Abstract
A large amount of the globally produced plastics are not treated and are eventually released into landfills or natural environments, including surface waters. The plastics that enter the aquatic environment are very often microplastics, which are produced in households by the slow degradation or abrasion of plastic products, or as whole plastic products, which eventually degrade (abrasion, photodegradation). Together with microplastics, other pollutants such as pharmaceuticals of various kinds enter surface waters—both of these counterparts can interact with each other as well as with organic and inorganic molecules available in the natural environment. The aim of this study was to identify the interaction of microplastics with pharmaceuticals, especially under conditions that are common in inland waters as well as the seas and oceans that the rivers feed their water into. It was found that salinity has a great impact on the sorption capacity of microplastics and pharmaceuticals. The sorption of naturally occurring humic substances (humic and fulvic acids) can greatly increase when the microplastic–pharmaceutical complex is formed; however, the priority of the interaction happens with pharmaceuticals and humic substances. Such complexes can influence the organisms that feed on small organic-matter particles, as they can be mistaken for food and thus be transferred throughout the food chain.
1. Introduction
With the global production of plastics rapidly increasing, it has been estimated that 79% of plastic products have not been efficiently treated and are eventually released into landfills or the natural environments [1,2]. An intermediate stage of the plastic waste degradation is the formation of small plastic particles [3]. Depending on the size, plastic waste-degradation products appearing in the environment can be classified as macroplastics (<2 cm), mesoplastics (5 mm–2 cm), microplastics (<5 mm), and nanoplastics (<1 μm) [4,5]. Considering the amounts that enter the environment and the possible threats to living organisms and ecosystems, microplastics are of special concern [6]. The main polymer constituents of microplastics found in the environment are synthetic polymers, such as polyethylene, polypropylene, polystyrene, and polyethylene terephthalate, accounting for 70% of the total, but polyvinylchloride, polyacrylonitrile, rubber, and different co-polymers are also common [7,8,9]. The high stability of the polymers forming microplastics makes them “forever chemicals” as their removal due to sedimentation, biodegradation, photo-oxidation and other degradation processes is slow and thus they are accumulated in the environment [10,11]. Nowadays, marine pollution with microplastics can be considered a major problem [12]; however, microplastics have also been found in inland waters [13], sediments of water bodies [14], drinking water [15], soil [16], air [17], and not only in industrialized territories, but also in remote places such as Antarctica [18].
The adverse impacts of microplastics on aquatic organisms due to impaired reproduction, malnutrition, internal abrasions, and blockages have been demonstrated [19,20,21], and adverse human health impacts have also been identified [22,23,24]. In addition, the toxicity of microplastics is a risk factor for their impacts on living organisms [12]. Considering their high surface area and the hydrophobicity of their surfaces, microplastics can act as sorbents and thus carriers for other environmental pollutants, for example, persistent organic pollutants [25], hydrocarbons [26], pharmaceuticals [27,28] and other pollutants [29]. The desorption of pollutants, if the particles enter the living body, is one of the microplastic toxicity mechanisms [12,21]. The sorption of pollutants onto microplastic particles influences the polymer and its additives, the particle size and surface area, as well as aging, thereby supporting the development of functional groups [30]. Microplastic–pollutant complexes can influence the persistence of both components in the environment, but also the fate of pollutants in the environment, and from this perspective the microplastic impacts have not been studied much. One of the priority emerging pollutant groups is pharmaceuticals, which can interact with microplastics during municipal wastewater treatment processes and can enter surface waters. The interaction of microplastics with pharmaceuticals can influence their properties, for example, the toxicity of microplastic particles, and facilitate their transport in aquatic environments.
Aquatic environment pollution with microplastics has been mostly studied in marine environments [29,31,32], similar to studies of microplastic interactions with pollutants of organic and inorganic origin. Inland waters are major conveyers of microplastics from generation sites (wastewaters, non-point pollution sources and others) to seas and oceans or coastal and inland seas; however, the microplastic interactions with pollutants in inland waters could be significantly different due to the higher concentrations of microplastic particles and pollutants, as well as the lower salinity than in seas and oceans. The interaction between microplastics and pollutants, especially pharmaceuticals, can significantly modify dissolved organic matter (DOM)—organic substances of natural and human made origin—for example, humic substances, which are present during wastewater treatment process as well as in inland waters at high concentrations [33]. In a few studies, it has been demonstrated that the interaction of DOM and microplastics significantly alters the properties of the latter [34,35].
The aim of this article was to study the interaction of microplastics with pharmaceuticals, especially under conditions that are common in inland waters, as well as the seas and oceans that the rivers feed their water into.
2. Materials and Methods
2.1. Microplastic Samples and Pharmaceuticals
Polyethylene (PE) and polyacrylonitrile (PN) were purchased directly from Sigma-Aldrich (St. Louis, MO, USA) with the particle sizes 40–48 µm and <50 µm, respectively. The acrylic fiber (PA) microplastic (MP) sample was prepared from a store-bought yarn. The sample was milled for 15 min in a blade mill and then sifted through a woven wire sieve with a 250 µm mesh size (Retsch, Haan, Germany). Polystyrene (PS) microplastic samples were prepared from fresh, single-use polystyrene food-storage containers. The food boxes were milled for 15 min and the resulting powdered microplastics were sifted through 125 µm mesh (Table 1).

Table 1.
Properties of microplastics used in this study.
During the initial experiments, it was determined that the different MPs contain water-soluble additives that influence the sorption of pharmaceuticals. During the initial experiments, these unidentified substances gave a background absorption that was larger or equal to the added analytes (blank samples had a higher absorption than the measured samples containing pharmaceuticals). Qualitative analysis using UPLC-PDA revealed that the water used for the washing of the MPs contained 40–75 different substances. All of the MPs were cleaned using single-use cellulose Soxhlet crucibles, placed into a Soxhlet unit and allowed to reflux for 24 h (approximately 50 cycles) with demineralized water (Behr Labortechnik, Düsseldorf, Germany). The washed MPs were air-dried at room temperature and used for all of the further experiments.
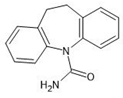
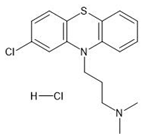
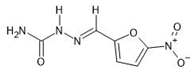
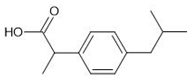
The sorption experiments in various aquatic environments were performed using four different pharmaceuticals: carbamazepine, chlorpromazine, nitrofurazone and ibuprofen (Sigma-Aldrich, purity ≥ 99.9%) (Table 2).

Table 2.
Chemical structures and selected properties of studied pharmaceuticals.
2.2. Aging of Microplastics
Microplastic samples were oxidized by the Fenton reaction to simulate the long-term natural oxidation of MPs according to a modified aging procedure [36]. The oxidation process was performed in a sealed glass batch reactor under constant stirring (200 rpm) at 80 °C and in an acidic environment (pH 3, acidified with HCl). The oxidation reaction was performed using 1 g of the respective MP in 1 L of solution. The initial concentrations of Fe2+ (added as FeCl2 × 6H2O) and H2O2 were 10 mg/L and 0.1%, respectively. An additional dose of Fe2+ (940 mg added as FeCl2 × 6H2O) and concentrated (30%) H2O2 (2.80 mL) were added every 24 h for a total of 5 days (120 h) to improve the oxidation rate and efficiency. After the oxidation with Fe2+, the reaction solution was heated to 100 °C and then 40 mL concentrated hydrochloric acid was added to separate the oxidized microplastic from the insoluble Fe(OH)3. Acid was added until the Fe(OH)3 (brownish residue) converted to soluble FeCl3, and the solution changed color to yellow. The final aged microplastics were then filtered through a Whatman 11 filter paper and air-dried at room temperature.
2.3. Sorption Experiments
2.3.1. General Procedure for Pharmaceutical Sorption on MPs
The sorption experiments of pharmaceuticals and metals were carried out following an optimized procedure, where the amount of MP, the length of sorption, and the shaking and concentration of the pharmaceutical was experimentally determined. The sorption experiments were performed in 20 mL glass vials capped with a stainless steel cap and PTFE septum. The volume of each sorption experiment was set to 10 mL. Each experiment contained 50 mg of the respective MP. The sorption experiments as well as all of the standard solutions were performed in 0.01 M CaCl2 buffer solution to ensure ionic strength. Standard solutions were prepared at the concentration of 100 mg/L and diluted according to the experimental design. The concentration ranges of the pharmaceuticals were determined to be up to 50 mg/L for ibuprofen and chlorpromazine, 20 mg/L for carbamazepine, and 10 mg/L for nitrofurazone. Each experimental set-up consisted of the samples where a varying concentration of the target compound was present, blank samples without the MP, and blank samples where only the MP was present in order to account for the matrix effects. All the sorption experiments were carried out at room temperature (21–23 °C) on an orbital shaker set at 190 rpm (reciprocating every 20 s, to ensure thorough mixing) for 48 h. After the incubation, all of the samples were filtered through a 0.45 µm PTFE syringe filter and measured immediately.
2.3.2. Sorption Experiments in Artificial Seawater
Artificial seawater was prepared as described by Lyman and Fleming [37]. The resulting artificial seawater was diluted to obtain a salinity similar to the above-ground salt waters (1–32 PSU). Salinity is expressed as PSU (practical salinity unit). The sorption efficiency of chlorpromazine was determined using 10 mg/L of the pharmaceutical with 50 mg of each respective MP, similar to the procedure described in Section 2.3.1.
2.3.3. Combined Humic Substance and Pharmaceutical Sorption Experiments
Humic substances obtained from Sigma-Aldrich were dissolved in demineralized water to the concentration of 50 mg/L. The sorption experiments were prepared as described in Section 2.3.1. and the necessary volume of humic substance stock solution was added to obtain a concentration of 10 mg/L. The samples were further analyzed using UPLC-PDA/FLR.
2.4. Instrumental Analysis
Analysis of Pharmaceuticals Using UPLC-PDA/FLR
The chromatographic separation and quantification was carried out using the Waters (Waters Corporation, Milford, MA, USA) Acquity UPLC H-class system consisting of an ultra-high-pressure gradient unit (Quaternary Solvent Manager), an auto injector (FTN), a column oven (CHA), a fluorescence detector (FLR) and a photodiode array detector (PDA). Chromatographic data were collected via Waters Empower chromatography software. The chromatography column was kept at 30 °C, while the samples were stored at 4 °C between the injections. All of the samples were injected after the sample was filtered using a 0.22 µm PTFE syringe filter and diluted where appropriate. Chromatographic conditions used for each of the pharmaceuticals analyzed in this study are summarized in Table 3.

Table 3.
Chromatographic conditions for the analyzed pharmaceutical substances.
Stock solution of the reference standard was prepared by dissolving an accurately weighed sample of the specific compound in 50 mM ammonium acetate solution at a concentration of 10,000 mg/mL. Working standard solutions (1.0–400 µg/mL) containing nitrofurazone, chlorpromazine, carbamazepine and ibuprofen (Sigma-Aldrich) were prepared by diluting the stock solutions. A sample of 2.0 µL was injected using an autosampler. The linear regression was not forced to a zero intercept. A linear least-squares regression of the peak areas as a function of the concentrations was performed to determine the correlation coefficients (R2 ≥ 0.9995). The precision of the method was assessed by triplicate analysis of the standard solutions at eight concentrations of each of the specific standards (1.0, 5.0, 10.0, 50.0, 100.0, 250.0 and 400.0 µg/mL).
3. Results and Discussion
3.1. Sorption Capacity Based on the Amount of Microplastics
Four pharmaceutical substances were dissolved in predetermined concentrations that fall within the linear range of UPLC-PDA—chlorpromazine 50 mg/L, carbamazepine 20 g/mL, ibuprofen 50 mg/L and nitrofurazone 10 mg/L. In the environment and wastewater treatment plant effluents, these substances are usually found in ng/L and µg/L; however, to effectively study the microplastic–pharmaceutical and DOM interactions, as well as present reproducible and representative data, the working concentrations were higher. The abovementioned concentrations were used to determine the sorption capacity of four different microplastics and the amount at which the adsorption was the highest. In this experiment, blank samples (MP samples without pharmaceuticals) and negative controls (pharmaceuticals without MPs) were also made to ensure that the pharmaceuticals did not degrade and that there were no interferences in the samples that could affect the obtained results.
The sorption of pharmaceuticals was tested using 20, 30, 40, 50, 100 and 150 mg of PE, PS, PA and PN. Chlorpromazine showed the highest sorption capacity at 50 mg for PS, PA, PN and especially high sorption for PE (Figure 1). PE had the highest sorption capacity of carbamazepine at 20 mg, which steadily decreased as the amount of MP was increased. PS, PA and PN, on the other hand, had the optimal sorption at 50 mg MP. Ibuprofen and nitrofurazone had the highest sorption capacity when 50 mg MP was used for all of the tested MPs (Figure 1). The experiments were performed during a 48 h period to ensure absorption equilibrium. To test this, an extended test (168 h, 7 days) was performed. The results of the prolonged sorption study showed no significant differences in the sorption capacity for either of the different concentrations of the MPs used. For all of the further experiments, a constant ratio of 20 mL pharmaceutical solution in 0.01 M CaCl2 (if not stated otherwise): 50 mg respective microplastic was used; this ratio ensured the complete immersion of the microplastics as well as the accurate determination of the concentration prior to and following the sorption experiments. The literature suggests that the ratio between the sorption solution and the microplastics can range within 10–100 mg/10–20 mL. Since these results vary so much, it is important to adjust and optimize the amount of microplastics used for the specific pharmaceuticals in the sorption experiments [38].
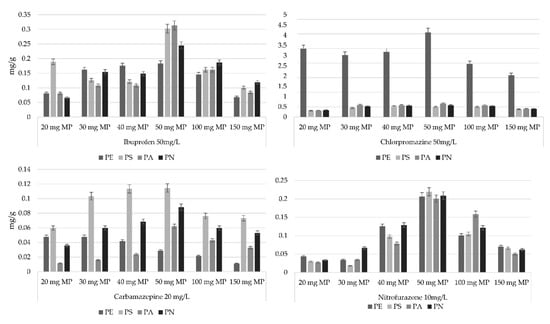
Figure 1.
Sorption capacity of four pharmaceuticals on four types of microplastics at different concentrations. N = 5.
3.2. Sorption Isotherms
The data of sorption experiments are often compared with theoretical sorption models such as the Langmuir, Freundlich, Dubinin–Radushkevich, Redlich–Peterson, and others. In this study, the obtained results were compared with the Langmuir and Freundlich theoretical models. Correspondence to one of the theoretical models was determined using linear forms of the Langmuir or Freundlich equations, respectively (Figure 2). Although the Langmuir model can be expressed in linear form using a number of different equations, in this study the Langmuir equation (Equation (1)), whichi is the most popular and most appropriate for the assessment of experimental data, was used, and it runs as follows:
where Ce is the equilibrium concentration, mg/L; qe is the amount of substance sorbed onto the solid phase, mg/g; qm and Ka are the Langmuir constants related to the sorption capacity and sorption energy, respectively. In addition, qm is qe for a complete monolayer (mg/g), and Ka is the sorption equilibrium constant (L/mg) [39,40]. Another frequently used sorption isotherm model is the Freundlich model. Its linearized form is shown in Equation (2):
where K and n are the Freundlich constants that correspond to the adsorption capacity and adsorption intensity, respectively. The Freundlich model assumes that the sorbent surface is heterogeneous and that the sorption sites have diverse energies.
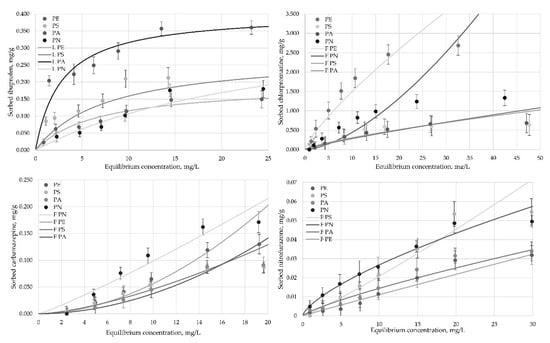
Figure 2.
Sorption isotherms of four pharmaceuticals on four types of microplastics according to Freundlich and Langmuir sorption models. N = 5.
Based on the correlation coefficient, the nitrofurazone sorption on PE, PS and PA (Table 4) corresponds to the Freundlich theoretical adsorption model, thus indicating adsorbate–adsorbent interactions on a heterogeneous surface with adsorbing particles having variable sorption affinities. This model is applicable for mono- and multilayer sorption [38]. Studies published to date indicate that the Freundlich sorption model can be used to explain the interaction between organic contaminants and micro (or nano) plastics [38]. Nevertheless, the correlation coefficient values were close for nitrofurazone sorption on PN, confirming that the experimentally obtained sorption data corresponded to both of the theoretical isotherm models. According to the Langmuir model, sorption occurs on the homogeneous surface, where pollutant molecules interact with specific sorption sites of the microplastic, thereby forming monolayers.

Table 4.
Determination coefficients of linear Freundlich (F) or Langmuir (L) sorption isotherm models.
Like nitrofurazone, the experimental sorption data for carbamazepine on PE, PA, PS and PN were consistent with the Freundlich isotherm model. However, the experimentally obtained data of chlorpromazine and ibuprofen sorption on the microplastics that were used in this study corresponded to both of the theoretical isotherm models, whereas in the case of chlorpromazine, the Freundlich model was predominant (with exception of PN), but Langmuir model predominated for ibuprofen (with exception of PN) (Table 5).

Table 5.
Conformity to sorption models of pharmaceuticals on microplastics.
Overall, from the obtained data (K from Freundlich equation and qm from Langmuir equation) it can be concluded that PN highly efficiently absorbs nitrofurazone, while carbamazepine and chlorpromazine showed high sorption abilities.
3.3. Chlorpromazine Sorption Based on Water Salinity
External factors such as salinity can significantly increase or decrease the sorption of different pharmaceuticals on microplastics. It has been previously determined that, generally, the most favorable conditions for the sorption of pharmaceuticals occur when the ionic strength of the solution is low (for example, in distilled water). In natural waters the salinity and thus the ionic strength differ greatly. Freshwater rivers have significantly less dissolved salts than sea or ocean waters; thus, when the microplastic enters the environment, depending on the entrance point, the sorption capacity of pharmaceuticals on MPs can significantly differ. Freshwater rivers have a salinity of <0.3 PSU compared to the salinity of 2 PSU in the Baltic Sea and the salinity of the Atlantic and Pacific Oceans, where the salinity reaches up to 35 PSU, or in extreme cases, more than 40 PSU in the Dead Sea. Microplastics have different entrance points into the natural environment; they are usually introduced through the ineffective wastewater treatment plants into the rivers and, in some cases, directly into the seas and oceans. Based on the MP entrance point, the MP particles can adsorb naturally occurring substances (humic substances), pharmaceuticals, or even play a role as points for microbial adsorption and colonization.
To test the effects of salinity on the adsorption of chlorpromazine, artificial sea water was prepared at 2–32 PSU, which covered the salinity levels of inland seas, river mouths as well as oceans. Chlorpromazine sorption on PE and PN gradually decreased with increasing salinity, indicating that better sorption could be achieved in freshwater and that these MPs would adsorb substances more effectively in freshwater rivers and lakes, rather than seas and oceans [41]. PA, which is a different form of PN, showed the lowest adsorption and did not change when the salinity increased, as opposed to PN. Interestingly, the sorption of chlorpromazine on PS increased with the increasing salinity. The obtained results indicated that there are different, complex interactions involved in the pharmaceutical–MP sorption. Usually, the hydrophobicity of the materials is assumed to be responsible for the interactions. In this case, the pharmaceutical (chlorpromazine) is hydrophobic in nature, which in general, indicates a rather higher sorption potential. The different MPs used (polymers) can have crystalline, semi-crystalline and amorphous states indicating the arrangement of molecular chains. The least crystalline plastics should be able to accumulate the highest amount of organic pollutants, as opposed to the amorphous glassy and rubbery fractions, which are rigid and flexible, respectively. The salinity and ionic strength of the medium greatly contributes to the sorption of chlorpromazine on different microplastics. As indicated by other authors, the increasing salinity in most cases decreases the sorption capacity; however, it has to be noted that the salt solutions used for the experiments do not represent the composition of the aquatic environment [42,43]. Very often solutions of NaCl or KCl have been used, despite the fact that the composition of natural saltwater is far more complex and thus the results could be different, as in the case of PS, where the sorption increased (Figure 3).
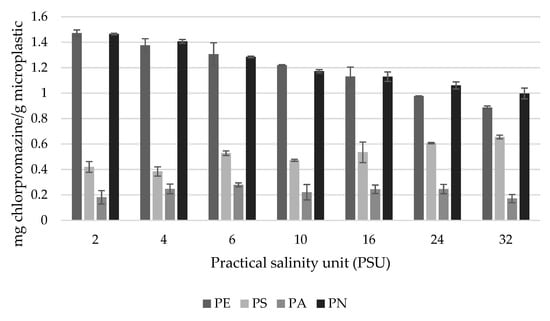
Figure 3.
Sorption of chlorpromazine on different microplastics depending on the salinity of water. N = 3.
3.4. Sorption of Pharmaceuticals on Aged Microplastics
Despite the high stability of most of microplastics in natural environment, changes to their surface, structure, and therefore their properties take place, resulting in full degradation and the transformation to mineral substances. During the aging of microplastic particles, their surface is changing (surface cracks appear) [44], and the degradation of polymeric structures results in the appearance of new functional groups (at first -COOH and -OH groups) [45]. Thus, the aging of microplastics can result in changes in the sorption behavior of pollutants, including pharmaceuticals. For example, an increase in metal sorption as a result of aging has been observed [36].
It is generally supposed that aging increases the sorption capacity of organic pollutants [46]; however, our results (Figure 4) demonstrated that the interactions are not so simple, as for some of the studied pharmaceuticals (in most cases for ibuprofen but for all studied microplastics for carbamazepine) the sorption decreased. However, the sorption of nitrofurazone on all the studied plastics after their aging increased. Thus, as previously suggested [45], the resulting impact of microplastic aging on the interaction with pharmaceuticals mainly depended on the changes in the hydrophobicity of the surface of the particles and the affinity of the more polar surface on the electrostatic interaction with sorbate.
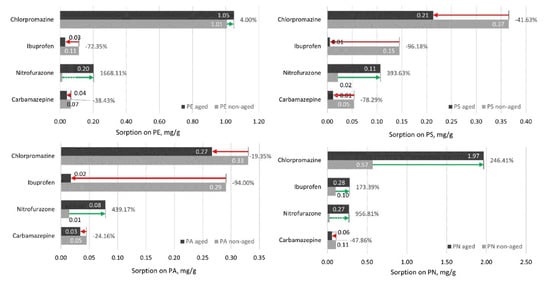
Figure 4.
Sorption of four pharmaceuticals on different aged and non-aged microplastics. The green arrows represent increased sorption of the respective microplastic onto aged microplastic; the red arrow represents decrease in sorption onto aged microplastic. N = 3.
3.5. Sorption of Pharmaceuticals in the Presence of Humic Substances
3.5.1. Simultaneous Sorption of Chlorpromazine and Humic Substances
Inland waters contain high concentrations of dissolved organic substances represented by low-molecular-weight compounds as well as refractory organic substances—humic substances. Concentrations of humic substances in inland waters can be as high as tens of milligrams per liter, and thus their presence can influence interactions between pharmaceuticals and microplastics. Our results indicated that humic substances are extremely effective in the sorption of pharmaceuticals (chlorpromazine) rather than the microplastics, indicating that in natural waterways rich in humic substances, the sorption and interaction with pharmaceuticals would primarily happen with the naturally occurring humic substances, rather than microplastics (Table 6).

Table 6.
Simultaneous sorption of humic substances (1 mg/L) and pharmaceuticals (three different concentrations) in the presence of four types of microplastics. RSD < 5%, N = 3.
The prepared humic substance solution at 1 mg/L could effectively adsorb all four of the pharmaceuticals used in this study. In experiments where the humic substances and pharmaceuticals were mixed without the presence of the microplastics (Table 6) only ibuprofen was not adsorbed completely (adsorption efficiency 99.5%); however, the rest of the pharmaceuticals could be adsorbed completely, leaving no detectable pharmaceutical residues. The humic substance–pharmaceutical complexes formed microscopic particles that could be filtered and thus removed from the solution. PE and PA did not affect the sorption of the humic substance with the pharmaceuticals. It cannot be concluded whether some of the pharmaceuticals or humic substances were not also adsorbed onto the microplastics; however, the control experiments (without MP) indicate that the priority of sorption happens between humic substances and pharmaceuticals. In the case where PN and PS were added to the samples, a slightly less adsorption rate was recorded, indicating that microplastics could hinder the humic substance–pharmaceutical adsorption; however, these differences are minimal, since the adsorption ranges from 90–99%.
3.5.2. Pharmaceutical–Microplastic Complex Effectively Adsorbs Humic Substances
As the results in Section 3.5.1. indicated that the sorption capacity between humic substances and pharmaceuticals is far greater than between microplastics and pharmaceuticals, additional experiments were set up to test whether the pharmaceuticals that adsorbed onto microplastics would retain the ability to effectively form complexes with humic substances.
To test this hypothesis, experiments were performed where microplastic samples were saturated with pharmaceuticals, washed with demineralized water and then mixed together with humic substance solution (1 mg/L). The obtained results clearly indicated that for all of the four tested microplastics, the sorption of humic substances significantly increased when the microplastics were modified with the pharmaceuticals beforehand, indicating that the humic substance–pharmaceutical sorption is not hindered even when the pharmaceutical is adsorbed onto the microplastic particle/fiber (Figure 5).
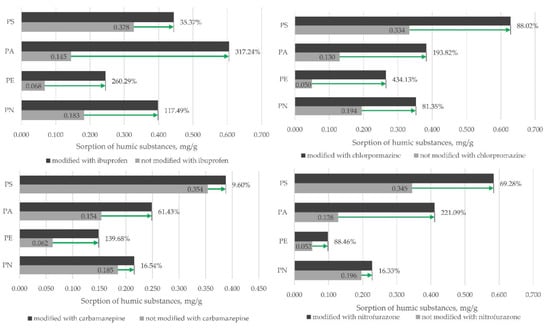
Figure 5.
Sorption of humic substances after pharmaceutical sorption onto four types of microplastics. The green arrows represent increased sorption of humic substances after the microplastic samples have been modified with chlorpromazine. N = 3.
For all of the tested MPs, the adsorbed amount of humic substance increased when the MP particles/fibers were modified with the four different pharmaceuticals. The largest increase (317.24%) was observed for ibuprofen on PA, while the smallest was observed for carbamazepine on PS (Figure 5). The results indicated that humic substances are a prerequisite for the effective sorption of pharmaceuticals; however, it is not known whether the humic substance–pharmaceutical complex can be adsorbed onto the MPs after the formation of the initial complex. Moreover, the humic substances can also be adsorbed onto the MPs, and more tests should be performed to explore whether microplastic–humic substance complexes can also effectively adsorb pharmaceuticals. Similar effects as these have also been proposed in other studies, where antibiotics have been examined for their effectiveness to adsorb onto microplastics and humic substances [47]. The complexation mechanism of the antibiotic (tetracycline) was proposed to be a cationic or zwitterionic interaction with the deprotonated site on the humic substance via H-bonding and cation exchange [48,49,50,51]. It has also been indicated that microplastics such as PE could decrease the sorption capacity of pharmaceutical–humic substance sorption [52]. PS microplastics together with organic matter (humic substances) could interact with the aromatic structure of humic substances via π–π conjugation, thereby producing a highly conjugated polymer complex with an increased electron density, thus increasing the electrostatic attraction for cationic pharmaceuticals [53].
Such complexes can have significant environmental implications: they can be ingested by a variety of aquatic animals—such as fish, benthic organisms, crustaceans, etc.—and due to the pharmaceutical activity hindering the development of these organisms, accumulation within and passing of the pharmaceuticals through the food chain can occur [54]. Fish populations could decrease due to the ingestion and presence of microplastics in the gastrointestinal tracts; the microplastic particles could cause nutritional and growth problems due to satiation and blocking of the digestive system [55]. Fish often ingest microplastics directly by mistaking these particles for food (filter- and deposit-feeding fish) or by consuming other, smaller organisms that contain microplastics (predatory fish) [56]. Most commonly, the ingested microplastics remain in the digestive tract; however, they can also adhere to the skin, gills, or translocate to other parts of the organism such as the liver or muscle (which are consumed by humans) [57]. Fine microplastics can also be translocated between cells and enter the circulatory or lymphatic systems, thus contaminating the whole organism with microplastics [58]. Since the plastics that are produced very often contain a variety of chemicals to improve the properties of the plastics, it is also important not only to study the effect of pure microplastics, but also the combined effects of the additives and MPs. For example, it has been demonstrated that commonly occurring PE increased the bioaccumulation of PCBs and aromatic hydrocarbons, which caused damage to the liver of fish in addition to glycogen depletion and cell necrosis [59]. As indicated by recent research, the bioaccumulation of MPs can cause disruptions in the circulatory system and hematological parameters, and induce oxidative, immune, and neurotoxicity [60]. Such results strongly suggest the ability of microplastic–pharmaceutical complexes to influence cellular mechanisms, leading to adverse effects. Since the consumption of fish and fish-related products is gradually increasing, measures to prevent microplastics from entering natural environments should be taken [61,62].
The most recent studies on microplastics and their impact on human health have revealed that, in fact, microplastics are bioavailable for uptake into the human bloodstream. In a study where the blood of 22 healthy volunteers was analyzed, it was found that the mean plastic concentration of PET, PE and styrene polymers was 1.6 µg/mL [63]. The found plastics are considered to be of high production volume and necessity, and evidently those are the plastics which can most often be found in natural aquatic environments. Once the microplastics have entered the bloodstream they can then be transported to the organs. For example, it has been shown that polystyrene beads of the size of 50–240 nm and polypropylene can be transported across the human placenta [64,65]. Polystyrene microparticles have also been demonstrated to be able to translocate to the placenta and fetal tissue in rats [66]. The ability of plastic particle translocation is being explored in pharmaceutical drug-delivery sciences, where these carriers would be used to carry delivery drugs across the blood–brain barrier [24]. However, in this case the drug delivery would be targeted and closely monitored, whereas in the case of microplastic–pharmaceutical complexes, this uptake is uncontrollable. It has been previously shown that microplastics can carry a variety of drugs—hormonal drugs [67], antibiotics [68], antidepressants, etc. [69]—which leads to serious effects on human health when unattended. For example, exposure to hormonal drugs can produce effects on the human endocrinal system, leading to hormone imbalance, infertility, etc. [67], while antibiotics could increase antibiotic resistance. These results show that proper wastewater management regarding the release of microplastics into the natural aquatic environment are a must, since through the food chain, where fish act as vectors to humans, microplastic–pharmaceutical complexes can be easily transferred, causing serious health issues.
4. Conclusions
Considering the amount of pharmaceuticals and microplastics entering the natural aquatic environment, it is crucial to monitor and investigate the environmental implications that these substances have individually, as complexes, and as possible pollutants that can form aggregates with naturally occurring organic matter. This study showed the sorption of pharmaceuticals based on the salinity of the aquatic environment, indicating that lower salinity, in general, increases the sorption ability of microplastics and thus the formation of microplastic–pharmaceutical complexes; this leads to the conclusion that freshwater bodies and low-salinity seas are at a greater risk of having their aquatic inhabitants influenced by these novel pollutants. The freshwater rivers that often have high contents of organic matter (humic substances) are especially at risk; as this study has indicated, the pharmaceuticals from treated wastewaters when entering rivers that feed into the sea can effectively form humic substance–pharmaceutical complexes. More importantly, since one of the main sources of microplastics in freshwater rivers is from wastewater treatment plants where discharged pharmaceuticals come in contact with microplastics and form microplastic–pharmaceutical complexes—as indicated by this study—these complexes can later effectively absorb humic substances, forming a large complex that can then be ingested by aquatic animals. The fate of these substances, their metabolism within an organism, or the biological effects of the adsorbed pharmaceuticals are not known; however, these aspects should be investigated, since pharmaceuticals of various kinds can be passed down the food chain through these complex structures.
Author Contributions
M.K.—Conceptualization, writing—original draft preparation, resources, supervision, funding acquisition; M.M.—methodology, formal analysis, investigation; V.S.—Conceptualization, writing—original draft preparation, supervision, funding acquisition; O.S.—resources, writing—original draft preparation; A.M.—data curation; L.K.—methodology, formal analysis, investigation, data curation, writing—review and editing; L.A.-B.—investigation, data curation; A.V.—writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
Research supported by Latvian-Ukrainian Joint Programme of Scientific and Technological Cooperation project No. LV-UA/2022/1 “The microbially-coated microplastics in neustonic water-air interphase and their environmental impacts on aquatic biotopes”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
The authors of the article would like to thank Chem., Jorens Kviesis for support with the analytical measurements of pharmaceuticals using LC.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- Statista 2020. Global Plastic Production 1950–2020. 2021. Available online: https://www.statista.com/statistics/282732/global-production-of-plastics-since-1950/ (accessed on 2 September 2021).
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.G.; McGonigle, D.; Russell, A.E. Lost at Sea: Where Is All the Plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef]
- Blettler, M.C.M.; Ulla, M.A.; Rabuffetti, A.P.; Garello, N. Plastic pollution in freshwater ecosystems: Macro-, meso-, and microplastic debris in a floodplain lake. Environ. Monit. Assess. 2017, 189, 581. [Google Scholar] [CrossRef] [PubMed]
- Boucher, J.; Friot, D. Primary Microplastics in the Oceans: A Global Evaluation of Sources; IUCN: Gland, Switzerland, 2017; Volume 10. [Google Scholar]
- Völker, C.; Kramm, J.; Wagner, M. On the Creation of Risk: Framing of Microplastics Risks in Science and Media. Glob. Chall. 2020, 4, 1900010. [Google Scholar] [CrossRef]
- Li, C.; Busquets, R.; Campos, L.C. Assessment of microplastics in freshwater systems: A review. Sci. Total Environ. 2020, 707, 135578. [Google Scholar] [CrossRef]
- Frias, J.; Nash, R. Microplastics: Finding a consensus on the definition. Mar. Pollut. Bull. 2019, 138, 145–147. [Google Scholar] [CrossRef]
- European Commission. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions. A European Strategy for Plastics in a Circular Economy; European Commission: Brussels, Belgium, 2018. [Google Scholar]
- Sharma, V.K.; Ma, X.; Guo, B.; Zhang, K. Environmental factors-mediated behavior of microplastics and nanoplastics in water: A review. Chemosphere 2021, 271, 129597. [Google Scholar] [CrossRef]
- Yuan, Z.; Nag, R.; Cummins, E. Ranking of potential hazards from microplastics polymers in the marine environment. J. Hazard. Mater. 2022, 429, 128399. [Google Scholar] [CrossRef]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef]
- Yan, M.; Wang, L.; Dai, Y.; Sun, H.; Liu, C. Behavior of Microplastics in Inland Waters: Aggregation, Settlement, and Transport. Bull. Environ. Contam. Toxicol. 2021, 107, 700–709. [Google Scholar] [CrossRef]
- Yao, P.; Zhou, B.; Lu, Y.; Yin, Y.; Zong, Y.; Chen, M.-T.; O’Donnell, Z. A review of microplastics in sediments: Spatial and temporal occurrences, biological effects, and analytic methods. Quat. Int. 2019, 519, 274–281. [Google Scholar] [CrossRef]
- Eerkes-Medrano, D.; Leslie, H.A.; Quinn, B. Microplastics in drinking water: A review and assessment. Curr. Opin. Environ. Sci. Health 2019, 7, 69–75. [Google Scholar] [CrossRef]
- Guo, J.-J.; Huang, X.-P.; Xiang, L.; Wang, Y.-Z.; Li, Y.-W.; Li, H.; Cai, Q.-Y.; Mo, C.-H.; Wong, M.-H. Source, migration and toxicology of microplastics in soil. Environ. Int. 2020, 137, 105263. [Google Scholar] [CrossRef] [PubMed]
- Gasperi, J.; Wright, S.L.; Dris, R.; Collard, F.; Mandin, C.; Guerrouache, M.; Langlois, V.; Kelly, F.J.; Tassin, B. Microplastics in air: Are we breathing it in? Curr. Opin. Environ. Sci. Health 2018, 1, 1–5. [Google Scholar] [CrossRef]
- Aves, A.R.; Revell, L.E.; Gaw, S.; Ruffell, H.; Schuddeboom, A.; Wotherspoon, N.E.; LaRue, M.; McDonald, A.J. First evidence of microplastics in Antarctic snow. Cryosphere 2022, 16, 2127–2145. [Google Scholar] [CrossRef]
- Kumar, M.; Xiong, X.; He, M.; Tsang, D.C.; Gupta, J.; Khan, E.; Harrad, S.; Hou, D.; Ok, Y.S.; Bolan, N.S. Microplastics as pollutants in agricultural soils. Environ. Pollut. 2020, 265, 114980. [Google Scholar] [CrossRef]
- Gola, D.; Tyagi, P.K.; Arya, A.; Chauhan, N.; Agarwal, M.; Singh, S.; Gola, S. The impact of microplastics on marine environment: A review. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100552. [Google Scholar] [CrossRef]
- Sun, T.; Zhan, J.; Li, F.; Ji, C.; Wu, H. Effect of microplastics on aquatic biota: A hormetic perspective. Environ. Pollut. 2021, 285, 117206. [Google Scholar] [CrossRef]
- Smith, M.; Love, D.C.; Rochman, C.M.; Neff, R.A. Microplastics in Seafood and the Implications for Human Health. Curr. Environ. Health Rep. 2018, 5, 375–386. [Google Scholar] [CrossRef]
- Prata, J.C.; da Costa, J.P.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Environmental exposure to microplastics: An overview on possible human health effects. Sci. Total Environ. 2020, 702, 134455. [Google Scholar] [CrossRef]
- Yee, M.; Hii, L.-W.; Looi, C.; Lim, W.-M.; Wong, S.-F.; Kok, Y.-Y.; Tan, B.-K.; Wong, C.-Y.; Leong, C.-O. Impact of Microplastics and Nanoplastics on Human Health. Nanomaterials 2021, 11, 496. [Google Scholar] [CrossRef] [PubMed]
- Bakir, A.; Rowland, S.J.; Thompson, R.C. Competitive sorption of persistent organic pollutants onto microplastics in the marine environment. Mar. Pollut. Bull. 2012, 64, 2782–2789. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Wu, X.; Song, X.; Shi, C.; Zhang, Z. Sorption and desorption of petroleum hydrocarbons on biodegradable and nondegradable microplastics. Chemosphere 2021, 273, 128553. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Liu, F.; Brookes, P.C.; Xu, J. Microplastics play a minor role in tetracycline sorption in the presence of dissolved organic matter. Environ. Pollut. 2018, 240, 87–94. [Google Scholar] [CrossRef]
- Akdogan, Z.; Guven, B. Microplastics in the environment: A critical review of current understanding and identification of future research needs. Environ. Pollut. 2019, 254, 113011. [Google Scholar] [CrossRef]
- Yu, F.; Yang, C.; Zhu, Z.; Bai, X.; Ma, J. Adsorption behavior of organic pollutants and metals on micro/nanoplastics in the aquatic environment. Sci. Total Environ. 2019, 694, 133643. [Google Scholar] [CrossRef]
- Torres, F.G.; Dioses-Salinas, D.C.; Pizarro-Ortega, C.I.; De-La-Torre, G.E. Sorption of chemical contaminants on degradable and non-degradable microplastics: Recent progress and research trends. Sci. Total Environ. 2020, 757, 143875. [Google Scholar] [CrossRef]
- Eriksen, M.; Lebreton, L.C.M.; Carson, H.S.; Thiel, M.; Moore, C.J.; Borerro, J.C.; Galgani, F.; Ryan, P.G.; Reisser, J. Plastic Pollution in the World’s Oceans: More than 5 Trillion Plastic Pieces Weighing over 250,000 Tons Afloat at Sea. PLoS ONE 2014, 9, e111913. [Google Scholar] [CrossRef]
- Van Sebille, E.; Wilcox, C.; Lebreton, L.; Maximenko, N.; Hardesty, B.D.; Van Franeker, J.A.; Eriksen, M.; Siegel, D.; Galgani, F.; Law, K. A global inventory of small floating plastic debris. Environ. Res. Lett. 2015, 10, 124006. [Google Scholar] [CrossRef]
- Xu, H.; Guo, L. Molecular size-dependent abundance and composition of dissolved organic matter in river, lake and sea waters. Water Res. 2017, 117, 115–126. [Google Scholar] [CrossRef]
- Abdurahman, A.; Cui, K.; Wu, J.; Li, S.; Gao, R.; Dai, J.; Liang, W.; Zeng, F. Adsorption of dissolved organic matter (DOM) on polystyrene microplastics in aquatic environments: Kinetic, isotherm and site energy distribution analysis. Ecotoxicol. Environ. Saf. 2020, 198, 110658. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Luo, Y.; Yu, X.; Ouyang, Z.; Liu, P.; Guo, X. Insight into interactions of polystyrene microplastics with different types and compositions of dissolved organic matter. Sci. Total Environ. 2022, 824, 153883. [Google Scholar] [CrossRef] [PubMed]
- Lang, M.; Yu, X.; Liu, J.; Xia, T.; Wang, T.; Jia, H.; Guo, X. Fenton aging significantly affects the heavy metal adsorption capacity of polystyrene microplastics. Sci. Total Environ. 2020, 722, 137762. [Google Scholar] [CrossRef]
- Lyman, J.; Fleming, R.H. Composition of sea water. J. Mar. Res. 1940, 3, 134–146. [Google Scholar]
- Agboola, O.D.; Benson, N.U. Physisorption and Chemisorption Mechanisms Influencing Micro (Nano) Plastics-Organic Chemical Contaminants Interactions: A Review. Front. Environ. Sci. 2021, 9, 678574. [Google Scholar] [CrossRef]
- Kuriakose, S.; Singh, T.S.; Pant, K.K. Adsorption of As(III) from Aqueous Solution onto Iron Oxide Impregnated Activated Alumina. Water Qual. Res. J. 2004, 39, 258–266. [Google Scholar] [CrossRef]
- Ho, Y.-S.; Ofomaja, A. Kinetics and thermodynamics of lead ion sorption on palm kernel fibre from aqueous solution. Process Biochem. 2005, 40, 3455–3461. [Google Scholar] [CrossRef]
- Wagner, M.; Scherer, C.; Alvarez-Muñoz, D.; Brennholt, N.; Bourrain, X.; Buchinger, S.; Fries, E.; Grosbois, C.; Klasmeier, J.; Marti, T.; et al. Microplastics in freshwater ecosystems: What we know and what we need to know. Environ. Sci. Eur. 2014, 26, 12. [Google Scholar] [CrossRef]
- Xu, B.; Liu, F.; Brookes, P.C.; Xu, J. The sorption kinetics and isotherms of sulfamethoxazole with polyethylene microplastics. Mar. Pollut. Bull. 2018, 131, 191–196. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.; Zhou, B.; Zhou, Y.; Dai, Z.; Zhou, Q.; Christie, P.; Luo, Y. Enhanced adsorption of oxytetracycline to weathered microplastic polystyrene: Kinetics, isotherms and influencing factors. Environ. Pollut. 2018, 243, 1550–1557. [Google Scholar] [CrossRef]
- Luo, H.; Zhao, Y.; Li, Y.; Xiang, Y.; He, D.; Pan, X. Aging of microplastics affects their surface properties, thermal decomposition, additives leaching and interactions in simulated fluids. Sci. Total Environ. 2020, 714, 136862. [Google Scholar] [CrossRef]
- Liu, P.; Lu, K.; Li, J.; Wu, X.; Qian, L.; Wang, M.; Gao, S. Effect of aging on adsorption behavior of polystyrene microplastics for pharmaceuticals: Adsorption mechanism and role of aging intermediates. J. Hazard. Mater. 2020, 384, 121193. [Google Scholar] [CrossRef] [PubMed]
- Bhagat, K.; Barrios, A.C.; Rajwade, K.; Kumar, A.; Oswald, J.; Apul, O.; Perreault, F. Aging of microplastics increases their adsorption affinity towards organic contaminants. Chemosphere 2022, 298, 134238. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, X.; Xia, S.; Zhao, J. New insights into oxytetracycline (OTC) adsorption behavior on polylactic acid microplastics undergoing microbial adhesion and degradation. Chem. Eng. J. 2021, 416, 129085. [Google Scholar] [CrossRef]
- Sun, H.; Shi, X.; Mao, J.; Zhu, D. Tetracycline sorption to coal and soil humic acids: An examination of humic structural heterogeneity. Environ. Toxicol. Chem. 2010, 29, 1934–1942. [Google Scholar] [CrossRef]
- Zhao, Y.; Geng, J.; Wang, X.; Gu, X.; Gao, S. Adsorption of tetracycline onto goethite in the presence of metal cations and humic substances. J. Colloid Interface Sci. 2011, 361, 247–251. [Google Scholar] [CrossRef]
- Zhao, Y.; Gu, X.; Gao, S.; Geng, J.; Wang, X. Adsorption of tetracycline (TC) onto montmorillonite: Cations and humic acid effects. Geoderma 2012, 183, 12–18. [Google Scholar] [CrossRef]
- Chen, W.; Ouyang, Z.-Y.; Qian, C.; Yu, H.-Q. Induced structural changes of humic acid by exposure of polystyrene microplastics: A spectroscopic insight. Environ. Pollut. 2018, 233, 1–7. [Google Scholar] [CrossRef]
- Seidensticker, S.; Zarfl, C.; Cirpka, O.A.; Fellenberg, G.; Grathwohl, P. Shift in Mass Transfer of Wastewater Contaminants from Microplastics in the Presence of Dissolved Substances. Environ. Sci. Technol. 2017, 51, 12254–12263. [Google Scholar] [CrossRef]
- Guo, X.; Wang, X.; Zhou, X.; Kong, X.; Tao, S.; Xing, B. Sorption of Four Hydrophobic Organic Compounds by Three Chemically Distinct Polymers: Role of Chemical and Physical Composition. Environ. Sci. Technol. 2012, 46, 7252–7259. [Google Scholar] [CrossRef]
- Rainieri, S.; Barranco, A. Microplastics, a food safety issue? Trends Food Sci. Technol. 2019, 84, 55–57. [Google Scholar] [CrossRef]
- Yin, L.; Chen, B.; Xia, B.; Shi, X.; Qu, K. Polystyrene microplastics alter the behavior, energy reserve and nutritional composition of marine jacopever (Sebastes schlegelii). J. Hazard. Mater. 2018, 360, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Batel, A.; Linti, F.; Scherer, M.; Erdinger, L.; Braunbeck, T. Transfer of benzo[a]pyrene from microplastics to Artemia nauplii and further to zebrafish via a trophic food web experiment: CYP1A induction and visual tracking of persistent organic pollutants. Environ. Toxicol. Chem. 2016, 35, 1656–1666. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, S.; Soltani, N.; Keshavarzi, B.; Moore, F.; Turner, A.; Hassanaghaei, M. Microplastics in different tissues of fish and prawn from the Musa Estuary, Persian Gulf. Chemosphere 2018, 205, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.L.; Kelly, F.J. Plastic and Human Health: A Micro Issue? Environ. Sci. Technol. 2017, 51, 6634–6647. [Google Scholar] [CrossRef]
- Rochman, C.M.; Hoh, E.; Kurobe, T.; Teh, S.J. Ingested plastic transfers hazardous chemicals to fish and induces hepatic stress. Sci. Rep. 2013, 3, 3263. [Google Scholar] [CrossRef]
- Kim, J.-H.; Yu, Y.-B.; Choi, J.-H. Toxic effects on bioaccumulation, hematological parameters, oxidative stress, immune responses and neurotoxicity in fish exposed to microplastics: A review. J. Hazard. Mater. 2021, 413, 125423. [Google Scholar] [CrossRef]
- Thiele, C.J.; Hudson, M.D.; Russell, A.E.; Saluveer, M.; Sidaoui-Haddad, G. Microplastics in fish and fishmeal: An emerging environmental challenge? Sci. Rep. 2021, 11, 2740. [Google Scholar] [CrossRef]
- Wootton, N.; Reis-Santos, P.; Gillanders, B.M. Microplastic in fish–A global synthesis. Rev. Fish Biol. Fish. 2021, 31, 753–771. [Google Scholar] [CrossRef]
- Leslie, H.A.; van Velzen, M.J.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and quantification of plastic particle pollution in human blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef]
- Wick, P.; Malek, A.; Manser, P.; Meili, D.; Maeder-Althaus, X.; Diener, L.; Diener, P.-A.; Zisch, A.; Krug, H.F.; Von Mandach, U. Barrier Capacity of Human Placenta for Nanosized Materials. Environ. Health Perspect. 2010, 118, 432–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ragusa, A.; Svelato, A.; Santacroce, C.; Catalano, P.; Notarstefano, V.; Carnevali, O.; Papa, F.; Rongioletti, M.C.A.; Baiocco, F.; Draghi, S.; et al. Plasticenta: First evidence of microplastics in human placenta. Environ. Int. 2021, 146, 106274. [Google Scholar] [CrossRef] [PubMed]
- Fournier, S.B.; D’Errico, J.N.; Adler, D.S.; Kollontzi, S.; Goedken, M.J.; Fabris, L.; Yurkow, E.J.; Stapleton, P.A. Nanopolystyrene translocation and fetal deposition after acute lung exposure during late-stage pregnancy. Part. Fibre Toxicol. 2020, 17, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wu, J.; Wu, J.; Zhang, C.; Luo, Y. Adsorption and Desorption of Steroid Hormones by Microplastics in Seawater. Bull. Environ. Contam. Toxicol. 2021, 107, 730–735. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Y.; Liu, X.; Zhao, J.; Liu, R.; Xing, B. Interaction of Microplastics with Antibiotics in Aquatic Environment: Distribution, Adsorption, and Toxicity. Environ. Sci. Technol. 2021, 55, 15579–15595. [Google Scholar] [CrossRef]
- Santos, L.H.; Rodríguez-Mozaz, S.; Barceló, D. Microplastics as vectors of pharmaceuticals in aquatic organisms–An overview of their environmental implications. Case Stud. Chem. Environ. Eng. 2021, 3, 100079. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).