Modelling the Photodegradation of Marine Microplastics by Means of Infrared Spectrometry and Chemometric Techniques
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Experimental Procedure
2.2. Analyses
- Keto-Carbonyl Bond Index (KCBI) = I1715/I1465
- Ester-Carbonyl Bond Index (ECBI) = I1740/I1465
- Vinyl Bond Index (VBI) = I1650/I1465
- Internal Double Bond Index (IDBI) = I908/I1465
- Ester-Carbonyl Bond Index (ECBI): I1748/I1456
- Methyl Group Index (MGI): I1377/I1456
- Isotacticity, I(%) computed as (I997/I973) × 100
2.3. Statistics
3. Results and Discussion
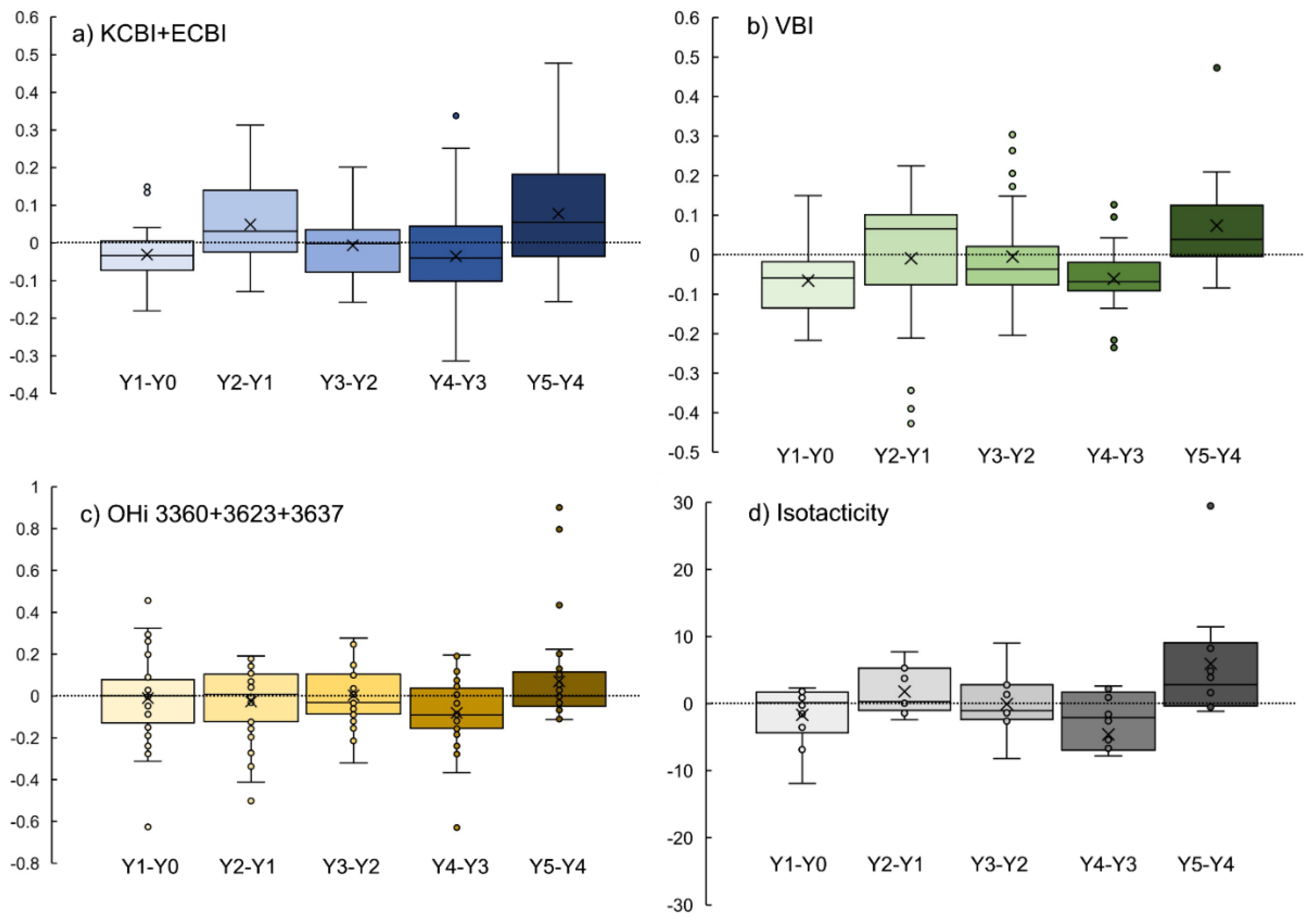
3.1. Photodegradation Indexes
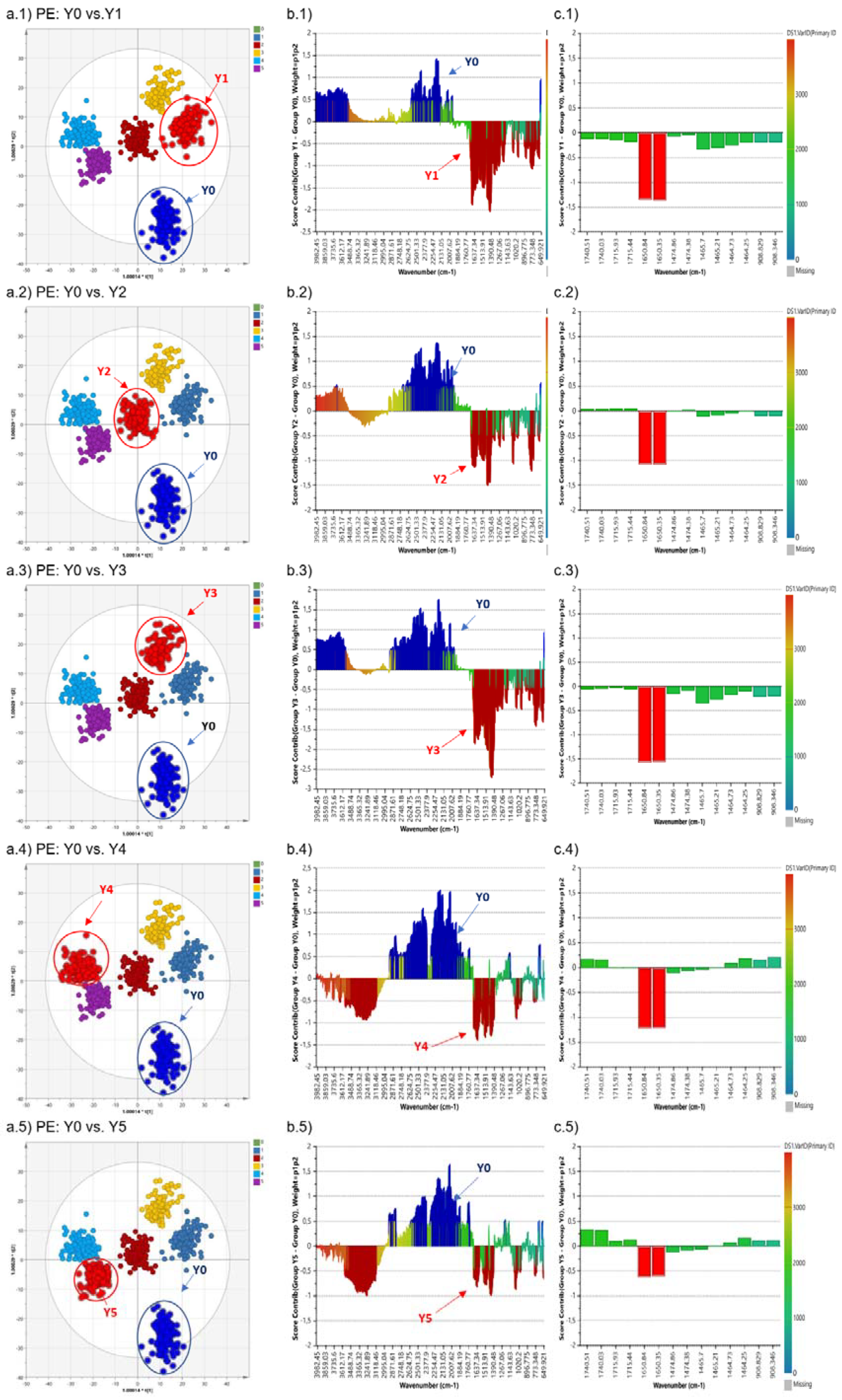
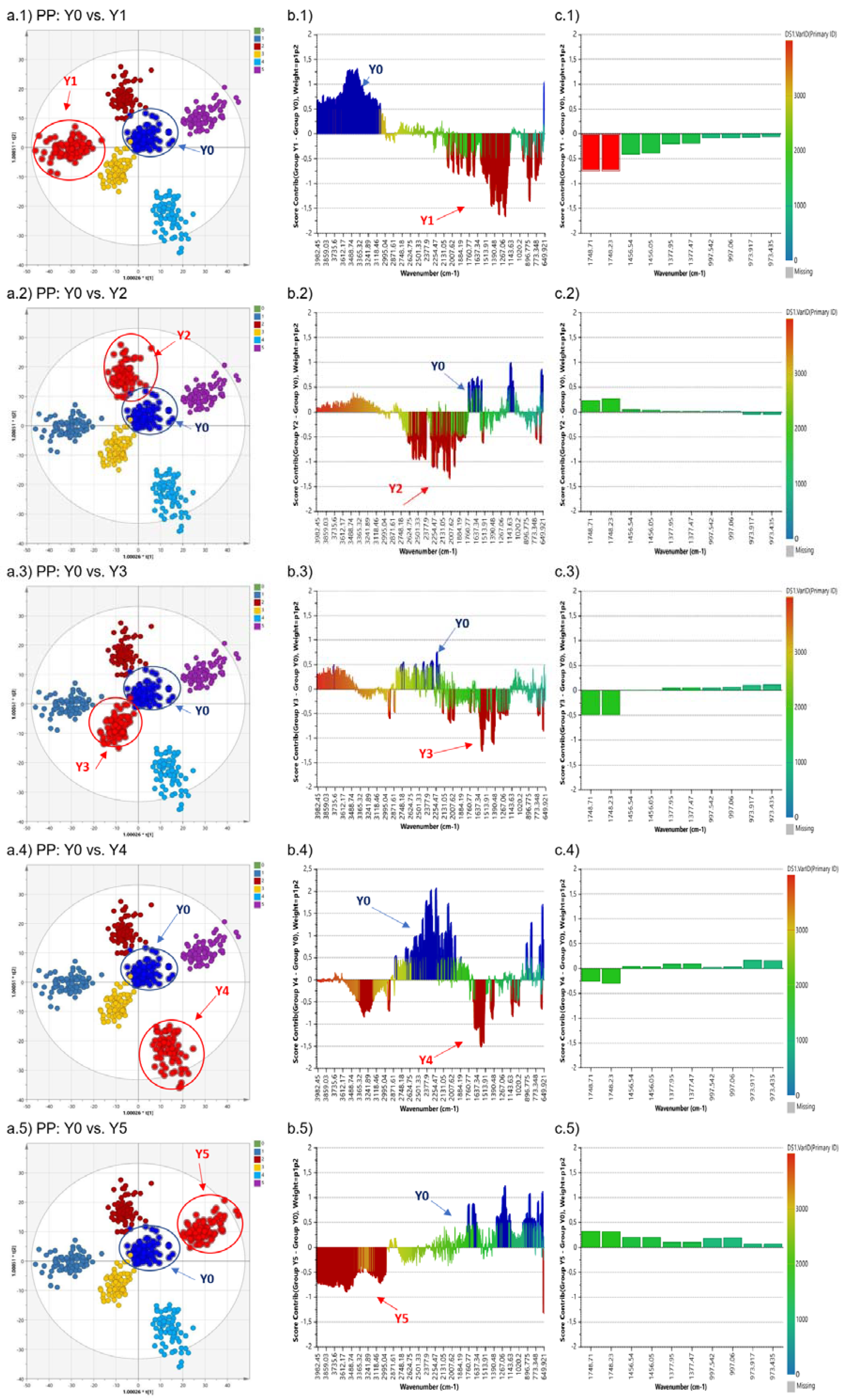
3.2. Orthogonal Partial Least Square-Discriminant Analysis (OPLS-DA)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ATR-FTIR | Attenuated Total Reflectance-Fourier Transform Infrared |
| CV-ANOVA | Analysis of Variance Testing of Cross-Validated Predictive Residuals |
| ECBI | Ester-Carbonyl Bond Index |
| HDPE | Hight Density Polyethylene |
| Hoi | Hydroxyl Index |
| Ia | Absorbance for FTIR band at 1474 cm−1 |
| Ib | Absorbance for FTIR band at 1464 cm−1 |
| IDBI | Internal Double Bond Index |
| KCBI | Keto-Carbonyl Bond Index |
| LDPE | Low-Density Polyethylene |
| (O)PLS-DA | (Orthogonal) Partial Least Squares-Discriminant Analysis |
| PE | Polyethylene |
| PP | Polypropylene |
| Q2 | Goodness-of-Prediction |
| R2P | Coefficient of Determination in Prediction |
| R2X/Y | Goodness-of-Fit |
| RMSECV | Root Mean Square Error of Cross-Validation |
| RMSEE | Root Mean Square Error of Estimation |
| SIMCA | Soft Independent Modelling of Class Analogy |
| SNV | Standard Normal Variate |
| VBI | Vinyl Bond Index |
| VIP | Analysis of Variable Importance of the Projection |
References
- PlasticsEurope. Plastics—The Facts 2021: An Analysis of European Plastics Production, Demand and Waste Data; PlasticsEurope; Association of Plastics Manufacturers: Brussels, Belgium, 2021. [Google Scholar]
- Lau, W.W.Y.; Shiran, Y.; Bailey, R.M.; Cook, E.; Stuchtey, M.R.; Koskella, J.; Velis, C.A.; Godfrey, L.; Boucher, J.; Murphy, M.B.; et al. Evaluating scenarios toward zero plastic pollution. Science 2020, 369, 1455–1461. [Google Scholar] [CrossRef] [PubMed]
- Pinto-da-Costa, J.; Rocha-Santos, T.; Duarte, A.C. The Environmental Impacts of Plastics and Micro-Plastics Use, Waste and Pollution: EU and National Measures; European Union; Policy Department for Citizens’ Rights and Constitutional Affairs: Brussels, Belgium, 2020; pp. 1–72. [Google Scholar]
- Napper, I.E.; Thompson, R.C. Release of synthetic microplastic plastic fibres from domestic washing machines: Effects of fabric type and washing conditions. Mar. Pollut. Bull. 2016, 112, 39–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- OECD. Global Plastics Outlook: Economic Drivers, Environmental Impacts and Policy Options; OECD Publishing: Paris, France, 2022. [Google Scholar] [CrossRef]
- North, E.J.; Halden, R.U. Plastics and environmental health: The road ahead. Rev. Environ. Health 2013, 28, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, E.J.; Smith, K.L. Plastics on the Sargasso Sea surface. Science 1972, 175, 1240–1241. [Google Scholar] [CrossRef]
- Chamas, A.; Moon, H.; Zheng, J.; Qiu, Y.; Tabassum, T.; Jang, J.H.; Abu-Omar, M.; Scott, S.L.; Suh, S. Degradation rates of plastics in the environment. ACS Sustain. Chem. Eng. 2020, 8, 3494–3511. [Google Scholar] [CrossRef] [Green Version]
- Gewert, B.; Plassmann, M.M.; MacLeod, M. Pathways for degradation of plastic polymers floating in the marine environment. Environ. Sci. Processes Impacts 2015, 17, 1513–1521. [Google Scholar] [CrossRef] [Green Version]
- Gopanna, A.; Mandapati, R.N.; Thomas, S.P.; Rajan, K.; Chavali, M. Fourier transform infrared spectroscopy (FTIR), Raman spectroscopy and wide-angle X-ray scattering (WAXS) of polypropylene (PP)/cyclic olefin copolymer (COC) blends for qualitative and quantitative analysis. Polym. Bull. 2019, 76, 4259–4274. [Google Scholar] [CrossRef]
- Brandon, J.; Goldstein, M.; Ohman, M.D. Long-term aging and degradation of microplastic particles: Comparing in situ oceanic and experimental weathering patterns. Mar. Pollut. Bull. 2016, 110, 299–308. [Google Scholar] [CrossRef] [Green Version]
- Edo, C.; Tamayo-Belda, M.; Martínez-Campos, S.; Martín-Betancor, K.; González-Pleiter, M.; Pulido-Reyes, G.; García-Ruiz, C.; Zapata, F.; Leganés, F.; Fernández-Piñas, F.; et al. Occurrence and identification of microplastics along a beach in the Biosphere Reserve of Lanzarote. Mar. Pollut. Bull. 2019, 143, 220–227. [Google Scholar] [CrossRef]
- ASTM D1141-98(2021); Standard Practice for Preparation of Substitute Ocean Water. ASTM International: West Conshohocken, PA, USA, 2021. [CrossRef]
- Rånby, B. Photodegradation and photo-oxidation of synthetic polymers. J. Anal. Appl. Pyrolysis 1989, 15, 237–247. [Google Scholar] [CrossRef]
- Jabarin, S.A.; Lofgren, E.A. Photooxidative effects on properties and structure of high-density polyethylene. J. Appl. Polym. Sci. 1994, 53, 411–423. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, N. Mechanistic implications of plastic degradation. Polym. Degrad. Stab. 2008, 93, 561–584. [Google Scholar] [CrossRef]
- Agboola, O.; Sadiku, R.; Mokrani, T.; Amer, I.; Imoru, O. Polyolefins and the environment. In Polyolefin Fibres, 2nd ed.; Ugbolue, S.C.O., Ed.; Woodhead Publishing: Sawston, UK, 2017; pp. 89–133. [Google Scholar]
- Albertsson, A.-C.; Andersson, S.O.; Karlsson, S. The mechanism of biodegradation of polyethylene. Polym. Degrad. Stab. 1987, 18, 73–87. [Google Scholar] [CrossRef]
- Zerbi, G.; Gallino, G.; Del Fanti, N.; Baini, L. Structural depth profiling in polyethylene films by multiple internal reflection infra-red spectroscopy. Polymer 1989, 30, 2324–2327. [Google Scholar] [CrossRef]
- Arkatkar, A.; Arutchelvi, J.; Bhaduri, S.; Uppara, P.V.; Doble, M. Degradation of unpretreated and thermally pretreated polypropylene by soil consortia. Int. Biodeterior. Biodegrad. 2009, 63, 106–111. [Google Scholar] [CrossRef]
- Adothu, B.; Costa, F.R.; Mallick, S. Damp heat resilient thermoplastic polyolefin encapsulant for photovoltaic module encapsulation. Sol. Energy Mater. 2021, 224, 111024. [Google Scholar] [CrossRef]
- Chung, I.-M.; Kim, J.-K.; Han, J.-G.; Kong, W.-S.; Kim, S.-Y.; Yang, Y.-J.; An, Y.-J.; Kwon, C.; Chi, H.-Y.; Yhung Jung, M.; et al. Potential geo-discriminative tools to trace the origins of the dried slices of shiitake (Lentinula edodes) using stable isotope ratios and OPLS-DA. Food Chem. 2019, 295, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Worley, B.; Powers, R. PCA as a practical indicator of OPLS-DA model reliability. Curr. Metab. 2016, 4, 97–103. [Google Scholar] [CrossRef] [Green Version]
- Bylesjö, M.; Rantalainen, M.; Cloarec, O.; Nicholson, J.K.; Holmes, E.; Trygg, J. OPLS discriminant analysis: Combining the strengths of PLS-DA and SIMCA classification. J. Chemom. 2006, 20, 341–351. [Google Scholar] [CrossRef]
- Hiejima, Y.; Kida, T.; Takeda, K.; Igarashi, T.; Nitta, K.-h. Microscopic structural changes during photodegradation of low-density polyethylene detected by Raman spectroscopy. Polym. Degrad. Stab. 2018, 150, 67–72. [Google Scholar] [CrossRef]
- Carrasco, F.; Pagès, P.; Pascual, S.; Colom, X. Artificial aging of high-density polyethylene by ultraviolet irradiation. Eur. Polym. J. 2001, 37, 1457–1464. [Google Scholar] [CrossRef]
- Iedema, P.D.; Remerie, K.; Seegers, D.; McAuley, K.B. Tacticity changes during controlled degradation of polypropylene. Macromolecules 2021, 54, 8921–8935. [Google Scholar] [CrossRef]
- Rosal, R. Morphological description of microplastic particles for environmental fate studies. Mar. Pollut. Bull. 2021, 171, 112716. [Google Scholar] [CrossRef] [PubMed]
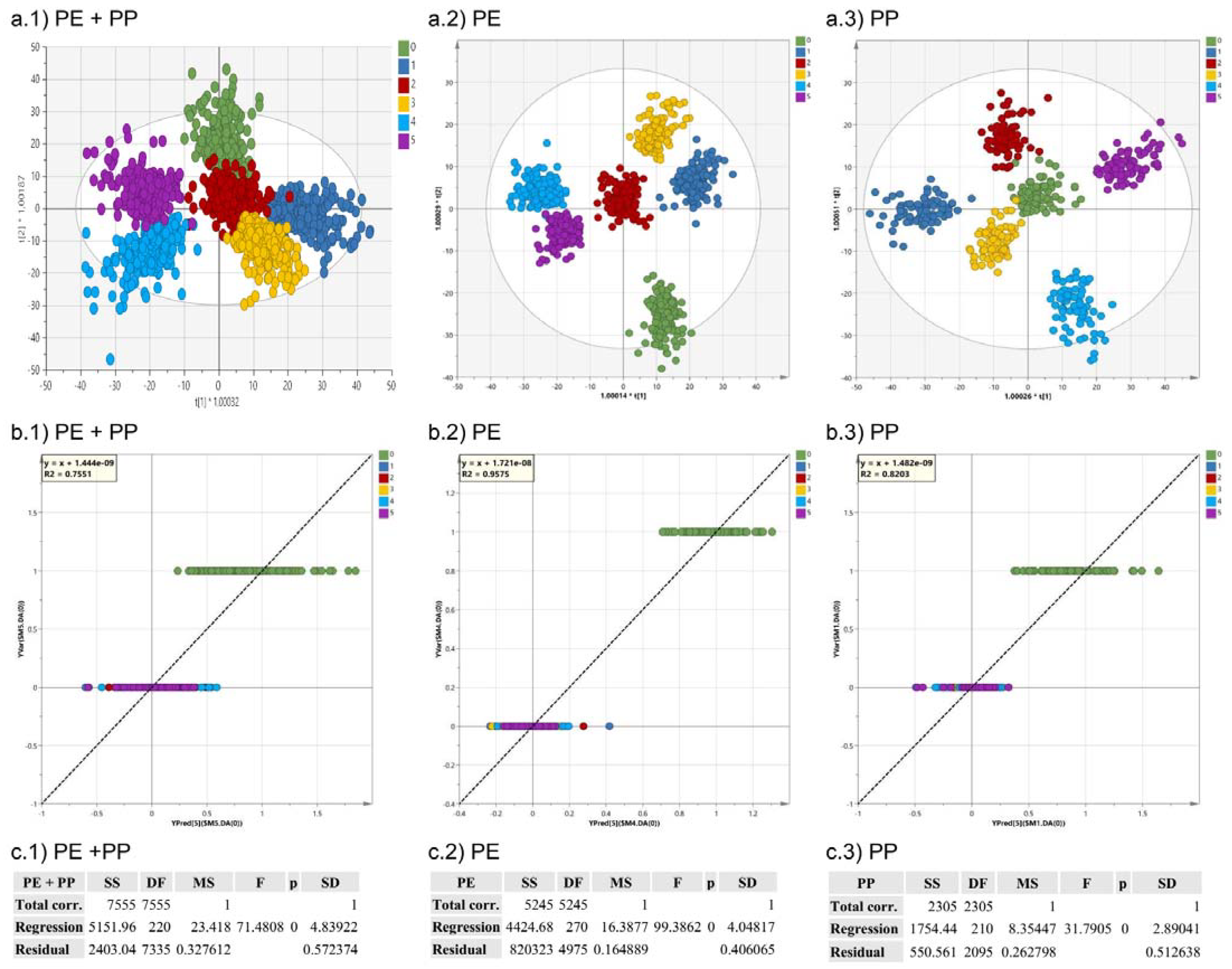

| Id. | Group Class | Spectra | Descriptive Components | Orthogonal Components | R2 X(cum) | R2 Y(cum) | Q2 (cum) | RMSEE | RMSECV |
|---|---|---|---|---|---|---|---|---|---|
| 1 | All Samples | ||||||||
| Polymer | 1512 | 6 | 16 | 0.931 | 0.749 | 0.710 | 0.094 | 0.102 | |
| Origin | 1512 | 2 | 13 | 0.912 | 0.946 | 0.938 | 0.051 | 0.051 | |
| Colour | 1512 | 5 | 14 | 0.926 | 0.576 | 0.534 | 0.250 | 0.259 | |
| Irradiation Time | 1512 | 5 | 17 | 0.932 | 0.714 | 0.679 | 0.186 | 0.201 | |
| Shape | 1512 | 2 | 14 | 0.915 | 0.967 | 0.962 | 0.033 | 0.367 | |
| 2 | Commercial pellets | ||||||||
| Polymer | 84 | 1 | 3 | 0.793 | 0.977 | 0.970 | 0.079 | 0.087 | |
| Irradiation Time | 84 | 5 | 5 | 0.925 | 0.714 | 0.602 | 0.246 | 0.264 | |
| 3 | Fragments of objects | ||||||||
| Polymer | 168 | 2 | 6 | 0.950 | 0.943 | 0.912 | 0.130 | 0.157 | |
| Colour | 168 | 1 | 3 | 0.882 | 0.970 | 0.958 | 0.088 | 0.103 | |
| Irradiation Time | 168 | 5 | 3 | 0.943 | 0.568 | 0.497 | 0.271 | 0.275 | |
| 4 | Marine debris | ||||||||
| Polymer | 1260 | 1 | 5 | 0.832 | 0.836 | 0.819 | 0.178 | 0.187 | |
| Colour | 1260 | 5 | 14 | 0.933 | 0.565 | 0.519 | 0.161 | 0.185 | |
| Irradiation time | 1260 | 5 | 17 | 0.939 | 0.752 | 0.710 | 0.161 | 0.180 | |
| 5 | PE | ||||||||
| Polymer | 1050 | 3 | 15 | 0.921 | 0.948 | 0.935 | 0.044 | 0.046 | |
| Origin | 1050 | 2 | 13 | 0.907 | 0.962 | 0.954 | 0.048 | 0.049 | |
| Irradiation Time | 1050 | 5 | 22 | 0.942 | 0.888 | 0.848 | 0.078 | 0.110 | |
| Colour | 1050 | 5 | 13 | 0.924 | 0.611 | 0.556 | 0.228 | 0.024 | |
| Shape | 1050 | 2 | 13 | 0.906 | 0.964 | 0.957 | 0.046 | 0.048 | |
| 6 | PP | ||||||||
| Polymer | 462 | 2 | 11 | 0.907 | 0.947 | 0.916 | 0.050 | 0.067 | |
| Origin | 462 | 2 | 11 | 0.907 | 0.947 | 0.916 | 0.050 | 0.067 | |
| Irradiation Time | 462 | 5 | 16 | 0.930 | 0.849 | 0.774 | 0.162 | 0.184 | |
| Colour | 462 | 3 | 9 | 0.902 | 0.755 | 0.693 | 0.185 | 0.210 | |
| Shape | 462 | 1 | 10 | 0.896 | 0.979 | 0.957 | 0.042 | 0.060 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sorasan, C.; Ortega-Ojeda, F.E.; Rodríguez, A.; Rosal, R. Modelling the Photodegradation of Marine Microplastics by Means of Infrared Spectrometry and Chemometric Techniques. Microplastics 2022, 1, 198-210. https://doi.org/10.3390/microplastics1010013
Sorasan C, Ortega-Ojeda FE, Rodríguez A, Rosal R. Modelling the Photodegradation of Marine Microplastics by Means of Infrared Spectrometry and Chemometric Techniques. Microplastics. 2022; 1(1):198-210. https://doi.org/10.3390/microplastics1010013
Chicago/Turabian StyleSorasan, Carmen, Fernando E. Ortega-Ojeda, Antonio Rodríguez, and Roberto Rosal. 2022. "Modelling the Photodegradation of Marine Microplastics by Means of Infrared Spectrometry and Chemometric Techniques" Microplastics 1, no. 1: 198-210. https://doi.org/10.3390/microplastics1010013
APA StyleSorasan, C., Ortega-Ojeda, F. E., Rodríguez, A., & Rosal, R. (2022). Modelling the Photodegradation of Marine Microplastics by Means of Infrared Spectrometry and Chemometric Techniques. Microplastics, 1(1), 198-210. https://doi.org/10.3390/microplastics1010013