients with osteoarthritis and rheumatoid arthritis. Selective inhibition of COX-2 [200]. | A recent meta-analysis demonstrated that celecoxib could be effective for improving depressive symptoms [201]. Antidepressant efficacy was demonstrated when used as an add-on treatment for MDD and mania, possibly by reducing inflammatory markers [202]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Correia, A.S.; Vale, N. Advancements Exploring Major Depressive Disorder: Insights on Oxidative Stress, Serotonin Metabolism, BDNF, HPA Axis Dysfunction, and Pharmacotherapy Advances. Int. J. Transl. Med. 2024, 4, 176-196. https://doi.org/10.3390/ijtm4010010
Correia AS, Vale N. Advancements Exploring Major Depressive Disorder: Insights on Oxidative Stress, Serotonin Metabolism, BDNF, HPA Axis Dysfunction, and Pharmacotherapy Advances. International Journal of Translational Medicine. 2024; 4(1):176-196. https://doi.org/10.3390/ijtm4010010
Chicago/Turabian StyleCorreia, Ana Salomé, and Nuno Vale. 2024. "Advancements Exploring Major Depressive Disorder: Insights on Oxidative Stress, Serotonin Metabolism, BDNF, HPA Axis Dysfunction, and Pharmacotherapy Advances" International Journal of Translational Medicine 4, no. 1: 176-196. https://doi.org/10.3390/ijtm4010010
APA StyleCorreia, A. S., & Vale, N. (2024). Advancements Exploring Major Depressive Disorder: Insights on Oxidative Stress, Serotonin Metabolism, BDNF, HPA Axis Dysfunction, and Pharmacotherapy Advances. International Journal of Translational Medicine, 4(1), 176-196. https://doi.org/10.3390/ijtm4010010







