Dexamethasone Treatment Preserves the Structure of Adult Cardiac Explants and Supports Their Long-Term Contractility In Vitro
Abstract
:1. Introduction
2. Material and Methods
2.1. Animal and Tissue Harvesting
2.2. Atrial Explant Culture
2.3. Isolation of and Culture of Rat Cardiomyocytes
2.4. Imaging of Live Cultures
2.5. Immunofluorescence Analysis
3. Results
3.1. DEX Supports the Functional Longevity of Cardiac Explant Cultures
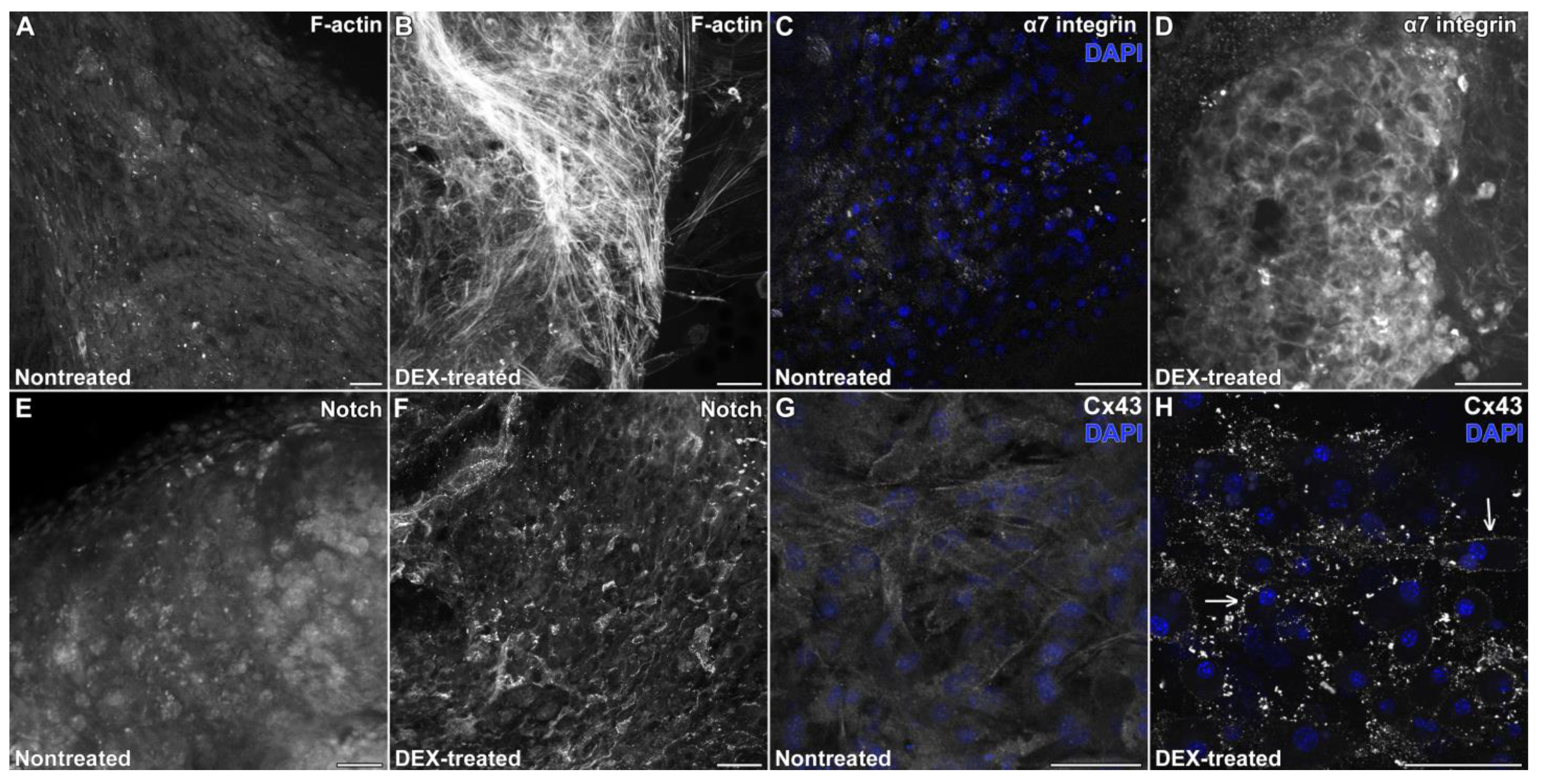
3.2. DEX Supports the Long-Term Retention of the Cardiac Phenotype of the Atrial Explants
3.3. DEX Treatment Helps Maintain the Cardiac Phenotype of Dissociated Cardiomyocytes In Vitro
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burrows, M.T. Rhythmical activity of isolated heart muscle cells in vitro. Science 1912, 36, 90–92. [Google Scholar] [CrossRef] [PubMed]
- Harary, I.; Farley, B. In vitro studies of single isolated beating heart cells. Science 1960, 131, 1674–1675. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, S.L. Culture of spontaneously contracting myocardial cells from adult rats. Cell Struct. Funct. 1977, 2, 1–9. [Google Scholar] [CrossRef]
- Ehler, E.; Moore-Morris, T.; Lange, S. Isolation and culture of neonatal mouse cardiomyocytes. J. Vis. Exp. 2013, 79, e50154. [Google Scholar] [CrossRef]
- Fu, J.; Gao, J.; Pi, R.; Liu, P. An optimized protocol for culture of cardiomyocyte from neonatal rat. Cytotechnology 2005, 49, 109–116. [Google Scholar] [CrossRef]
- Nag, A.C.; Cheng, M.; Fischman, D.A.; Zak, R. Long-term cell culture of adult mammalian cardiac myocytes: Electron microscopic and immunofluorescent analyses of myofibrillar structure. J. Mol. Cell. Cardiol. 1983, 15, 301–317. [Google Scholar] [CrossRef]
- Piper, H.M.; Jacobson, S.L.; Schwartz, P. Determinants of cardiomyocyte development in long-term primary culture. J. Mol. Cell. Cardiol. 1988, 20, 825–835. [Google Scholar] [CrossRef]
- Das, M.; Molnar, P.; Gregory, C.; Riedel, L.; Jamshidi, A.; Hickman, J.J. Long-term culture of embryonic rat cardiomyocytes on an organosilane surface in a serum-free medium. Biomaterials 2004, 25, 5643–5647. [Google Scholar] [CrossRef]
- Fukushima, H.; Yoshioka, M.; Kawatou, M.; López-Dávila, V.; Takeda, M.; Kanda, Y.; Sekino, Y.; Yoshida, Y.; Yamashita, J.K. Specific induction and long-term maintenance of high purity ventricular cardiomyocytes from human induced pluripotent stem cells. PLoS ONE 2020, 15, e0241287. [Google Scholar] [CrossRef]
- Kaur, K.; Yang, J.; Edwards, J.; Eisenberg, C.; Eisenberg, L. G9a histone methyltransferase inhibitor BIX 01294 promotes expansion of adult cardiac progenitor cells without changing their phenotype or differentiation potential. Cell Prolif. 2016, 49, 373–385. [Google Scholar] [CrossRef]
- Kaur, K.; Yang, J.; Eisenberg, C.A.; Eisenberg, L.M. 5-azacytidine promotes the transdifferentiation of cardiac cells to skeletal myocytes. Cell Reprogram. 2014, 16, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, L.M.; Eisenberg, C.A. Embryonic myocardium shows increased longevity as a functional tissue when cultured in the presence of a noncardiac tissue layer. Tissue Eng. 2006, 12, 853–865. [Google Scholar] [CrossRef] [PubMed]
- Rémond, M.C.; Iaffaldano, G.; O’Quinn, M.P.; Mezentseva, N.V.; Garcia, V.; Harris, B.S.; Gourdie, R.G.; Eisenberg, C.A.; Eisenberg, L.M. GATA6 reporter gene reveals myocardial phenotypic heterogeneity that is related to variations in gap junction coupling. Am. J. Physiol. Heart 2011, 301, H1952–H1964. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Kaur, K.; Edwards, J.G.; Eisenberg, C.A.; Eisenberg, L.M. Inhibition of histone methyltransferase, histone deacetylase, and β-catenin synergistically enhance the cardiac potential of bone marrow cells. Stem Cells Int. 2017. [Google Scholar] [CrossRef] [PubMed]
- Davis, D.R.; Kizana, E.; Terrovitis, J.; Barth, A.S.; Zhang, Y.; Smith, R.R.; Miake, J.; Marbán, E. Isolation and expansion of functionally-competent cardiac progenitor cells directly from heart biopsies. J. Mol. Cell. Cardiol. 2010, 49, 312–321. [Google Scholar] [CrossRef]
- Messina, E.; De Angelis, L.; Frati, G.; Morrone, S.; Chimenti, S.; Fiordaliso, F.; Salio, M.; Battaglia, M.; Latronico, M.V.; Coletta, M. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ. Res. 2004, 95, 911–921. [Google Scholar] [CrossRef]
- Cartwright, T.; Shah, G.P. Culture media. In Basic Cell Culture: A Practical Approach, 2nd ed.; Davis, J.M., Ed.; Practical Approach; Oxford University Press: Oxford, UK, 2004; pp. 69–105. [Google Scholar]
- Fredin, B.L.; Seifert, S.C.; Gelehrter, T.D. Dexamethasone-induced adhesion in hepatoma cells: The role of plasminogen activator. Nature 1979, 277, 312–313. [Google Scholar] [CrossRef]
- Lin, J.-W.; Huang, Y.-M.; Chen, Y.-Q.; Chuang, T.-Y.; Lan, T.-Y.; Liu, Y.-W.; Pan, H.-W.; You, L.-R.; Wang, Y.-K.; Lin, K.-H. Dexamethasone accelerates muscle regeneration by modulating kinesin-1-mediated focal adhesion signals. Cell Death Discov. 2021, 7, 35. [Google Scholar] [CrossRef]
- Richman, R.A.; Claus, T.H.; Pilkis, S.J.; Friedman, D.L. Hormonal stimulation of DNA synthesis in primary cultures of adult rat hepatocytes. Proc. Natl. Acad. Sci. USA 1976, 73, 3589–3593. [Google Scholar] [CrossRef]
- Freshney, R.I. Defined media and supplements. In Culture of Animal Cells: A Manual of Basic Technique and Specialized Applications, 7th ed.; Freshney, R.I., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 125–148. [Google Scholar]
- Kurpińska, A.; Skrzypczak, W. Hormonal changes in dairy cows during periparturient period. Acta Sci. Pol. Zootech. 2020, 18, 13–22. [Google Scholar] [CrossRef]
- Shenavai, S.; Preissing, S.; Hoffmann, B.; Dilly, M.; Pfarrer, C.; Schuler, G. Investigations into the mechanisms controlling parturition in cattle. Reproduction 2012, 144, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Yao, T.; Asayama, Y. Animal-cell culture media: History, characteristics, and current issues. Reprod. Med. Biol. 2017, 16, 99–117. [Google Scholar] [CrossRef]
- Park, Y.-K.; Ge, K. Glucocorticoid receptor accelerates, but is dispensable for, adipogenesis. Mol. Cell. Biol. 2016, 37, e00260-16. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, R.H.; Leach, K.L.; La Forest, A.; O’Toole, T.E.; Wagner, R.; Pratt, W. Glucocorticoid receptor activation and inactivation in cultured human lymphocytes. J. Biol. Chem. 1981, 256, 434–441. [Google Scholar] [CrossRef]
- Zhang, C.; Tannous, E.; Thomas, A.; Jung, N.; Ma, E.; Zheng, J.J. Dexamethasone Modulates the Dynamics of Wnt Signaling in Human Trabecular Meshwork Cells. Vision 2023, 7, 43. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Otto, P.S.; Kennedy, D.L.; Whayne, T.F. The effects of dexamethasone on metabolic activity of hepatocytes in primary monolayer culture. In Vitro 1980, 16, 559–570. [Google Scholar] [CrossRef]
- Akazawa, H.; Komuro, I. Cardiac transcription factor Csx/Nkx2-5: Its role in cardiac development and diseases. Pharmacol. Ther. 2005, 107, 252–268. [Google Scholar] [CrossRef]
- Jamali, M.; Rogerson, P.J.; Wilton, S.; Skerjanc, I.S. Nkx2–5 activity is essential for cardiomyogenesis. J. Biol. Chem. 2001, 276, 42252–42258. [Google Scholar] [CrossRef]
- Toko, H.; Zhu, W.; Takimoto, E.; Shiojima, I.; Hiroi, Y.; Zou, Y.; Oka, T.; Akazawa, H.; Mizukami, M.; Sakamoto, M. Csx/Nkx2-5 is required for homeostasis and survival of cardiac myocytes in the adult heart. J. Biol. Chem. 2002, 277, 24735–24743. [Google Scholar] [CrossRef]
- Yuan, F.; Qiu, X.-B.; Li, R.-G.; Qu, X.-K.; Wang, J.; Xu, Y.-J.; Liu, X.; Fang, W.-Y.; Yang, Y.-Q.; Liao, D.-N. A novel NKX2-5 loss-of-function mutation predisposes to familial dilated cardiomyopathy and arrhythmias. Int. J. Mol. Med. 2015, 35, 478–486. [Google Scholar] [CrossRef]
- Genead, R.; Danielsson, C.; Andersson, A.B.; Corbascio, M.; Franco-Cereceda, A.; Sylven, C.; Grinnemo, K.-H. Islet-1 cells are cardiac progenitors present during the entire lifespan: From the embryonic stage to adulthood. Stem Cells Dev. 2010, 19, 1601–1615. [Google Scholar] [CrossRef]
- Borghetti, G.; Eisenberg, C.A.; Signore, S.; Sorrentino, A.; Kaur, K.; Andrade-Vicenty, A.; Edwards, J.G.; Nerkar, M.; Qanud, K.; Sun, D. Notch signaling modulates the electrical behavior of cardiomyocytes. Am. J. Physiol. Heart 2018, 314, H68–H81. [Google Scholar] [CrossRef] [PubMed]
- Bugiardini, E.; Nunes, A.M.; Oliveira-Santos, A.; Dagda, M.; Fontelonga, T.M.; Barraza-Flores, P.; Pittman, A.M.; Morrow, J.M.; Parton, M.; Houlden, H. Integrin α7 Mutations Are Associated With Adult-Onset Cardiac Dysfunction in Humans and Mice. J. Am. Heart Assoc. 2022, 11, e026494. [Google Scholar] [CrossRef] [PubMed]
- Gourdie, R.G.; Green, C.R.; Severs, N.J.; Thompson, R.P. Immunolabelling patterns of gap junction connexins in the developing and mature rat heart. Anat. Embryol. 1992, 185, 363–378. [Google Scholar] [CrossRef]
- Banyasz, T.; Lozinskiy, I.; Payne, C.E.; Edelmann, S.; Norton, B.; Chen, B.; Chen-Izu, Y.; Izu, L.T.; Balke, C.W. Transformation of adult rat cardiac myocytes in primary culture. Exp. Physiol. 2008, 93, 370–382. [Google Scholar] [CrossRef]
- Bird, S.; Doevendans, P.; Van Rooijen, M.; Brutel De La Riviere, A.; Hassink, R.; Passier, R.; Mummery, C. The human adult cardiomyocyte phenotype. Cardiovasc. Res. 2003, 58, 423–434. [Google Scholar] [CrossRef]
- Grafi, G. The complexity of cellular dedifferentiation: Implications for regenerative medicine. Trends Biotechnol. 2009, 27, 329–332. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, C. Dedifferentiation: Inspiration for devising engineering strategies for regenerative medicine. NPJ Regen. Med. 2020, 5, 14. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, T.-S.; Lee, S.-T.; Wawrowsky, K.A.; Cheng, K.; Galang, G.; Malliaras, K.; Abraham, M.R.; Wang, C.; Marbán, E. Dedifferentiation and proliferation of mammalian cardiomyocytes. PLoS ONE 2010, 5, e12559. [Google Scholar] [CrossRef] [PubMed]
- Kubin, T.; Pöling, J.; Kostin, S.; Gajawada, P.; Hein, S.; Rees, W.; Wietelmann, A.; Tanaka, M.; Lörchner, H.; Schimanski, S. Oncostatin M is a major mediator of cardiomyocyte dedifferentiation and remodeling. Cell Stem Cell 2011, 9, 420–432. [Google Scholar] [CrossRef]
- Zhu, Y.; Do, V.D.; Richards, A.M.; Foo, R. What we know about cardiomyocyte dedifferentiation. J. Mol. Cell. Cardiol. 2021, 152, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Ren, R.; Oakley, R.H.; Cruz-Topete, D.; Cidlowski, J.A. Dual role for glucocorticoids in cardiomyocyte hypertrophy and apoptosis. Endocrinology 2012, 153, 5346–5360. [Google Scholar] [CrossRef] [PubMed]
- Severinova, E.; Alikunju, S.; Deng, W.; Dhawan, P.; Sayed, N.; Sayed, D. Glucocorticoid receptor-binding and transcriptome signature in cardiomyocytes. J. Am. Heart Assoc. 2019, 8, e011484. [Google Scholar] [CrossRef]
- Yang, J.; Chen, Y.; Li, X.; Xu, D. New insights into the roles of glucocorticoid signaling dysregulation in pathological cardiac hypertrophy. Heart Fail. Rev. 2022, 27, 1431–1441. [Google Scholar] [CrossRef] [PubMed]
- Oakley, R.H.; Ren, R.; Cruz-Topete, D.; Bird, G.S.; Myers, P.H.; Boyle, M.C.; Schneider, M.D.; Willis, M.S.; Cidlowski, J.A. Essential role of stress hormone signaling in cardiomyocytes for the prevention of heart disease. Proc. Natl. Acad. Sci. USA 2013, 110, 17035–17040. [Google Scholar] [CrossRef]
- Rog-Zielinska, E.; Craig, M.; Manning, J.; Richardson, R.; Gowans, G.; Dunbar, D.; Gharbi, K.; Kenyon, C.; Holmes, M.; Hardie, D. Glucocorticoids promote structural and functional maturation of foetal cardiomyocytes: A role for PGC-1α. Cell Death Differ. 2015, 22, 1106–1116. [Google Scholar] [CrossRef]
- Rog-Zielinska, E.A.; Thomson, A.; Kenyon, C.J.; Brownstein, D.G.; Moran, C.M.; Szumska, D.; Michailidou, Z.; Richardson, J.; Owen, E.; Watt, A. Glucocorticoid receptor is required for foetal heart maturation. Hum. Mol. Genet. 2013, 22, 3269–3282. [Google Scholar] [CrossRef]
- West, Z.E.; Dove, M.; Kochilas, L.K.; Oster, M.E.; Oster, M. Transient Global Ventricular Hypertrophy in a Patient With Multisystem Inflammatory Syndrome in Children (MIS-C) Correlated With High-Dose Glucocorticoid Treatment: A Case Report. Cureus 2022, 14, e32139. [Google Scholar] [CrossRef]
- Samarel, A.M. Costameres, focal adhesions, and cardiomyocyte mechanotransduction. Am. J. Physiol. Heart 2005, 289, H2291–H2301. [Google Scholar] [CrossRef]
- Clark, A.F.; Brotchie, D.; Read, A.T.; Hellberg, P.; English-Wright, S.; Pang, I.H.; Ethier, C.R.; Grierson, I. Dexamethasone alters F-actin architecture and promotes cross-linked actin network formation in human trabecular meshwork tissue. Cell Motil. Cytoskelet. 2005, 60, 83–95. [Google Scholar] [CrossRef]
- Yang, N.; Li, Y.-C.; Xiong, T.-Q.; Chen, L.-M.; Zhai, Y.; Liang, J.-M.; Hao, Y.-P.; Ma, D.-H.; Zhang, Y.-F. Dexamethasone ameliorates the damage of hippocampal filamentous actin cytoskeleton but is not sufficient to cease epileptogenesis in pilocarpine induced epileptic mice. Epilepsy Res. 2019, 154, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-R.; Kim, H.-K.; Song, I.-S.; Youm, J.; Dizon, L.A.; Jeong, S.-H.; Ko, T.-H.; Heo, H.-J.; Ko, K.S.; Rhee, B.D. Glucocorticoids and their receptors: Insights into specific roles in mitochondria. Prog. Biophys. Mol. Biol. 2013, 112, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Ramachandra, C.J.; Mehta, A.; Wong, P.; Ja, K.M.M.; Fritsche-Danielson, R.; Bhat, R.V.; Hausenloy, D.J.; Kovalik, J.-P.; Shim, W. Fatty acid metabolism driven mitochondrial bioenergetics promotes advanced developmental phenotypes in human induced pluripotent stem cell derived cardiomyocytes. Int. J. Cardiol. 2018, 272, 288–297. [Google Scholar] [CrossRef] [PubMed]




| Months | DEX (10 nmol/L) | % Wells Containing Beating Tissue |
|---|---|---|
| 1 | – | 0.0% (n = 27) |
| 1 | + | 66.7% (n = 36) |
| 2 | + | 62.9% (n = 35) |
| 4 | + | 73.5% (n = 34) |
| 6 | + | 71.4% (n = 7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eisenberg, L.M.; Kaur, K.; Castillo, J.M.; Edwards, J.G.; Eisenberg, C.A. Dexamethasone Treatment Preserves the Structure of Adult Cardiac Explants and Supports Their Long-Term Contractility In Vitro. Int. J. Transl. Med. 2023, 3, 360-373. https://doi.org/10.3390/ijtm3030025
Eisenberg LM, Kaur K, Castillo JM, Edwards JG, Eisenberg CA. Dexamethasone Treatment Preserves the Structure of Adult Cardiac Explants and Supports Their Long-Term Contractility In Vitro. International Journal of Translational Medicine. 2023; 3(3):360-373. https://doi.org/10.3390/ijtm3030025
Chicago/Turabian StyleEisenberg, Leonard M., Keerat Kaur, John M. Castillo, John G. Edwards, and Carol A. Eisenberg. 2023. "Dexamethasone Treatment Preserves the Structure of Adult Cardiac Explants and Supports Their Long-Term Contractility In Vitro" International Journal of Translational Medicine 3, no. 3: 360-373. https://doi.org/10.3390/ijtm3030025
APA StyleEisenberg, L. M., Kaur, K., Castillo, J. M., Edwards, J. G., & Eisenberg, C. A. (2023). Dexamethasone Treatment Preserves the Structure of Adult Cardiac Explants and Supports Their Long-Term Contractility In Vitro. International Journal of Translational Medicine, 3(3), 360-373. https://doi.org/10.3390/ijtm3030025







