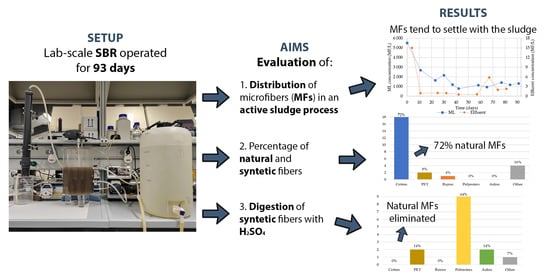
Assessment of Microplastics Distribution in a Biological Wastewater Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Wastewater and Activated Sludge
2.2. Experimental Setup
2.3. SBR Performance Monitoring
2.4. Microfiber Analyses
2.4.1. Sulfuric Acid Digestion Protocol
2.4.2. Physical Characterization
2.4.3. Chemical Characterization
2.4.4. Microfiber Distribution Model
2.5. Quality Assurance and Quality Control
3. Results and Discussions
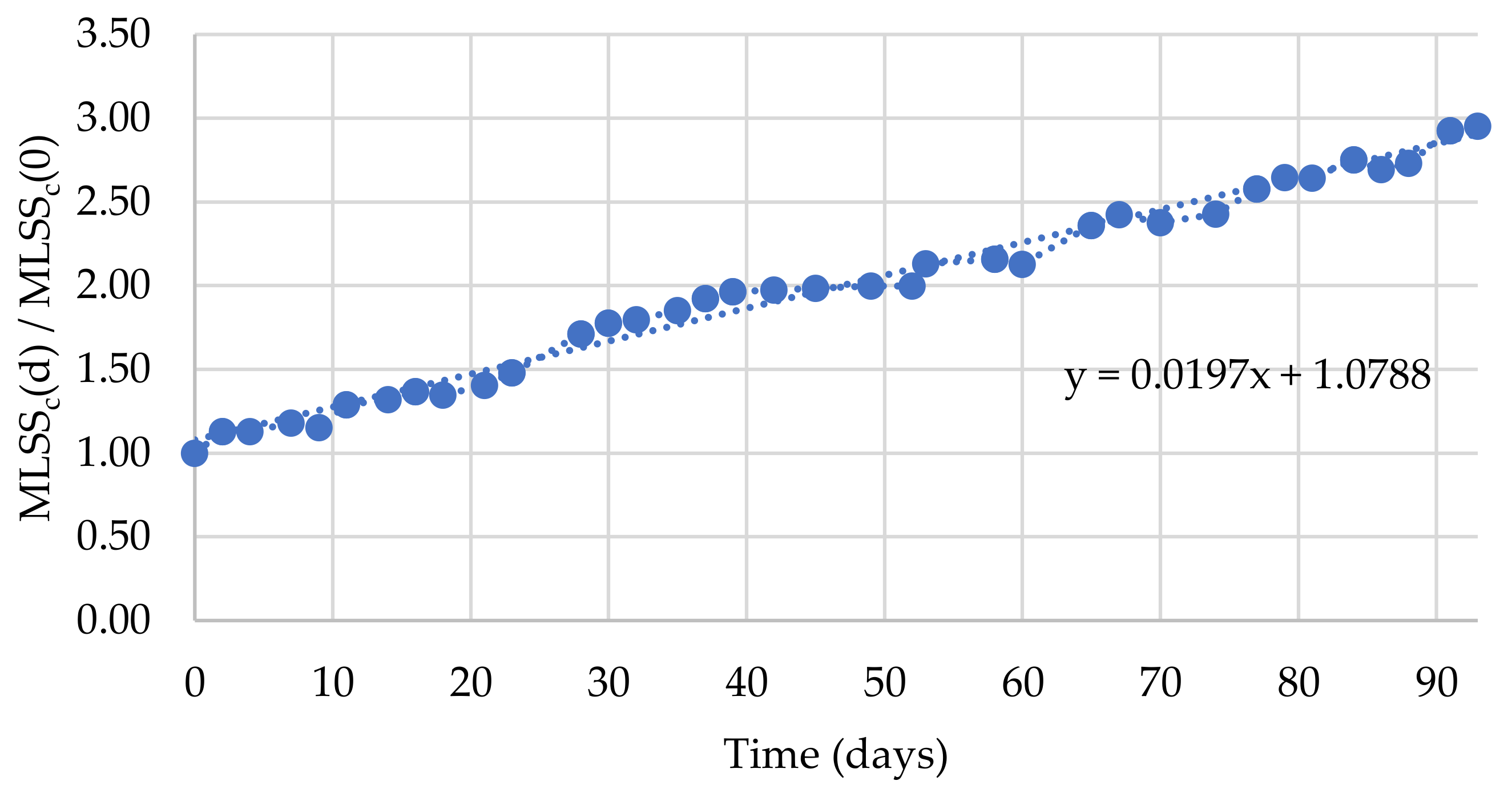
3.1. SBR Performance Monitoring
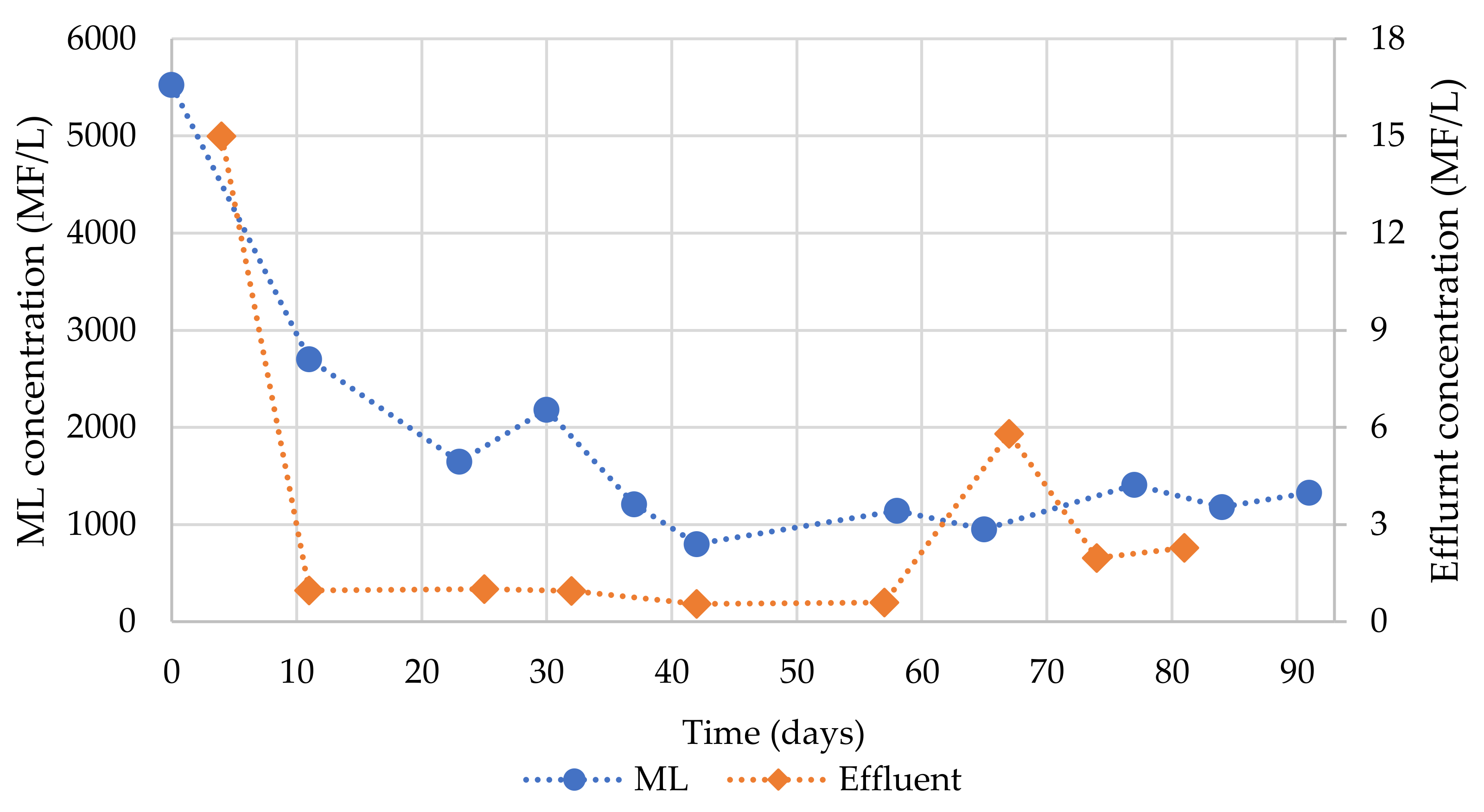
3.2. Microfiber Analyses
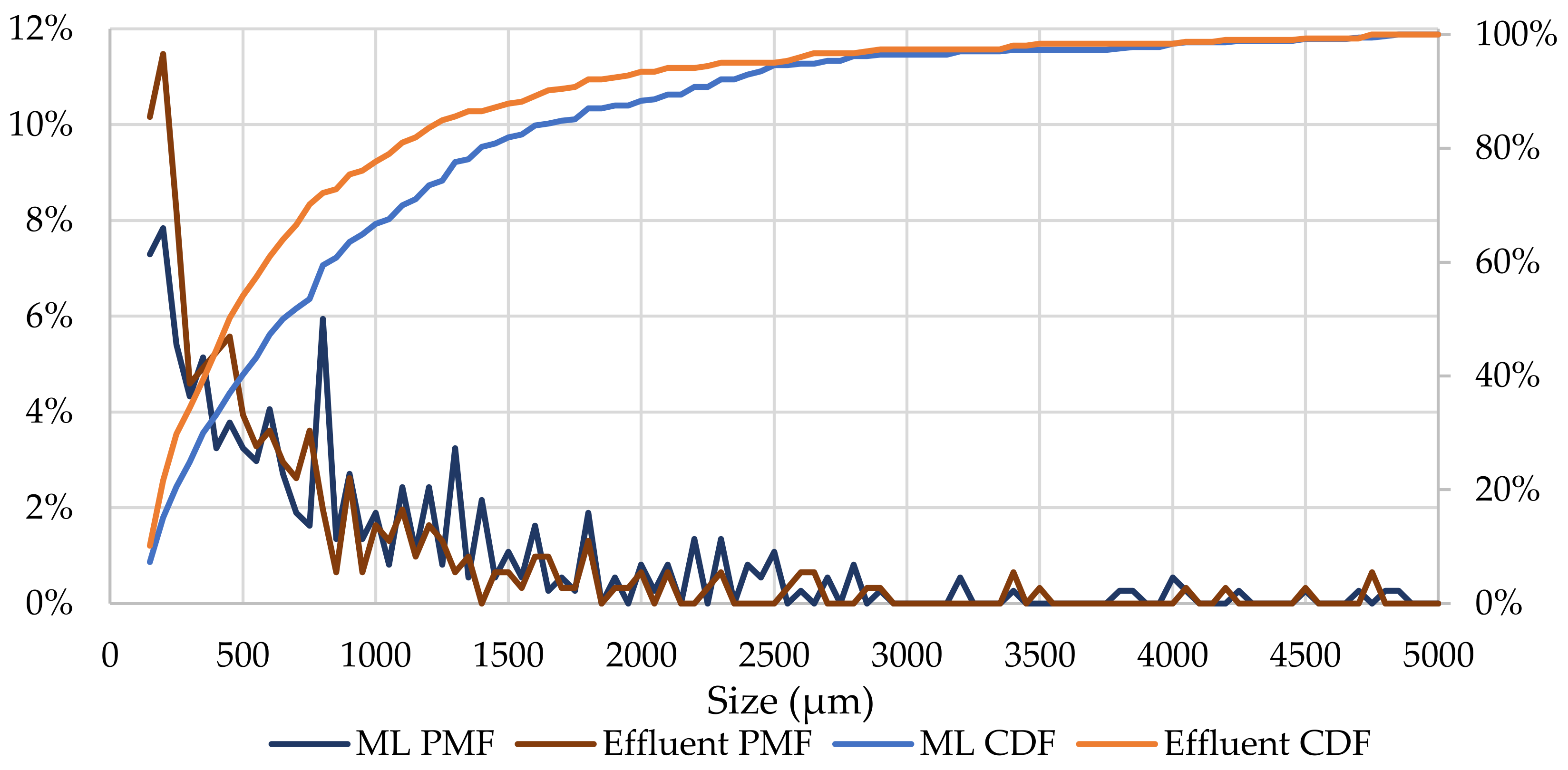
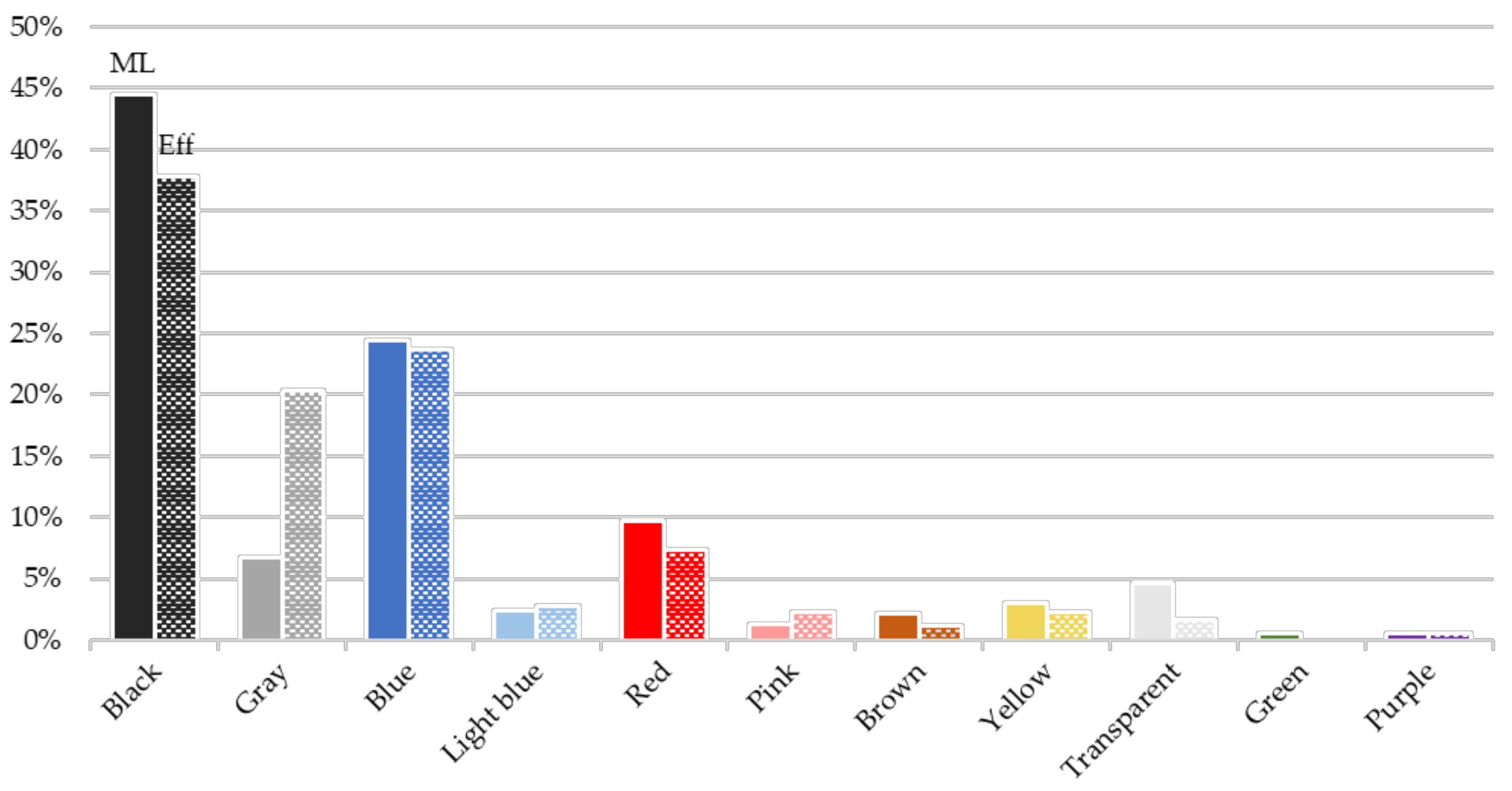
3.2.1. Physical Characterization
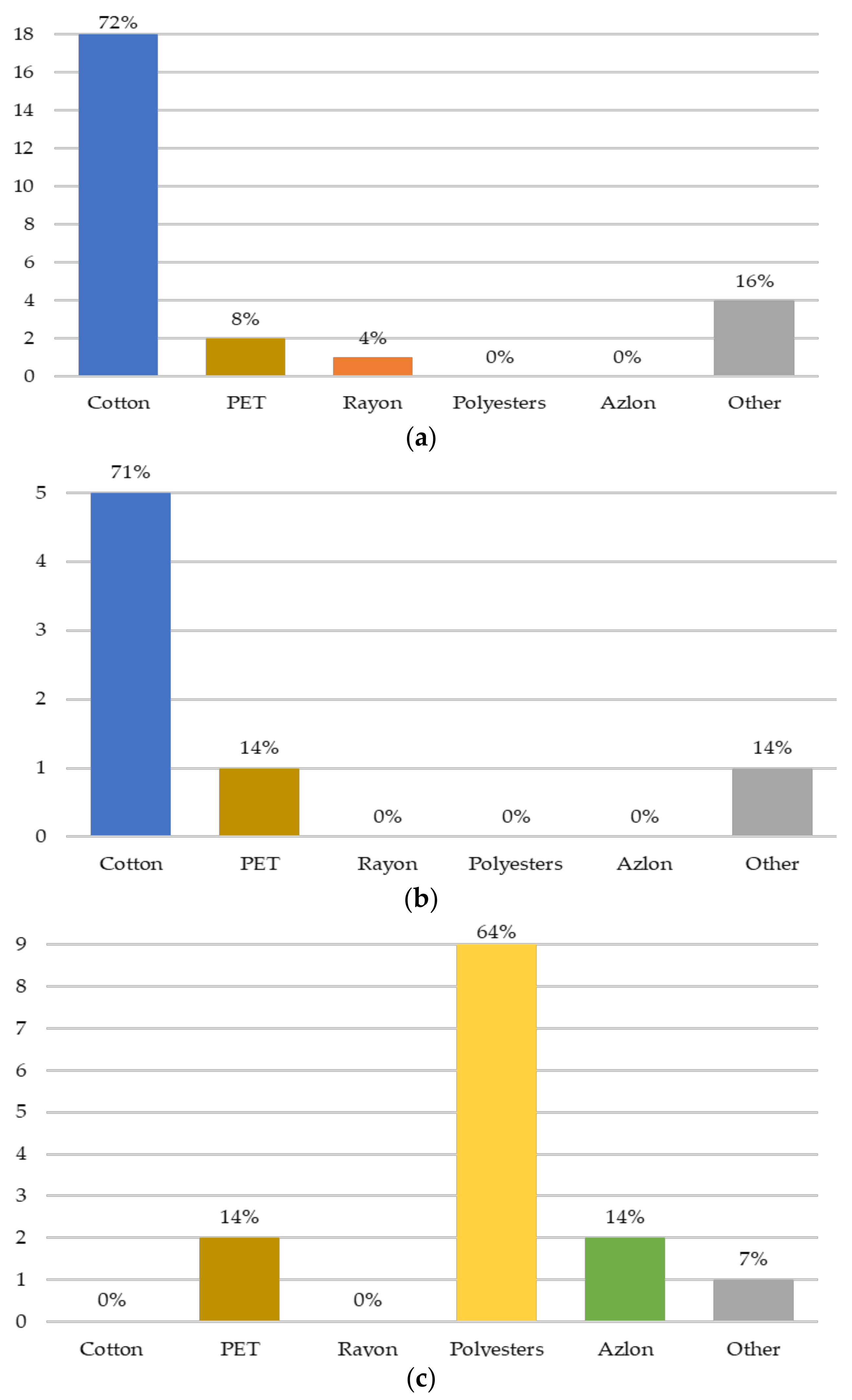
3.2.2. Chemical Characterization
3.3. MF Distribution Model
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ngo, P.L.; Pramanik, B.K.; Shah, K.; Roychand, R. Pathway, classification and removal efficiency of microplastics in wastewater treatment plants. Environ. Pollut. 2019, 255, 113326. [Google Scholar] [CrossRef] [PubMed]
- Vivekanand, A.C.; Mohapatra, S.; Tyagi, V.K. Microplastics in aquatic environment: Challenges and perspectives. Chemosphere 2021, 282, 131151. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Huang, J.; Zhang, W.; Shi, L.; Yi, K.; Yu, H.; Zhang, C.; Li, S.; Li, J. Microplastics as a vehicle of heavy metals in aquatic environments: A review of adsorption factors, mechanisms, and biological effects. J. Environ. Manag. 2021, 302, 113995. [Google Scholar] [CrossRef] [PubMed]
- Frias, J.P.G.L.; Nash, R. Microplastics: Finding a consensus on the definition. Mar. Pollut. Bull. 2019, 138, 145–147. [Google Scholar] [CrossRef]
- Wang, T.; Li, B.; Yu, W.; Zou, X. Microplastic pollution and quantitative source apportionment in the Jiangsu coastal area, China. Mar. Pollut. Bull. 2021, 166, 112237. [Google Scholar] [CrossRef]
- Luo, Z.; Zhou, X.; Su, Y.; Wang, H.; Yu, R.; Zhou, S.; Xu, E.G.; Xing, B. Environmental occurrence, fate, impact, and potential solution of tire microplastics: Similarities and differences with tire wear particles. Sci. Total Environ. 2021, 795, 148902. [Google Scholar] [CrossRef]
- Galvão, A.; Aleixo, M.; De Pablo, H.; Lopes, C.; Raimundo, J. Microplastics in wastewater: Microfiber emissions from common household laundry. Environ. Sci. Pollut. Res. 2020, 27, 26643–26649. [Google Scholar] [CrossRef]
- Kurniawan, S.B.; Said, N.S.M.; Imron, M.F.; Abdullah, S.R.S. Microplastic pollution in the environment: Insights into emerging sources and potential threats. Environ. Technol. Innov. 2021, 23, 101790. [Google Scholar] [CrossRef]
- Sun, J.; Dai, X.; Wang, Q.; van Loosdrecht, M.C.; Ni, B.-J. Microplastics in wastewater treatment plants: Detection, occurrence and removal. Water Res. 2019, 152, 21–37. [Google Scholar] [CrossRef]
- Li, X.; Mei, Q.; Chen, L.; Zhang, H.; Dong, B.; Dai, X.; He, C.; Zhou, J. Enhancement in adsorption potential of microplastics in sewage sludge for metal pollutants after the wastewater treatment process. Water Res. 2019, 157, 228–237. [Google Scholar] [CrossRef]
- Avio, C.G.; Gorbi, S.; Milan, M.; Benedetti, M.; Fattorini, D.; D’Errico, G.; Pauletto, M.; Bargelloni, L.; Regoli, F. Pollutants bioavailability and toxicological risk from microplastics to marine mussels. Environ. Pollut. 2015, 198, 211–222. [Google Scholar] [CrossRef]
- Carbery, M.; O’Connor, W.; Palanisami, T. Trophic transfer of microplastics and mixed contaminants in the marine food web and implications for human health. Environ. Int. 2018, 115, 400–409. [Google Scholar] [CrossRef] [Green Version]
- Hermabessiere, L.; Dehaut, A.; Paul-Pont, I.; Lacroix, C.; Jezequel, R.; Soudant, P.; Duflos, G. Occurrence and effects of plastic additives on marine environments and organisms: A review. Chemosphere 2017, 182, 781–793. [Google Scholar] [CrossRef] [Green Version]
- Ou, H.; Zeng, E.Y. Chapter 10—Occurrence and Fate of Microplastics in Wastewater Treatment Plants. In Microplastic Contamination in Aquatic Environments; Zeng, E.Y., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 317–338. [Google Scholar]
- Sol, D.; Laca, A.; Laca, A.; Díaz, M. Approaching the environmental problem of microplastics: Importance of WWTP treatments. Sci. Total Environ. 2020, 740, 140016. [Google Scholar] [CrossRef]
- Corradini, F.; Meza, P.; Eguiluz, R.; Casado, F.; Huerta-Lwanga, E.; Geissen, V. Evidence of microplastic accumulation in agricultural soils from sewage sludge disposal. Sci. Total Environ. 2019, 671, 411–420. [Google Scholar] [CrossRef]
- Schell, T.; Hurley, R.; Buenaventura, N.T.; Mauri, P.V.; Nizzetto, L.; Rico, A.; Vighi, M. Fate of microplastics in agricultural soils amended with sewage sludge: Is surface water runoff a relevant environmental pathway? Environ. Pollut. 2021, 293, 118520. [Google Scholar] [CrossRef]
- van Den Berg, P.; Huerta-Lwanga, E.; Corradini, F.; Geissen, V. Sewage sludge application as a vehicle for microplastics in eastern Spanish agricultural soils. Environ. Pollut. 2020, 261, 114198. [Google Scholar] [CrossRef]
- Tagg, A.S.; Brandes, E.; Fischer, F.; Fischer, D.; Brandt, J.; Labrenz, M. Agricultural application of microplastic-rich sewage sludge leads to further uncontrolled contamination. Sci. Total Environ. 2021, 806, 150611. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, J.; Liu, H.; Guo, X.; Zhang, X.; Yao, X.; Cao, Z.; Zhang, T. A review of the removal of microplastics in global wastewater treatment plants: Characteristics and mechanisms. Environ. Int. 2021, 146, 106277. [Google Scholar] [CrossRef]
- Tirkey, A.; Upadhyay, L.S.B. Microplastics: An overview on separation, identification and characterization of microplastics. Mar. Pollut. Bull. 2021, 170, 112604. [Google Scholar] [CrossRef]
- Institute for Environment and Sustainability (Joint Research Centre), MSFD Technical Subgroup on Marine Litter. Guidance on Monitoring of Marine Litter in European Seas; Publications Office of the European Union: Luxembourg, 2013. [Google Scholar]
- Riani, E.; Cordova, M.R. Microplastic ingestion by the sandfish Holothuria scabra in Lampung and Sumbawa, Indonesia. Mar. Pollut. Bull. 2021, 2021, 113134. [Google Scholar] [CrossRef]
- Suteja, Y.; Atmadipoera, A.S.; Riani, E.; Nurjaya, I.W.; Nugroho, D.; Cordova, M.R. Spatial and temporal distribution of microplastic in surface water of tropical estuary: Case study in Benoa Bay, Bali, Indonesia. Mar. Pollut. Bull. 2021, 163, 111979. [Google Scholar] [CrossRef]
- Hidalgo-Ruz, V.; Gutow, L.; Thompson, R.C.; Thiel, M. Microplastics in the Marine Environment: A Review of the Methods Used for Identification and Quantification. Environ. Sci. Technol. 2012, 46, 3060–3075. [Google Scholar] [CrossRef]
- Gies, E.A.; LeNoble, J.L.; Noël, M.; Etemadifar, A.; Bishay, F.; Hall, E.R.; Ross, P.S. Retention of microplastics in a major secondary wastewater treatment plant in Vancouver, Canada. Mar. Pollut. Bull. 2018, 133, 553–561. [Google Scholar] [CrossRef]
- Ziajahromi, S.; Neale, P.A.; Rintoul, L.; Leusch, F.D.L. Wastewater treatment plants as a pathway for microplastics: Development of a new approach to sample wastewater-based microplastics. Water Res. 2017, 112, 93–99. [Google Scholar] [CrossRef]
- Yang, L.; Li, K.; Cui, S.; Kang, Y.; An, L.; Lei, K. Removal of microplastics in municipal sewage from China’s largest water reclamation plant. Water Res. 2019, 155, 175–181. [Google Scholar] [CrossRef]
- Prata, J.C.; Reis, V.; Matos, J.T.; da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T. A new approach for routine quantification of microplastics using Nile Red and automated software (MP-VAT). Sci. Total Environ. 2019, 690, 1277–1283. [Google Scholar] [CrossRef]
- Alvim, C.B.; Mendoza-Roca, J.-A.; Bes-Piá, A. Wastewater treatment plant as microplastics release source—Quantification and identification techniques. J. Environ. Manag. 2020, 255, 109739. [Google Scholar] [CrossRef]
- Prata, J.C.; da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T. Methods for sampling and detection of microplastics in water and sediment: A critical review. TrAC Trends Anal. Chem. 2019, 110, 150–159. [Google Scholar] [CrossRef]
- Shim, W.J.; Hong, S.H.; Eo, S.E. Identification methods in microplastic analysis: A review. Anal. Methods 2017, 9, 1384–1391. [Google Scholar] [CrossRef]
- Alvim, C.B.; Castelluccio, S.; Ferrer-Polonio, E.; Bes-Piá, M.; Mendoza-Roca, J.; Fernández-Navarro, J.; Alonso, J.; Amorós, I. Effect of polyethylene microplastics on activated sludge process—Accumulation in the sludge and influence on the process and on biomass characteristics. Process Saf. Environ. Prot. 2021, 148, 536–547. [Google Scholar] [CrossRef]
- GESAMP. Guidelines or the Monitoring and Assessment of Plastic Litter and Microplastics in the Ocean; GESAMP Joint Group of Experts on the Scientific Aspects of Marine Environmental Protection: London, UK, 2019; 130p. [Google Scholar]
- American Association of Textile Chemists and Colorists (AATCC). AATCC 20A: Test Method for Fiber Analysis: Quantitative 2020; American Association of Textile Chemists and Colorists (AATCC): Durham, NC, USA, 2021. [Google Scholar]
- Ferrer-Polonio, E.; White, K.; Mendoza-Roca, J.-A.; Bes-Piá, A. The role of the operating parameters of SBR systems on the SMP production and on membrane fouling reduction. J. Environ. Manag. 2018, 228, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Sol, D.; Laca, A.; Laca, A.; Díaz, M. Microplastics in Wastewater and Drinking Water Treatment Plants: Occurrence and Removal of Microfibres. Appl. Sci. 2021, 11, 10109. [Google Scholar] [CrossRef]
- American Public Health Association (APHA); American Water Works Association (AWWA); Water Environment Federation (WEF). Standard Methods for the Examination of Water and Wastewater, 22nd ed.; APHA: Washington, DC, USA, 2012. [Google Scholar]
- Avio, C.G.; Gorbi, S.; Regoli, F. Experimental development of a new protocol for extraction and characterization of microplastics in fish tissues: First observations in commercial species from Adriatic Sea. Mar. Environ. Res. 2015, 111, 18–26. [Google Scholar] [CrossRef]
- Cole, M.; Webb, H.; Lindeque, P.K.; Fileman, E.S.; Halsband, C.; Galloway, T.S. Isolation of microplastics in biota-rich seawater samples and marine organisms. Sci. Rep. 2014, 4, 4528. [Google Scholar] [CrossRef] [Green Version]
- Carr, S.A.; Liu, J.; Tesoro, A.G. Transport and fate of microplastic particles in wastewater treatment plants. Water Res. 2016, 91, 174–182. [Google Scholar] [CrossRef]
- DeCoursey, W. Chapter 5—Probability Distributions of Discrete Variables: For this chapter the reader should have a solid understanding of sections 2.1, 2.2, 3.1, and 3.2. In Statistics and Probability for Engineering Applications; DeCoursey, W.J., Ed.; Burlington: Newnes, Australia, 2003; pp. 84–140. [Google Scholar]
- Lasch, P. Spectral pre-processing for biomedical vibrational spectroscopy and microspectroscopic imaging. Chemom. Intell. Lab. Syst. 2012, 117, 100–114. [Google Scholar] [CrossRef] [Green Version]
- Institute of Chemistry University of Tartu. Textile Fibres—Database of ATR-FT-IR Spectra of Various Materials 2021. Available online: https://spectra.chem.ut.ee/textile-fibres/ (accessed on 10 December 2021).
- Ali, M.U.; Lin, S.; Yousaf, B.; Abbas, Q.; Munir, M.A.M.; Rasihd, A.; Zheng, C.; Kuang, X.; Wong, M.H. Environmental emission, fate and transformation of microplastics in biotic and abiotic compartments: Global status, recent advances and future perspectives. Sci. Total Environ. 2021, 791, 148422. [Google Scholar] [CrossRef]
- Metcalf & Eddy. Wastewater Engineering: Treatment and Reuse, 4th ed.; McGraw-Hill: New York, NY, USA, 2003. [Google Scholar]
- Lares, M.; Ncibi, M.C.; Sillanpää, M.; Sillanpää, M. Occurrence, identification and removal of microplastic particles and fibers in conventional activated sludge process and advanced MBR technology. Water Res. 2018, 133, 236–246. [Google Scholar] [CrossRef]
- Mahon, A.M.; O’Connell, B.; Healy, M.; O’Connor, I.; Officer, R.; Nash, R.; Morrison, L. Microplastics in Sewage Sludge: Effects of Treatment. Environ. Sci. Technol. 2017, 51, 810–818. [Google Scholar] [CrossRef]
- Liu, X.; Yuan, W.; Di, M.; Li, Z.; Wang, J. Transfer and fate of microplastics during the conventional activated sludge process in one wastewater treatment plant of China. Chem. Eng. J. 2019, 362, 176–182. [Google Scholar] [CrossRef]
- Murphy, F.; Ewins, C.; Carbonnier, F.; Quinn, B. Wastewater Treatment Works (WwTW) as a Source of Microplastics in the Aquatic Environment. Environ. Sci. Technol. 2016, 50, 5800–5808. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Chen, L.; Mei, Q.; Dong, B.; Dai, X.; Ding, G.; Zeng, E.Y. Microplastics in sewage sludge from the wastewater treatment plants in China. Water Res. 2018, 142, 75–85. [Google Scholar] [CrossRef]
- Nuelle, M.-T.; Dekiff, J.H.; Remy, D.; Fries, E. A new analytical approach for monitoring microplastics in marine sediments. Environ. Pollut. 2014, 184, 161–169. [Google Scholar] [CrossRef]



| Parameter | Average Value | SD |
|---|---|---|
| pH | 7.31 | ± 0.20 |
| EC (mS/cm) | 1.10 | ± 0.07 |
| Turbidity (NTU) | 1.50 | ± 1.27 |
| COD (mg O2/L) | 18.24 | ± 7.78 |
| COD removal efficiency (%) | 96.35 | ± 1.56 |
| Ntot (mg/L) | 44.64 | ± 7.73 |
| N-NO2−(mg/L) | 0.11 | ± 0.11 |
| N-NO3− (mg/L) | 38.38 | ± 5.05 |
| N-NH4+ (mg/L) | < 4.00 | - |
| Ptot (mg/L) | 5.52 | ± 1.34 |
| P-PO43− (mg/L) | 5.70 | ± 1.58 |
| TOC (mg/L) | 5.41 | ± 1.52 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castelluccio, S.; Alvim, C.B.; Bes-Piá, M.A.; Mendoza-Roca, J.A.; Fiore, S. Assessment of Microplastics Distribution in a Biological Wastewater Treatment. Microplastics 2022, 1, 141-155. https://doi.org/10.3390/microplastics1010009
Castelluccio S, Alvim CB, Bes-Piá MA, Mendoza-Roca JA, Fiore S. Assessment of Microplastics Distribution in a Biological Wastewater Treatment. Microplastics. 2022; 1(1):141-155. https://doi.org/10.3390/microplastics1010009
Chicago/Turabian StyleCastelluccio, Stefano, Clara Bretas Alvim, María Amparo Bes-Piá, José Antonio Mendoza-Roca, and Silvia Fiore. 2022. "Assessment of Microplastics Distribution in a Biological Wastewater Treatment" Microplastics 1, no. 1: 141-155. https://doi.org/10.3390/microplastics1010009
APA StyleCastelluccio, S., Alvim, C. B., Bes-Piá, M. A., Mendoza-Roca, J. A., & Fiore, S. (2022). Assessment of Microplastics Distribution in a Biological Wastewater Treatment. Microplastics, 1(1), 141-155. https://doi.org/10.3390/microplastics1010009