The First Approved COVID-19 Vaccines: The Road to Cancer Vaccines
Abstract
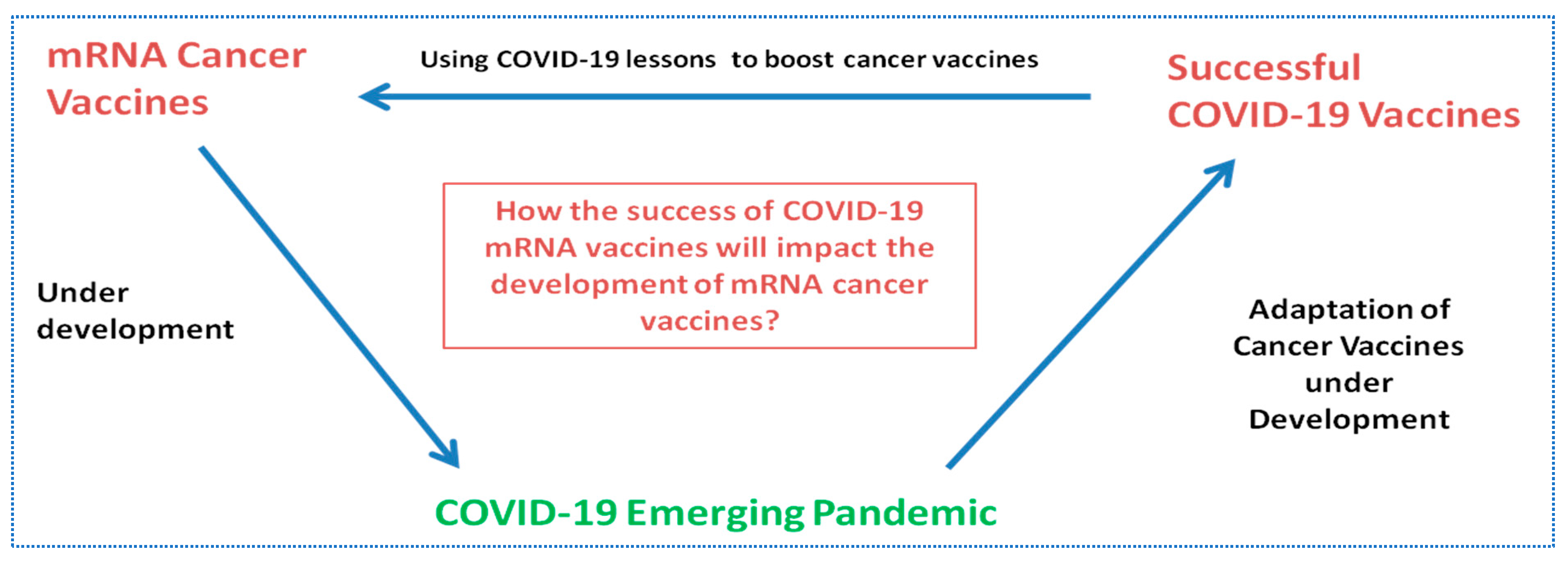
:1. Introduction
2. Search Strategy
2.1. Data Collection Process for COVID-19 Vaccines
2.2. Data Collection Process for mRNA Cancer Vaccines
2.3. Data Collection for Immunology Concepts
3. Data Analysis and Study Limitations
4. Results
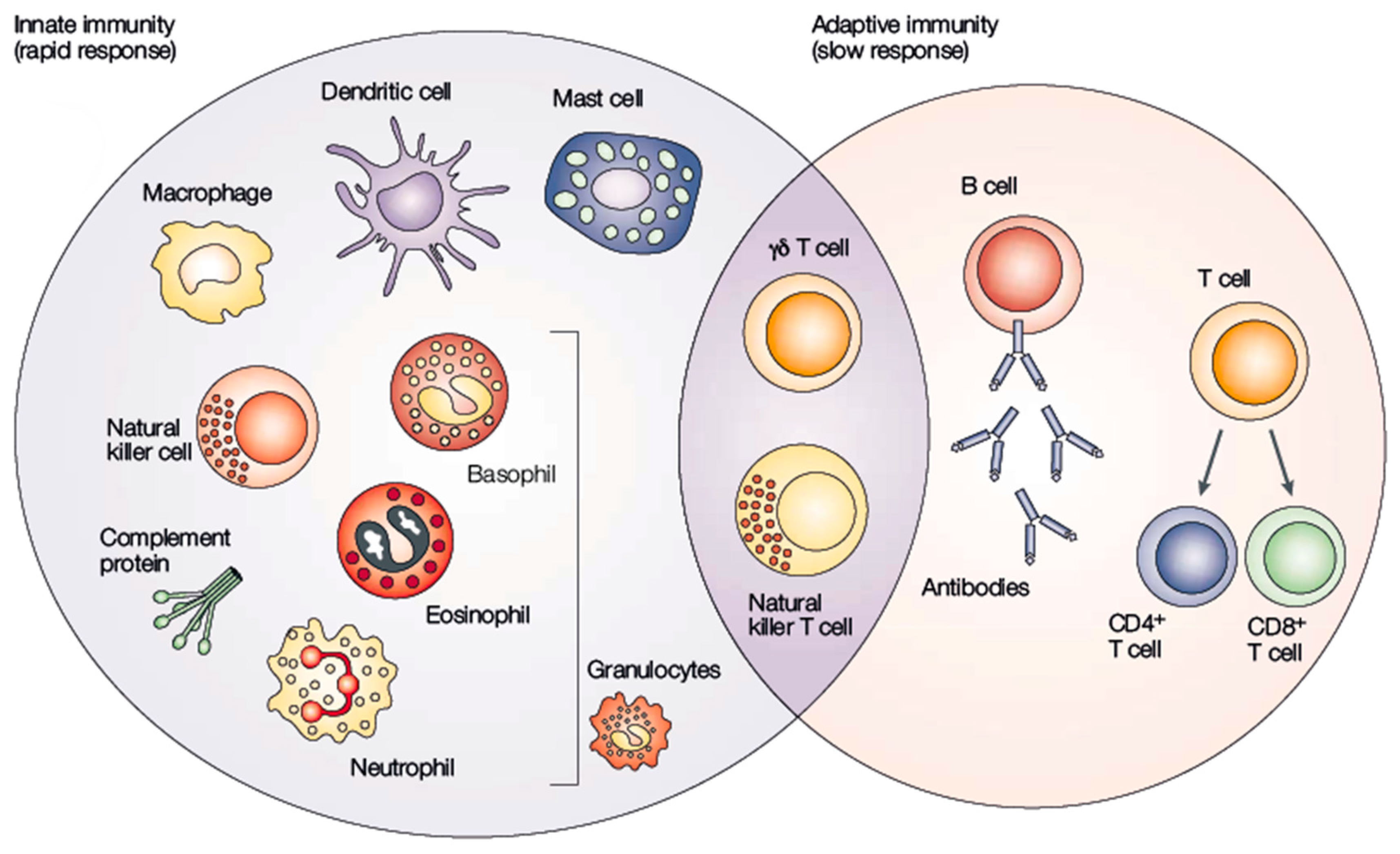
4.1. Immunology Concepts
4.1.1. Innate Immune Responses against mRNA Vaccines
4.1.2. Differences and Similarities across Immune Responses between Infectious Diseases and Cancer
4.2. Vaccine Techonologies for SARS-CoV-2 Candidates
4.3. mRNA Platform for Vaccines—Advantages and Disadvantages
4.4. mRNA Cancer Vaccines
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Greenwood, B. The contribution of vaccination to global health: Past, present and future. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2014, 369, 20130433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. Ten Threats to Global Health in 2019; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- Dong, Y.; Dai, T.; Wei, Y.; Zhang, L.; Zheng, M.; Zhou, F. A systematic review of SARS-CoV-2 vaccine candidates. Signal Transduct. Target. Ther. 2020, 5, 237. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Gao, G.F. Viral targets for vaccines against COVID-19. Nat. Rev. Immunol. 2020, 21, 73–82. [Google Scholar] [CrossRef] [PubMed]
- CDC. Varicella. In Epidemiology and Prevention of Vaccine-Preventable Diseases; CDC: Atlanta, GA, USA, 2021. [Google Scholar]
- Wolf, J.; Bruno, S.; Eichberg, M.; Jannat, R.; Rudo, S.; VanRheenen, S.; Coller, B.A. Applying lessons from the Ebola vaccine experience for SARS-CoV-2 and other epidemic pathogens. Npj Vaccines 2020, 5, 51. [Google Scholar] [CrossRef]
- Bok, K.; Sitar, S.; Graham, B.S.; Mascola, J.R. Accelerated COVID-19 vaccine development: Milestones, lessons, and prospects. Immunity 2021, 54, 1636–1651. [Google Scholar] [CrossRef]
- Ball, P. The lightning-fast quest for COVID vaccines—And what it means for other diseases. Nature 2020, 589, 16–18. [Google Scholar] [CrossRef]
- Dolgin, E. How COVID unlocked the power of RNA vaccines. Nature 2021, 589, 189–191. [Google Scholar] [CrossRef]
- Lythgoe, M.P.; Middleton, P. Comparison of COVID-19 Vaccine Approvals at the US Food and Drug Administration, European Medicines Agency, and Health Canada. JAMA Netw. Open 2021, 4, e2114531. [Google Scholar] [CrossRef]
- Saadi, F.; Pal, D.; Sarma, J.D. Spike Glycoprotein Is Central to Coronavirus Pathogenesis-Parallel Between m-CoV and SARS-CoV-2. Ann. Neurosci. 2021, 28, 201–218. [Google Scholar] [CrossRef]
- Badgujar, K.C.; Badgujar, V.C.; Badgujar, S.B. Vaccine development against coronavirus (2003 to present): An overview, recent advances, current scenario, opportunities and challenges. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 1361–1376. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Du, L.; Jiang, S. Learning from the past: Development of safe and effective COVID-19 vaccines. Nat. Rev. Genet. 2020, 19, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Riley, T.P.; Chou, H.T.; Hu, R.; Bzymek, K.P.; Correia, A.R.; Partin, A.C.; Li, D.; Gong, D.; Wang, Z.; Yu, X.; et al. Enhancing the Prefusion Conformational Stability of SARS-CoV-2 Spike Protein Through Structure-Guided Design. Front. Immunol. 2021, 12, 660198. [Google Scholar] [CrossRef] [PubMed]
- Khandker, S.S.; Godman, B.; Jawad, I.; Meghla, B.A.; Tisha, T.A.; Khondoker, M.U.; Haq, A.; Charan, J.; Talukder, A.A.; Azmuda, N.; et al. A Systematic Review on COVID-19 Vaccine Strategies, Their Effectiveness, and Issues. Vaccines 2021, 9, 1387. [Google Scholar] [CrossRef] [PubMed]
- Fang, E.; Liu, X.; Li, M.; Zhang, Z.; Song, L.; Zhu, B.; Wu, X.; Liu, J.; Zhao, D.; Li, Y. Advances in COVID-19 mRNA vaccine development. Signal Transduct. Target. Ther. 2022, 7, 94. [Google Scholar] [CrossRef] [PubMed]
- Granados-Riveron, J.T.; Aquino-Jarquin, G. Engineering of the current nucleoside-modified mRNA-LNP vaccines against SARS-CoV-2. Biomed. Pharmacother. 2021, 142, 111953. [Google Scholar] [CrossRef]
- DDolgin, E. Unlocking the potential of vaccines built on messenger RNA. Nature 2019, 574, S10–S12. [Google Scholar] [CrossRef] [Green Version]
- British Society for Immunology, What Is Immunology? Available online: https://www.immunology.org/public-information/what-is-immunology (accessed on 5 February 2022).
- PParkin, J.; Cohen, B. An overview of the immune system. Lancet 2001, 357, 1777–1789. [Google Scholar] [CrossRef]
- Eftimie, R.; Gillard, J.J.; Cantrell, D.A. Mathematical Models for Immunology: Current State of the Art and Future Research Directions. Bull. Math. Biol. 2016, 78, 2091–2134. [Google Scholar] [CrossRef] [Green Version]
- Marshall, J.S.; Warrington, R.; Watson, W.; Kim, H.L. An introduction to immunology and immunopathology. Allergy, Asthma Clin. Immunol. 2018, 14, 49. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Yang, W.; Li, W.; Zhao, Y. NLRP3 Inflammasome: Checkpoint Connecting Innate and Adaptive Immunity in Autoimmune Diseases. Front. Immunol. 2021, 12, 732933. [Google Scholar] [CrossRef]
- Sherwood, E.R.; Burelbach, K.R.; McBride, M.A.; Stothers, C.L.; Owen, A.M.; Hernandez, A.; Patil, N.K.; Williams, D.L.; Bohannon, J.K. Innate Immune Memory and the Host Response to Infection. J. Immunol. 2022, 208, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Dranoff, G. Cytokines in cancer pathogenesis and cancer therapy. Nat. Cancer 2004, 4, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Haseeb, M.; Anwar, M.A.; Choi, S. Molecular Interactions Between Innate and Adaptive Immune Cells in Chronic Lymphocytic Leukemia and Their Therapeutic Implications. Front. Immunol. 2018, 9, 2720. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, T.; Kitamura, H.; Iwakabe, K.; Yahata, T.; Ohta, A.; Sato, M.; Takeda, K.; Okumura, K.; Van Kaer, L.; Kawano, T.; et al. The interface between innate and acquired immunity: Glycolipid antigen presentation by CD1d-expressing dendritic cells to NKT cells induces the differentiation of antigen-specific cytotoxic T lymphocytes. Int. Immunol. 2000, 12, 987–994. [Google Scholar] [CrossRef] [Green Version]
- Wieczorek, M.; Abualrous, E.T.; Sticht, J.; Álvaro-Benito, M.; Stolzenberg, S.; Noé, F.; Freund, C. Major Histocompatibility Complex (MHC) Class I and MHC Class II Proteins: Conformational Plasticity in Antigen Presentation. Front. Immunol. 2017, 8, 292. [Google Scholar] [CrossRef] [Green Version]
- Spellberg, B.; Edwards, J.E., Jr. Type 1/Type 2 Immunity in Infectious Diseases. Clin. Infect. Dis. 2001, 32, 76–102. [Google Scholar] [CrossRef]
- Sahin, U.; Muik, A.; Derhovanessian, E.; Vogler, I.; Kranz, L.M.; Vormehr, M.; Baum, A.; Pascal, K.; Quandt, J.; Maurus, D.; et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T-cell responses. Nature 2020, 586, 594–599. [Google Scholar] [CrossRef]
- Lee, S.; Ryu, J.-H. Influenza Viruses: Innate Immunity and mRNA Vaccines. Front. Immunol. 2021, 12, 710647. [Google Scholar] [CrossRef]
- Minnaert, A.-K.; Vanluchene, H.; Verbeke, R.; Lentacker, I.; De Smedt, S.C.; Raemdonck, K.; Sanders, N.N.; Remaut, K. Strategies for controlling the innate immune activity of conventional and self-amplifying mRNA therapeutics: Getting the message across. Adv. Drug Deliv. Rev. 2021, 176, 113900. [Google Scholar] [CrossRef]
- Vance, R.E.; Eichberg, M.J.; Portnoy, D.A.; Raulet, D.H. Listening to each other: Infectious disease and cancer immunology. Sci. Immunol. 2017, 2, eaai9339. [Google Scholar] [CrossRef] [Green Version]
- Benharroch, D.; Osyntsov, L. Infectious Diseases Are Analogous With Cancer. Hypothesis And Implications. J. Cancer 2012, 3, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Trinchieri, G. Cancer Immunity: Lessons From Infectious Diseases. J. Infect. Dis. 2015, 212, S67–S73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EMA. COVID-19 Vaccines: Development, Evaluation, Approval and Monitoring. 2020. Available online: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/vaccines-covid-19/covid-19-vaccines-development-evaluation-approval-monitoring (accessed on 3 February 2022).
- Marinus, R.; Mofid, S.; Mpandzou, M.; Kühler, T.C. Rolling Reviews During COVID-19: The European Union Experience in a Global Context. Clin. Ther. 2022, 44, 352–363. [Google Scholar] [CrossRef] [PubMed]
- CDC. Pfizer-BioNTech COVID-19 Vaccine. Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/Pfizer-BioNTech.html (accessed on 2 February 2022).
- CDC. Moderna COVID-19 Vaccine. Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/Moderna.html (accessed on 2 February 2022).
- EMA. EPAR Comirnaty. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/comirnaty (accessed on 3 February 2022).
- EMA. EPAR Spikevax. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/spikevax (accessed on 3 February 2022).
- EMA. EPAR Vaxzevria. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/vaxzevria-previously-covid-19-vaccine-astrazeneca (accessed on 3 February 2022).
- AstraZeneca, A.B. Summary of Product Characteristics, Vaxzevria. 2021. Available online: https://www.ema.europa.eu/en/documents/product-information/vaxzevria-previously-covid-19-vaccine-astrazeneca-epar-product-information_en.pdf (accessed on 3 February 2022).
- EMA. EPAR COVID-19 Vaccine Janssen. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/covid-19-vaccine-janssen (accessed on 4 February 2022).
- CDC. Johnson & Johnson’s Janssen COVID-19 Vaccine. Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/janssen.html (accessed on 4 February 2022).
- Janssen-Cilag International, N.V. Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/covid-19-vaccine-janssen-epar-product-information_en.pdf (accessed on 3 February 2022).
- Wang, Y.; Zhang, Z.; Luo, J.; Han, X.; Wei, Y.; Wei, X. mRNA vaccine: A potential therapeutic strategy. Mol. Cancer 2021, 20, 33. [Google Scholar] [CrossRef]
- Chakraborty, C.; Sharma, A.R.; Bhattacharya, M.; Lee, S.-S. From COVID-19 to Cancer mRNA Vaccines: Moving From Bench to Clinic in the Vaccine Landscape. Front. Immunol. 2021, 12, 679344. [Google Scholar] [CrossRef]
- Rosa, S.S.; Prazeres, D.M.F.; Azevedo, A.M.; Marques, M.P.C. mRNA vaccines manufacturing: Challenges and bottlenecks. Vaccine 2021, 39, 2190–2200. [Google Scholar] [CrossRef]
- Conditional Marketing Authorisation, EMA. Available online: https://www.ema.europa.eu/en/human-regulatory/marketing-authorisation/conditional-marketing-authorisation (accessed on 7 May 2022).
- Heine, A.; Juranek, S.; Brossart, P. Clinical and immunological effects of mRNA vaccines in malignant diseases. Mol. Cancer 2021, 20, 52. [Google Scholar] [CrossRef]
- Chaudhary, N.; Weissman, D.; Whitehead, K.A. mRNA vaccines for infectious diseases: Principles, delivery and clinical translation. Nat. Rev. Drug Discov. 2021, 20, 817–838. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef] [Green Version]
- Benteyn, D.; Heirman, C.; Bonehill, A.; Thielemans, K.; Breckpot, K. mRNA-based dendritic cell vaccines. Expert Rev. Vaccines 2015, 14, 161–176. [Google Scholar] [CrossRef]
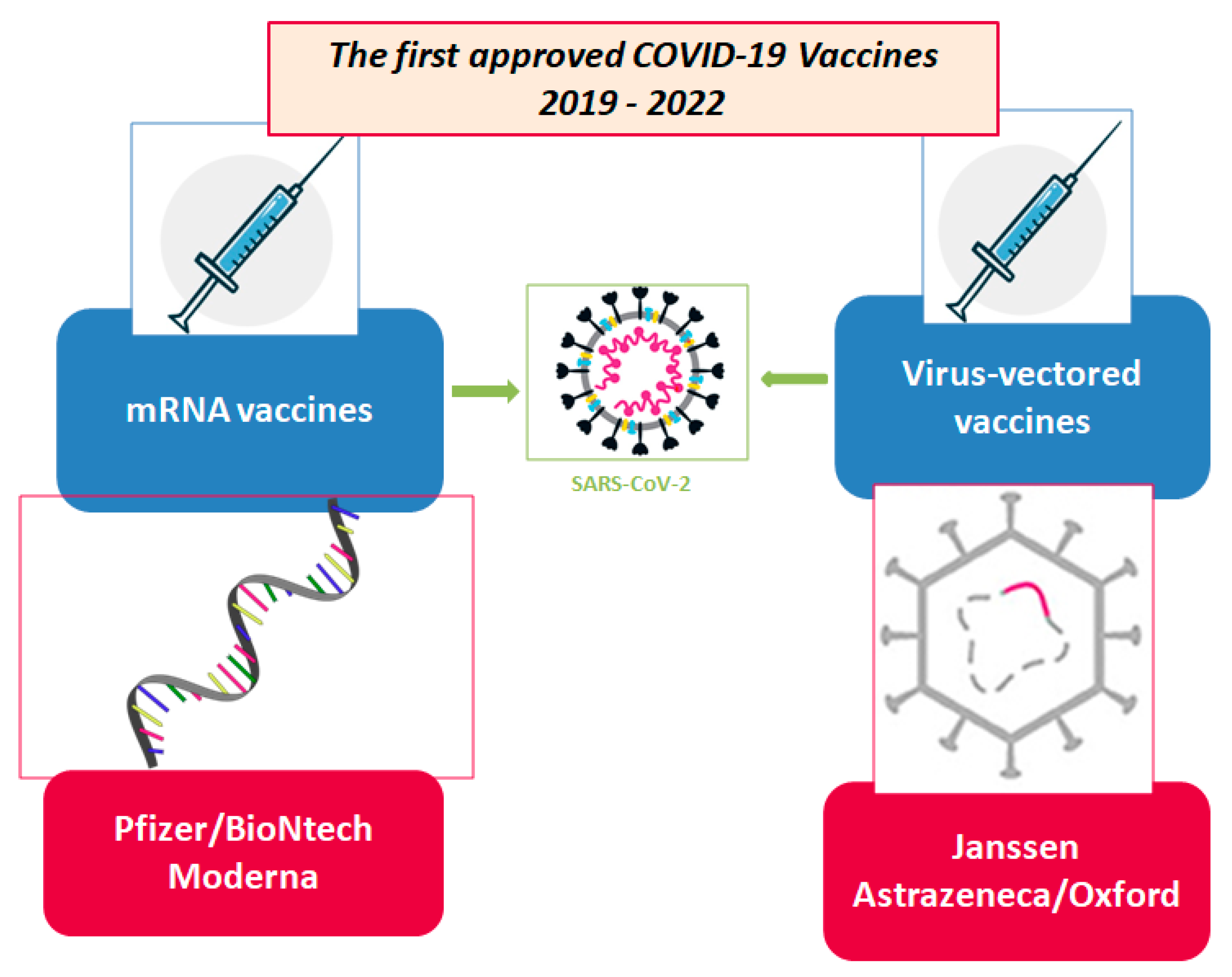
| MAH | Name | Strategy | Active Component | Formulation | Dosage | How It Works |
|---|---|---|---|---|---|---|
| Pfizer BioNTech | BNT162b2 | mRNA vaccines. The mRNA provides instructions the body uses to build a harmless piece of a protein from the virus that causes COVID-19. This protein causes an immune response that helps protect the body from getting sick with COVID-19 in the future [38,39]. | Nucleoside modified mRNA (Single-stranded, 5′-capped messenger RNA produced using a cell-free in vitro transcription from the corresponding DNA templates, encoding the viral spike (S) protein of SARS-CoV-2) Prefusion stabilized spike protein [40] | Individuals 12 years of age and older Lipids (help the mRNA to enter the cells)
| Individuals 12 years of age and older 2 shots, 21 days apart [38] One dose (0.3 mL) contains 30 micrograms of tozinameran, a COVID-19 mRNA Vaccine (embedded in lipid nanoparticles) [40] | Comirnaty and Spikevax work by preparing the body to defend itself against COVID-19. It contains a molecule called mRNA which has instructions for making the spike protein. This is a protein on the surface of the SARS-CoV-2 virus which the virus needs to enter the body’s cells. When a person is given the vaccine, some of their cells will read the mRNA instructions and temporarily produce the spike protein. The person’s immune system will then recognise this protein as foreign and produce antibodies and activate T-cells (white blood cells) to attack it. If, later on, the person comes into contact with SARS-CoV-2 virus, their immune system will recognise it and be ready to defend the body against it. The mRNA from the vaccine does not stay in the body but is broken down shortly after vaccination [40,41]. |
| Moderna | RNA-1273 | CX-024414 Nucleoside modified mRNA (single-stranded, 5′-capped messenger RNA (mRNA) produced using a cell-free in vitro transcription from the corresponding DNA templates, encoding the viral spike (S) protein of SARS-CoV-2) Prefusion stabilized spike protein [41] | Individuals 12 years of age and older Lipids (help the mRNA to enter the cells)
| Individuals 12 years of age and older One dose (0.5 mL) contains 100 micrograms of messenger RNA (mRNA) (embedded in SM-102 lipid nanoparticles) Spikevax is administered as a course of 2 (two) 100 microgram doses (0.5 mL each). It is recommended to administer the second dose 28 days after the first dose [41] | ||
| AstraZeneca | AZD1222 | Vaxzevria is made up of another virus (of the adenovirus family) that has been modified to contain the gene for making a protein from SARS-CoV-2. Vaxzevria does not contain the virus itself and cannot cause COVID-19 [42]. | ChAdOx1-SARS-CoV-2 Prefusion stabilized spike protein [42] | L-Histidine L-Histidine hydrochloride monohydrate Magnesium chloride hexahydrate Polysorbate 80 (E 433) Ethanol Sucrose Sodium chloride Disodium edetate (dihydrate) Water for injections [43] | Individuals 18 years of age and older One dose (0.5 mL) contains: Chimpanzee Adenovirus encoding the SARS-CoV-2 Spike glycoprotein (ChAdOx1-S), not less than 2.5 × 108 infectious units (Inf.U) * Produced in genetically modified human embryonic kidney (HEK) 293 cells and by recombinant DNA technology. This product contains genetically modified organisms (GMOs). Individuals 18 years of age and older The Vaxzevria vaccination course consists of two separate doses of 0.5 mL each. The second dose should be administered between 4 and 12 weeks (28 to 84 days) after the first dose [43] | Vaxzevria works by preparing the body to defend itself against COVID-19. It is made up of another virus (adenovirus) that has been modified to contain the gene for making the SARS-CoV-2 spike protein. This is a protein on the surface of the SARS-CoV-2 virus which the virus needs to enter the body’s cells. Once it has been given, the vaccine delivers the SARS-CoV-2 gene into cells in the body. The cells will use the gene to produce the spike protein. The person’s immune system will then recognise this protein as foreign and produce antibodies and activate T-cells (white blood cells) to attack it. If, later on, the person comes into contact with SARS-CoV-2 virus, their immune system will recognise it and be ready to defend the body against it. The adenovirus in the vaccine cannot reproduce and does not cause disease [42]. |
| Janssen Pharmaceuticals Companies of Johnson & Johnson | JNJ-78436735 | COVID-19 Vaccine Janssen is made up of another virus (of the adenovirus family) that has been modified to contain the gene for making a protein found on SARS-CoV-2 [44]. This harmless version of a virus unrelated to the COVID-19 virus provides instructions the body uses to build a harmless piece of a protein from the virus that causes COVID-19. This protein causes an immune response that helps protect the body from getting sick with COVID-19 in the future [45]. | Adenovirus type 26 encoding the SARS-CoV-2 spike glycoprotein (Ad26.COV2-S) Prefusion stabilized spike protein [44] | Sugars, salts, acid, and acid stabilizer:
| Individuals 18 years of age and older 1 shot requiring a booster dose Everyone ages 18 years and older should get a booster dose of either Pfizer-BioNTech or Moderna (mRNA COVID-19 vaccines) at least 2 months after receiving the Johnson & Johnson’s Janssen (J&J/Janssen) vaccine in most situations [45]. One dose (0.5 mL) contains: Adenovirus type 26 encoding the SARS-CoV-2 spike glycoprotein * (Ad26.COV2-S), not less than 8.92 log10 infectious units (Inf.U). [46] | COVID-19 Vaccine Janssen works by preparing the body to defend itself against COVID-19. It is made up of another virus (an adenovirus) that has been modified to contain the gene for making the SARS-CoV-2 spike protein. This is a protein on the SARS-CoV-2 virus which it needs to enter the body’s cells. The adenovirus passes the SARS-CoV-2 gene into the vaccinated person’s cells. The cells can then use the gene to produce the spike protein. The person’s immune system will recognise the spike protein as foreign and produce antibodies and activate T-cells (white blood cells) to target it. Later, if the person comes into contact with SARS-CoV-2 virus, the person’s immune system will recognise the spike protein on the virus and be ready to defend the body against it. The adenovirus in the vaccine cannot reproduce and does not cause the disease [44] |
| Title | Status | Study Results | Conditions | Interventions | Characteristics | Dates—Study Start | Locations | Trial Identifier |
|---|---|---|---|---|---|---|---|---|
| Trial of Vaccine Therapy With mRNA—Transfected Dendritic Cells in Patients with Advanced Malignant Melanoma | Completed | No Results Available |
|
| Phase:
| March 2002 | NCT01278940 | |
| Carcinoembryonic Antigen-loaded Dendritic Cells in Advanced Colorectal Cancer Patients | Completed | No Results Available |
|
| Phase:
| December 2003 |
| NCT00228189 |
| Peptide-pulsed vs. RNA-transfected Dendritic Cell Vaccines in Melanoma Patients | Completed | No Results Available |
|
| Phase:
| April 2004 |
| NCT00243529 |
| Peptide-specific Vaccination in HLA-A*02 Positive Patients with Biochemical Recurrence After Radical Prostatectomy | Unknown status | No Results Available |
|
| Phase:
| April 2004 | NCT02452307 | |
| Intradermal Vaccination with Stabilized Tumor mRNA—a Clinical Phase I/II Trial in Melanoma Patients | Completed | No Results Available |
|
| Phase:
| July 2004 |
| NCT00204607 |
| Dendritic Cell Vaccination for Patients with Acute Myeloid Leukemia in Remission | Completed | No Results Available |
|
| Phase: Phase 1 | March 2005 |
| NCT00834002 |
| Vaccine Therapy in Treating Patients with Newly Diagnosed Glioblastoma Multiforme | Active, not recruiting | No Results Available |
|
| Phase: Phase 1 | January 2006 |
| NCT00639639 |
| Vaccination With Tumor mRNA in Metastatic Melanoma—Fixed Combination Versus Individual Selection of Targeted Antigens | Completed | No Results Available |
|
| Phase:
| April 2007 |
| NCT00204516 |
| Basiliximab in Treating Patients With Newly Diagnosed Glioblastoma Multiforme Undergoing Targeted Immunotherapy and Temozolomide-Caused Lymphopenia | Completed | No Results Available |
|
| Phase: Phase 1 | April 2007 |
| NCT00626483 |
| A Study of Active Immunotherapy with GRNVAC1 in Patients With Acute Myelogenous Leukemia (AML) | Completed | No Results Available |
|
| Phase: Phase 2 | July 2007 |
| NCT00510133 |
| Feasibility Study of Acute Myelogenous Leukemia mRNA Plus Lysate Loaded Dendritic Cell Vaccines | Terminated | No Results Available |
|
| Phase: Phase 1 | July 2007 |
| NCT00514189 |
| Human Telomerase Reverse Transcriptase Messenger RNA (hTERT mRNA) Transfected Dendritic Cell Vaccines | Withdrawn | No Results Available |
|
| Phase:
| January 2008 | NCT01153113 | |
| Trial for Vaccine Therapy with Dendritic Cells in Patients With Metastatic Malignant Melanoma | Terminated | No Results Available |
|
| Phase:
| August 2009 |
| NCT00961844 |
| A Study on the Safety and Immunogenicity of Combined Intradermal and Intravenous Administration of an Autologous mRNA Electroporated Dendritic Cell Vaccine in Patients with Previously Treated Unresectable Stage III or IV Melanoma | Completed | No Results Available |
|
| Phase: Phase 1 | December 2009 |
| NCT01066390 |
| Trial of Bi-shRNA-furin and GMCSF Augmented Autologous Tumor Cell Vaccine for Advanced Cancer | Completed | No Results Available |
|
| Phase: Phase 1 | December 2009 |
| NCT01061840 |
| Safe Study of Dendritic Cell (DC) Based Therapy Targeting Tumor Stem Cells in Glioblastoma | Completed | No Results Available |
|
| Phase:
| January 2009 | NCT00846456 | |
| Messenger Ribonucleic Acid (mRNA) Transfected Dendritic Cell Vaccination in High Risk Uveal Melanoma Patients | Terminated | No Results Available |
|
| Phase:
| June 2009 |
| NCT00929019 |
| Toll-like Receptor (TLR) Ligand Matured Dendritic Cell Vaccination in Melanoma Patients | Completed | No Results Available |
| Biological: autologous dendritic cell vaccination | Phase:
| June 2009 |
| NCT00940004 |
| Trial of an RNActive®-Derived Cancer Vaccine in Stage IIIB/IV Non-Small Cell Lung Cancer (NSCLC) | Completed | No Results Available |
| Biological: CV9201 | Phase:
| May 2009 |
| NCT00923312 |
| Transfected Dendritic Cell Based Therapy for Patients with Breast Cancer or Malignant Melanoma | Completed | No Results Available |
|
| Phase: Phase 1 | September 2009 |
| NCT00978913 |
| Vaccine Therapy in Treating Patients Undergoing Surgery for Recurrent Glioblastoma Multiforme | Completed | No Results Available |
|
| Phase: Phase 1 | September 2009 |
| NCT00890032 |
| Single-step Antigen Loading and TLR Activation of Dendritic Cells in Melanoma Patients | Completed | No Results Available |
|
| Phase:
| April 2010 |
| NCT01530698 |
| Efficacy of Dendritic Cell Therapy for Myeloid Leukemia and Myeloma | Unknown status | No Results Available |
|
| Phase: Phase 2 | January 2010 |
| NCT00965224 |
| Safety of Active Immunotherapy in Subjects with Ovarian Cancer | Unknown status | No Results Available |
|
| Phase: Phase 1 | September 2010 |
| NCT01456065 |
| Vaccine Therapy in Curative Resected Prostate Cancer Patients | Active, not recruiting | No Results Available |
|
| Phase:
| September 2010 |
| NCT01197625 |
| Trial of Vaccine Therapy in Recurrent Platinum Sensitive Ovarian Cancer Patients | Terminated | No Results Available |
|
| Phase:
| April 2011 |
| NCT01334047 |
| Dendritic Cell Vaccination for Patients with Solid Tumors | Unknown status | No Results Available |
|
| Phase:
| February 2011 |
| NCT01291420 |
| Platin-based Chemotherapeutics to Enhance Dendritic Cell Vaccine Efficacy in Melanoma Patients | Completed | No Results Available |
|
| Phase: Phase 2 | February 2011 |
| NCT02285413 |
| Trial of Vaccine Therapy With mRNA- Transfected Dendritic Cells in Patients with Androgen Resistant Metastatic Prostate Cancer | Completed | No Results Available |
|
| Phase:
| No information available |
| NCT01278914 |
| Immune Responses to Autologous Langerhans-type Dendritic Cells Electroporated with mRNA Encoding a Tumor-associated Antigen in Patients With Malignancy: A Single- arm Phase I Trial in Melanoma | Active, not recruiting | No Results Available |
| Biological: Langerhanstype dendritic cells (a.k.a. Langerhans cells or LCs) | Phase: Phase 1 | October 2011 |
| NCT01456104 |
| Dendritic Cell Vaccination and Docetaxel for Patients with Prostate Cancer | Completed | No Results Available |
|
| Phase: Phase 2 | October 2011 |
| NCT01446731 |
| Efficacy Study of Dendritic Cell Vaccination in Patients with Acute Myeloid Leukemia in Remission | Recruiting | No Results Available |
|
| Phase: Phase 2 | October 2012 |
| NCT01686334 |
| DC Vaccination for Postremission Therapy in AML | Completed | No Results Available |
|
| Phase:
| November 2013 |
| NCT01734304 |
| DC Vaccine Combined with CIK Cells in Patients With Esophagus Cancer | Unknown status | No Results Available |
|
| Phase:
| August 2014 |
| NCT02693236 |
| CT7, MAGE-A3, and WT1 mRNA- electroporated Autologous Langerhans-type Dendritic Cells as Consolidation for Multiple Myeloma Patients Undergoing Autologous Stem Cell Transplantation | Active, not recruiting | No Results Available |
|
| Phase: Phase 1 | January 2014 |
| NCT01995708 |
| An Open Label Randomised Trial of RNActive® Cancer Vaccine in High Risk and Intermediate Risk Patients with Prostate Cancer | Terminated | No Results Available |
|
| Phase: Phase 2 | June 2014 |
| NCT02140138 |
| Adjuvant Dendritic Cell-immunotherapy Plus Temozolomide in Glioblastoma Patients | Recruiting | No Results Available |
|
| Phase:
| December 2015 |
| NCT02649582 |
| Natural Dendritic Cells for Immunotherapy of Chemo-naive Metastatic Castration-resistant Prostate Cancer Patients | Completed | No Results Available |
|
| Phase:Phase 2 | September 2015 |
| NCT02692976 |
| Cellular Immunotherapy for Patients with High Risk Myelodysplastic Syndromes and Acute Myeloid Leukemia | Active, not recruiting | No Results Available |
|
| Phase:
| August 2016 |
| NCT03083054 |
| Vaccine Therapy for the Treatment of Newly Diagnosed Glioblastoma Multiforme | Recruiting | No Results Available |
|
| Phase: Phase 2 | August 2016 |
| NCT02465268 |
| Safety and Efficacy of DC-CIK in Patients with Advanced Non-Small-Cell Lung Cancer With Bone Metastases | Unknown status | No Results Available |
|
| Phase:
| February 2016 |
| NCT02688686 |
| MiHA-loaded PD-L-silenced DC Vaccination After Allogeneic SCT | Completed | No Results Available |
|
| Phase:
| January 2016 |
| NCT02528682 |
| Nivolumab With DC Vaccines for Recurrent Brain Tumors | Completed | Has Results |
|
| Phase: Phase 1 | January 2016 |
| NCT02529072 |
| Personalized Cellular Vaccine for Brain Metastases (PERCELLVAC3) | Unknown status | No Results Available |
|
| Phase: Phase 1 | June 2016 |
| NCT02808416 |
| Personalized Cellular Vaccine for Recurrent Glioblastoma (PERCELLVAC2) | Unknown status | No Results Available |
|
| Phase: Phase 1 | June 2016 |
| NCT02808364 |
| Personalized Cellular Vaccine for Glioblastoma (PERCELLVAC) | Unknown status | No Results Available |
|
| Phase: Phase 1 | March 2016 |
| NCT02709616 |
| Autologous Dendritic Cell Vaccination in Mesothelioma | Recruiting | No Results Available |
|
| Phase:
| August 2017 |
| NCT02649829 |
| Safety, Tolerability, and Immunogenicity of mRNA-4157 Alone in Participants With Resected Solid Tumors and in Combination with Pembrolizumab in Participants With Unresectable Solid Tumors | Recruiting | No Results Available |
|
| Phase: Phase 1 | August 2017 |
| NCT03313778 |
| Phase 1/2 Study of Combination Immunotherapy and mRNA Vaccine in Subjects With NSCLC | Completed | No Results Available |
|
| Phase:
| December 2017 |
| NCT03164772 |
| Dendritic Cell Immunotherapy Against Cancer Stem Cells in Glioblastoma Patients Receiving Standard Therapy | Recruiting | No Results Available |
|
| Phase:
| April 2018 |
| NCT03548571 |
| Clinical Study of Personalized mRNA Vaccine Encoding Neoantigen in Patients with Advanced Digestive System Neoplasms | Unknown status | No Results Available |
| Biological: Personalized mRNA Tumor Vaccine | Phase: Not Applicable | May 2018 |
| NCT03468244 |
| Messenger RNA (mRNA)-Based, Personalized Cancer Vaccine Against Neoantigens Expressed by the Autologous Cancer | Terminated | Has Results |
| Biological: National Cancer Institute (NCI)-4650, a messenger ribonucleic acid (mRNA)-based, Personalized Cancer Vaccine | Phase:
| May 2018 |
| NCT03480152 |
| PRO-MERIT (Prostate Cancer Messenger RNA Immunotherapy) | Recruiting | No Results Available |
|
| Phase:
| December 2019 |
| NCT04382898 |
| An Efficacy Study of Adjuvant Treatment with the Personalized Cancer Vaccine mRNA-4157 and Pembrolizumab in Participants With High- Risk Melanoma (KEYNOTE-942) | Active, not recruiting | No Results Available |
|
| Phase: Phase 2 | July 2019 |
| NCT03897881 |
| A Study of mRNA-5671/V941 as Monotherapy and in Combination With Pembrolizumab (V941-001) | Active, not recruiting | No Results Available |
| Biological: V941
| Phase: Phase 1 | June 2019 |
| NCT03948763 |
| Clinical Study of Personalized mRNA Vaccine Encoding Neoantigen in Patients with Advanced Esophageal Cancer and Non-small Cell Lung Cancer | Not yet recruiting | No Results Available |
| Biological: Personalized mRNA Tumor Vaccine | Phase: Not Applicable | May 2019 | NCT03908671 | |
| Ovarian Cancer Treatment with a Liposome Formulated mRNA Vaccine in Combination With (Neo-)Adjuvant Chemotherapy | Recruiting | No Results Available |
|
| Phase: Phase 1 | November 2019 |
| NCT04163094 |
| Immunotherapy Targeted Against Cytomegalovirus in Patients with Newly- Diagnosed WHO Grade IV Unmethylated Glioma | Suspended | No Results Available |
|
| Phase: Phase 2 | September 2019 |
| NCT03927222 |
| A Study of RNA-lipid Particle (RNA-LP) Vaccines for Newly Diagnosed Pediatric High- Grade Gliomas (pHGG) and Adult Glioblastoma (GBM) | Recruiting | No Results Available |
|
| Phase: Phase 1 | October 2021 |
| NCT04573140 |
| Adjuvant Dendritic Cell Immunotherapy for Pediatric Patients with High-grade Glioma or Diffuse Intrinsic Pontine Glioma | Recruiting | No Results Available |
|
| Phase:
| September 2021 |
| NCT04911621 |
| Safety and Efficacy of Personalized Neoantigen Vaccine in Advanced Gastric Cancer, Esophageal Cancer and Liver Cancer | Recruiting | No Results Available |
|
| Phase: Not Applicable | February 2022 |
| NCT05192460 |
| A Study of Neoantigen mRNA Personalised Cancer in Patients with Advanced Solid Tumors | Not yet recruiting | No Results Available |
|
| Phase: Phase 1 | March 2022 | NCT05198752 | |
| Monocyte Antigen Carrier Cells for Newly Diagnosed GBM | Not yet recruiting | No Results Available |
|
| Phase: Phase 1 | March 2022 |
| NCT04741984 |
| Safety and Efficacy of Personalized Neoantigen Vaccine in Advanced Gastric Cancer | Not yet recruiting | No Results Available |
|
| Phase: Not Applicable | March 2022 |
| NCT05227378 |
| Novel RNA-nanoparticle Vaccine for the Treatment of Early Melanoma Recurrence Following Adjuvant Anti-PD-1 Antibody Therapy | Not yet recruiting | No Results Available |
|
| Phase: Phase 1 | May 2022 | NCT05264974 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saldanha, L.; Vale, N. The First Approved COVID-19 Vaccines: The Road to Cancer Vaccines. Int. J. Transl. Med. 2022, 2, 309-331. https://doi.org/10.3390/ijtm2030025
Saldanha L, Vale N. The First Approved COVID-19 Vaccines: The Road to Cancer Vaccines. International Journal of Translational Medicine. 2022; 2(3):309-331. https://doi.org/10.3390/ijtm2030025
Chicago/Turabian StyleSaldanha, Leonor, and Nuno Vale. 2022. "The First Approved COVID-19 Vaccines: The Road to Cancer Vaccines" International Journal of Translational Medicine 2, no. 3: 309-331. https://doi.org/10.3390/ijtm2030025
APA StyleSaldanha, L., & Vale, N. (2022). The First Approved COVID-19 Vaccines: The Road to Cancer Vaccines. International Journal of Translational Medicine, 2(3), 309-331. https://doi.org/10.3390/ijtm2030025







