On The Biophysical Complexity of Brain Dynamics: An Outlook
Abstract
1. Introduction
2. Nonlinear Biological Interactions
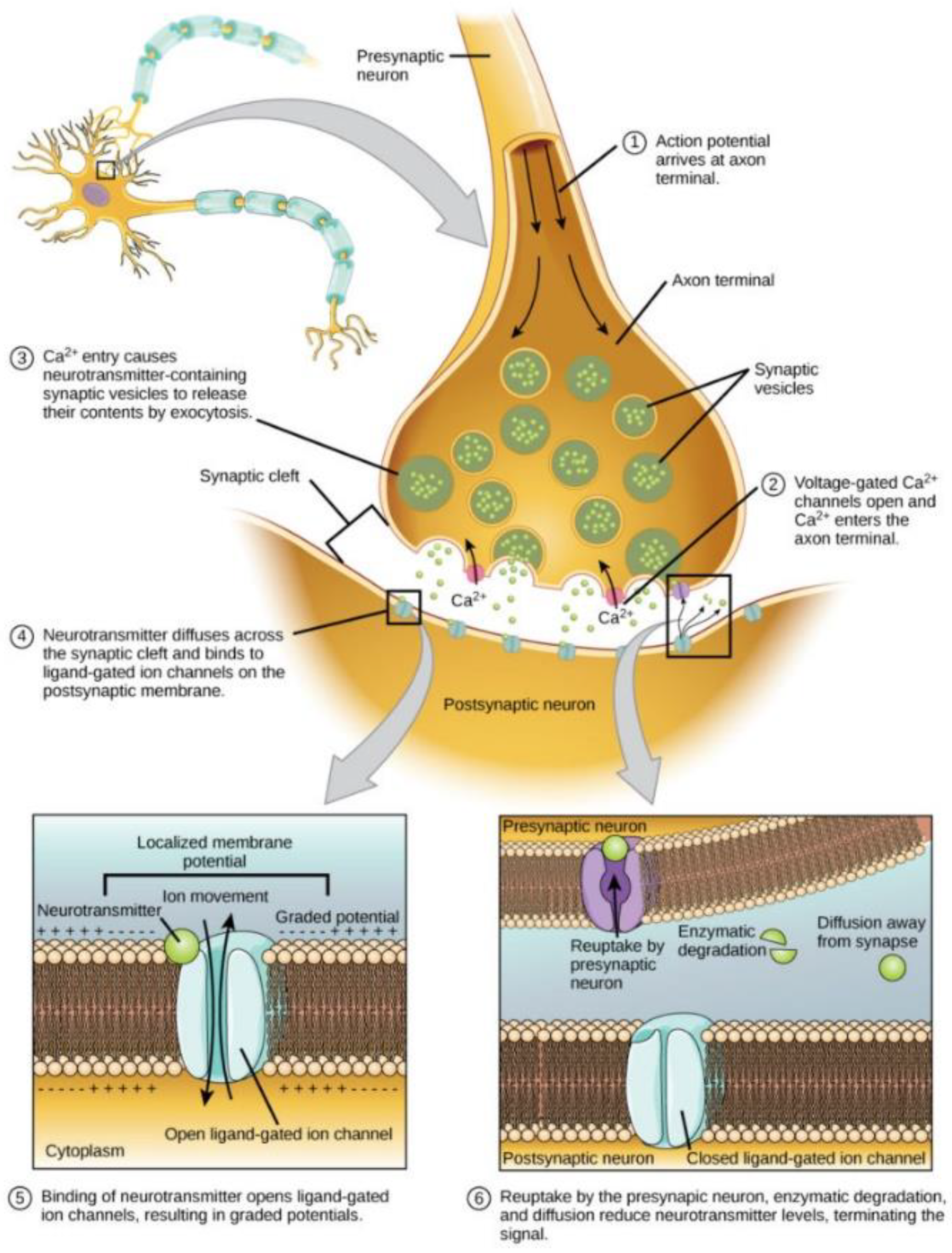
2.1. Synaptic Plasticity
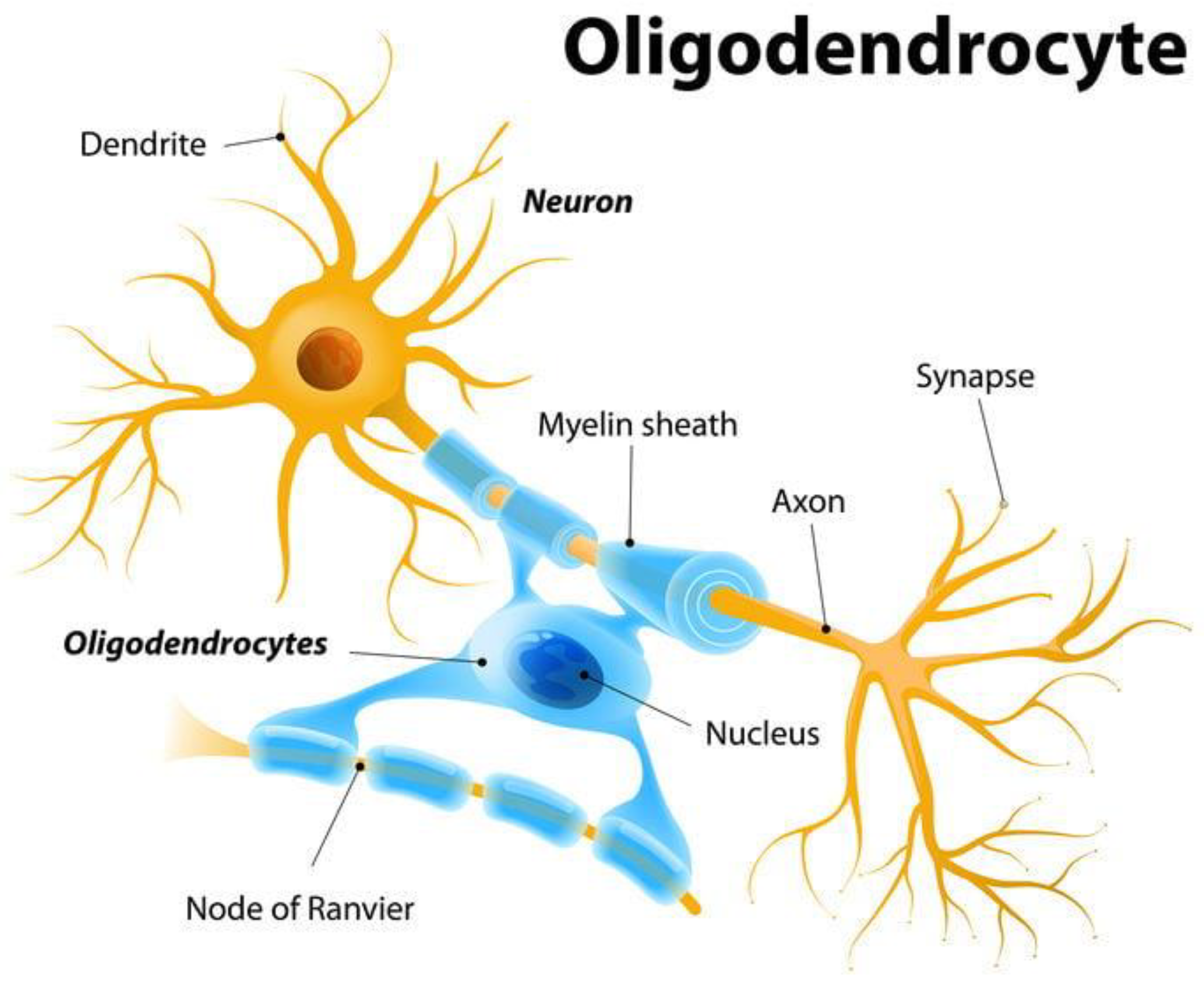
2.2. Axonal and Dendritic Structural Plasticity
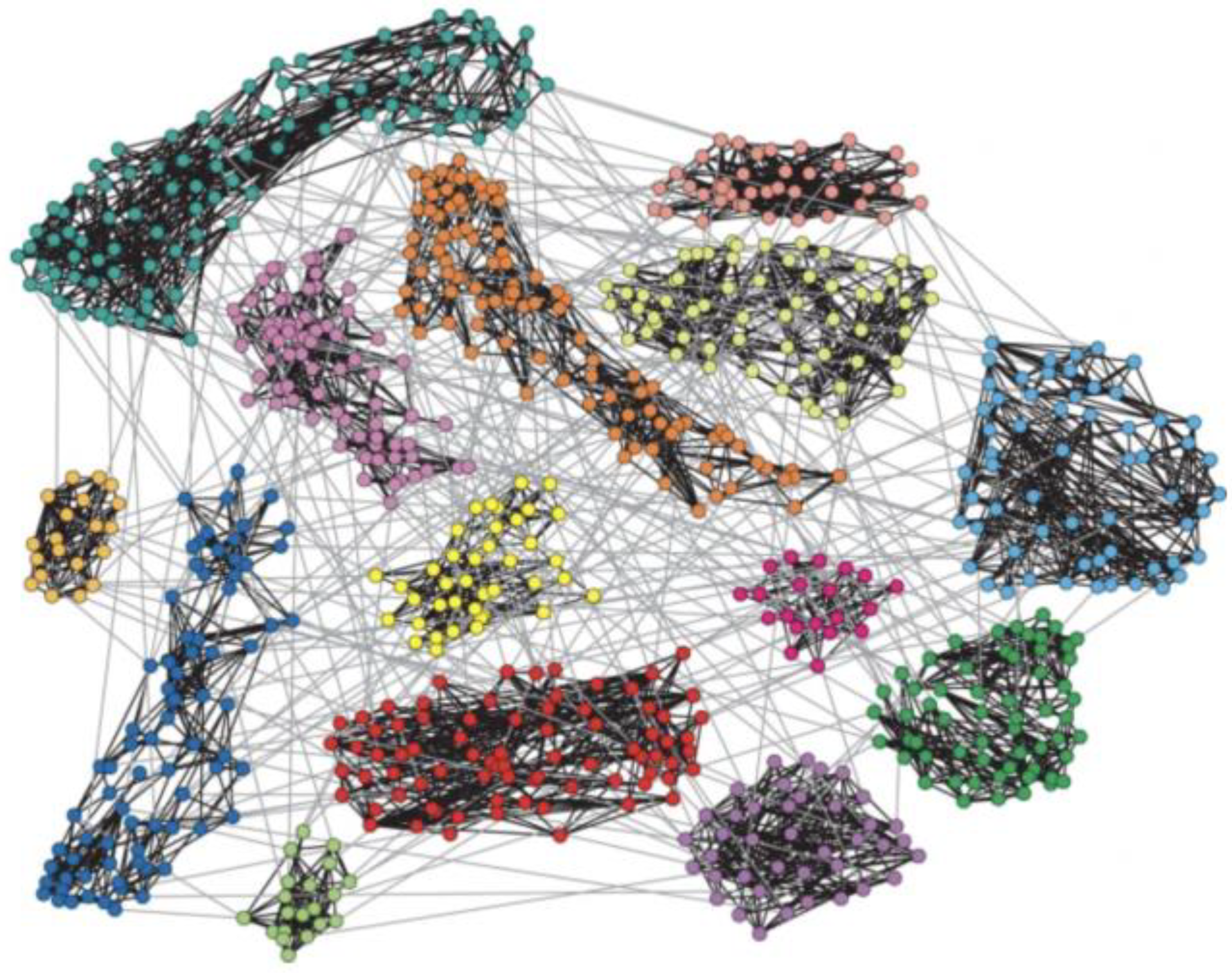
2.3. Quantifying Dynamical Local Coupling
2.4. Local Interaction-Induced Global Characteristics
3. Complex Global Multimodal Synchronization from Local Nonlinear Interactions
3.1. Synchronization
3.2. Multimodal Synchronization
3.3. Complex Forms of Self-Organization
3.4. Examples Observed in the Brain
3.5. Defining the Brain Quantitatively
4. Concluding Remarks
Funding
Conflicts of Interest
References
- Park, H.-J.; Friston, K. Structural and functional brain networks: From connections to cognition. Science 2013, 342, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Bassett, D.S.; Sporns, O. Network neuroscience. Nat. Neurosci. 2017, 20, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Bressler, S.L.; Menon, V. Large-scale brain networks in cognition: Emerging methods and principles. Trends Cogn. Sci. 2010, 14, 277–290. [Google Scholar] [CrossRef]
- Sporns, O. Structure and function of complex brain networks. Dialog Clin. Neurosci. 2013, 15, 247–262. [Google Scholar] [CrossRef]
- Shine, J.M.; Poldrack, R.A. Principles of dynamic network reconfiguration across diverse brain states. NeuroImage 2018, 180, 396–405. [Google Scholar] [CrossRef]
- Lin, A.; Liu, K.K.L.; Bartsch, R.P.; Ivanov, P.C. Dynamic network interactions among distinct brain rhythms as a hallmark of physiologic state and function. Commun. Biol. 2020, 3, 1–11. [Google Scholar]
- Davison, E.N.; Schlesinger, K.J.; Bassett, D.S.; Lynall, M.-E.; Miller, M.B.; Grafton, S.T.; Carlson, J.M. Brain Network Adaptability across Task States. PLOS Comput. Biol. 2015, 11, e1004029. [Google Scholar] [CrossRef]
- Wang, X.-J.; Kennedy, H. Brain structure and dynamics across scales: In search of rules. Curr. Opin. Neurobiol. 2016, 37, 92–98. [Google Scholar] [CrossRef]
- Bar, M. The proactive brain: Memory for predictions. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1235–1243. [Google Scholar] [CrossRef]
- Geary, D.C. The Origin of Mind; American Psychological Association: Washington, DC, USA, 2005. [Google Scholar]
- Harrison, B.J.; Pujol, J.; López-Solà, M.; Hernández-Ribas, R.; Deus, J.; Ortiz, H.; Soriano-Mas, C.; Yücel, M.; Pantelis, C.; Cardoner, N. Consistency and functional specialization in the default mode brain network. Proc. Natl. Acad. Sci. USA 2008, 105, 9781–9786. [Google Scholar] [CrossRef]
- Pessoa, L. Understanding brain networks and brain organization. Phys. Life Rev. 2014, 11, 400–435. [Google Scholar] [CrossRef]
- Cocchi, L.; Zalesky, A.; Fornito, A.; Mattingley, J. Dynamic cooperation and competition between brain systems during cognitive control. Trends Cogn. Sci. 2013, 17, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Hariri, A.R.; Mattay, V.S.; Tessitore, A.; Fera, F.; Weinberger, D.R. Neocortical modulation of the amygdala response to fearful stimuli. Biol. Psychiatry 2003, 53, 494–501. [Google Scholar] [CrossRef]
- Barbas, H. Anatomic basis of cognitive-emotional interactions in the primate prefrontal cortex. Neurosci. Biobehav. Rev. 1995, 19, 499–510. [Google Scholar] [CrossRef]
- Wolff, M.; Alcaraz, F.; Marchand, A.R.; Coutureau, E. Functional heterogeneity of the limbic thalamus: From hippocampal to cortical functions. Neurosci. Biobehav. Rev. 2015, 54, 120–130. [Google Scholar] [CrossRef]
- Tozzi, A. The multidimensional brain. Phys. Life Rev. 2019, 31, 86–103. [Google Scholar] [CrossRef] [PubMed]
- Székely, G. An approach to the complexity of the brain. Brain Res. Bull. 2001, 55, 11–28. [Google Scholar] [CrossRef]
- Rolls, E.T. Information representation, processing and storage in the brain: Analysis at the single neuron level. In The Neural and Molecular Bases of Learning; John Wiley & Sons: Hoboken, NJ, USA, 1987; pp. 503–540. [Google Scholar]
- Nieder, A.; Dehaene, S. Representation of number in the brain. Annu. Rev. Neurosci. 2009, 32, 185–208. [Google Scholar] [CrossRef] [PubMed]
- Martin, A. The Representation of Object Concepts in the Brain. Annu. Rev. Psychol. 2007, 58, 25–45. [Google Scholar] [CrossRef] [PubMed]
- Bisiach, E.; Capitani, E.; Luzzatti, C.G.; Perani, D. Brain and conscious representation of outside reality. Neuropsychologia 1981, 19, 543–551. [Google Scholar] [CrossRef]
- Hofstadter, D.R. Gödel, Escher, Bach; Harvester Press: London, UK, 1979. [Google Scholar]
- Purves, D. Neural Activity and the Growth of the Brain; Cambridge University Press: Cambridge, UK, 1994. [Google Scholar]
- Cramer, S.C.; Sur, M.; Dobkin, B.H.; O’Brien, C.; Sanger, T.D.; Trojanowski, J.Q.; Rumsey, J.M.; Hicks, R.; Cameron, J.; Chen, D.; et al. Harnessing neuroplasticity for clinical applications. Brain 2011, 134, 1591–1609. [Google Scholar] [CrossRef] [PubMed]
- Takesian, A.E.; Hensch, T.K. Balancing Plasticity/Stability Across Brain Development. Prog. Brain Res. 2013, 207, 3–34. [Google Scholar] [PubMed]
- Michel, C.M.; Seeck, M.; Landis, T. Spatiotemporal Dynamics of Human Cognition. News Physiol. Sci. Int. J. Physiol. Prod. Jt. Int. Union Physiol. Sci. Am. Physiol. Soc. 1999, 14, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Canolty, R.T.; Soltani, M.; Dalal, S.S.; Edwards, E.; Dronkers, N.F.; Nagarajan, S.S.; E Kirsch, H.; Barbaro, N.M.; Knight, R.T. Spatiotemporal dynamics of word processing in the human brain. Front. Neurosci. 2007, 1, 185–196. [Google Scholar] [CrossRef]
- Stevens, R.H.; Galloway, T.L. Are neurodynamic organizations a fundamental property of teamwork? Front. Psychol. 2017, 8, 644. [Google Scholar] [CrossRef]
- Gray, C.M. The Temporal Correlation Hypothesis of Visual Feature Integration: Still Alive and Well. Neuron 1999, 24, 31–47. [Google Scholar] [CrossRef]
- Stein, B.E.; Wallace, M.T.; Stanford, T.R. Development of multisensory integration: Transforming sensory input into motor output. Ment. Retard. Dev. Disabil. Res. Rev. 1999, 5, 72–85. [Google Scholar] [CrossRef]
- Harris, J.; Petersen, R.; Diamond, M.E. The Cortical Distribution of Sensory Memories. Neuron 2001, 30, 315–318. [Google Scholar] [CrossRef]
- Sporns, O. Network attributes for segregation and integration in the human brain. Curr. Opin. Neurobiol. 2013, 23, 162–171. [Google Scholar] [CrossRef]
- Landauer, R. The physical nature of information. Phys. Lett. A 1996, 217, 188–193.1. [Google Scholar] [CrossRef]
- Simon, H.A. The architecture of complexity. In Facets of Systems Science; Springer: Boston, MA, USA, 1991; pp. 457–476. [Google Scholar]
- Kiselev, V.G.; Hahn, K.R.; Auer, D.P. Is the brain cortex a fractal? NeuroImage 2003, 20, 1765–1774. [Google Scholar] [CrossRef]
- Yang, C.-L.; Suh, C.S. A General Framework for Dynamic Complex Networks. J. Vib. Test. Syst. Dyn. 2021, 5, 87–111. [Google Scholar] [CrossRef]
- Buzsaki, G. Rhythms of the Brain; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- da Silva, F.L. Neural mechanisms underlying brain waves: From neural membranes to networks. Electroencephalogr. Clin. Neurophysiol. 1991, 79, 81–93. [Google Scholar] [CrossRef]
- Klimesch, W.; Sauseng, P.; Hanslmayr, S. EEG alpha oscillations: The inhibition–timing hypothesis. Brain Res. Rev. 2007, 53, 63–88. [Google Scholar] [CrossRef]
- Cox, D.D.; Savoy, R.L. Functional magnetic resonance imaging (fMRI) “brain reading”: Detecting and classifying distributed patterns of fMRI activity in human visual cortex. NeuroImage 2003, 19, 261–270. [Google Scholar] [CrossRef]
- Logothetis, N.K. What we can do and what we cannot do with fMRI. Nature 2008, 453, 869–878. [Google Scholar] [CrossRef]
- Buzsáki, G.; Anastassiou, C.A.; Koch, C. The origin of extracellular fields and currents—EEG, ECoG, LFP and spikes. Nat. Rev. Neurosci. 2012, 13, 407–420. [Google Scholar] [CrossRef]
- Pfurtscheller, G.; Lopes da Silva, F.H. Event-related EEG/MEG synchronization and desynchronization: Basic principles. Clin. Neurophysiol. 1999, 110, 1842–1857. [Google Scholar] [CrossRef]
- Ayaz, H.; Onaral, B.; Izzetoglu, K.; Shewokis, P.A.; McKendrick, R.; Parasuraman, R. Continuous monitoring of brain dynamics with functional near infrared spectroscopy as a tool for neuroergonomic research: Empirical examples and a technological development. Front. Hum. Neurosci. 2013, 7, 871. [Google Scholar] [CrossRef]
- Horwitz, B.; Poeppel, D. How can EEG/MEG and fMRI/PET data be combined? Hum. Brain Mapp. 2002, 17, 1–3. [Google Scholar] [CrossRef]
- Song, S.; Sjostrom, P.J.; Reigl, M.; Nelson, S.; Chklovski, D.B. Highly nonrandom features of synaptic connectivity in local cortical circuits. PLoS Biol. 2005, 3, e68. [Google Scholar]
- Freeman, W.J. Nonlinear dynamics of paleocortex manifested in the olfactory EEG. Biol. Cybern. 1979, 35, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.; Myers, R.; Frackowiak, R.; Hajnal, J.; Woods, R.P.; Mazziotta, J.C.; Shipp, S.; Zeki, S. Area V5 of the Human Brain: Evidence from a Combined Study Using Positron Emission Tomography and Magnetic Resonance Imaging. Cereb. Cortex 1993, 3, 79–94. [Google Scholar] [CrossRef] [PubMed]
- Iturria-Medina, Y.; Canales-Rodríguez, E.J.; Melie-Garcia, L.; Valdés-Hernández, P.A.; Martínez-Montes, E.; Alemán-Gómez, Y.; Sanchez-Bornot, J.M. Characterizing brain anatomical connections using diffusion weighted MRI and graph theory. NeuroImage 2007, 36, 645–660. [Google Scholar] [CrossRef] [PubMed]
- Knott, G.; Marchman, H.; Wall, D.; Lich, B. Serial Section Scanning Electron Microscopy of Adult Brain Tissue Using Focused Ion Beam Milling. J. Neurosci. 2008, 28, 2959–2964. [Google Scholar] [CrossRef]
- Barabási, A.-L. The network takeover. Nat. Phys. 2012, 8, 14–16. [Google Scholar] [CrossRef]
- Erdős, P.; Rényi, A. On the evolution of random graphs. Publ. Math. Inst. Hung. Acad. Sci. 1960, 5, 17–60. [Google Scholar]
- Watts, D.J.; Strogatz, S.H. Collective dynamics of ‘small-world’networks. Nature 1998, 393, 440–442. [Google Scholar] [CrossRef]
- Rubinov, M.; Sporns, O. Complex network measures of brain connectivity: Uses and interpretations. NeuroImage 2010, 52, 1059–1069. [Google Scholar] [CrossRef]
- Goldman-Rakic, P.S. Modular organization of prefrontal cortex. Trends Neurosci. 1984, 7, 419–424. [Google Scholar] [CrossRef]
- Bassett, D.S.; Bullmore, E. Small-World Brain Networks. Neurosci. 2006, 12, 512–523. [Google Scholar] [CrossRef] [PubMed]
- White, J.G.; Southgate, E.; Thomson, J.N.; Brenner, S. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos. Trans. R. Soc. B Biol. Sci. 1986, 314, 1–340. [Google Scholar]
- Iturria-Medina, Y.; Sotero, R.C.; Canales-Rodríguez, E.J.; Alemán-Gómez, Y.; Melie-Garcia, L. Studying the human brain anatomical network via diffusion-weighted MRI and Graph Theory. NeuroImage 2008, 40, 1064–1076. [Google Scholar] [CrossRef] [PubMed]
- Lynn, C.W.; Bassett, D.S. The physics of brain network structure, function and control. Nat. Rev. Phys. 2019, 1, 318–332. [Google Scholar] [CrossRef]
- Menon, V. Large-scale brain networks and psychopathology: A unifying triple network model. Trends Cogn. Sci. 2011, 15, 483–506. [Google Scholar] [CrossRef]
- Sporns, O. The human connectome: A complex network. Ann. N. Y. Acad. Sci. 2011, 1224, 109–125. [Google Scholar] [CrossRef]
- Bassett, D.S.; Zurn, P.; Gold, J.I. On the Nature and use of Models in Network Neuroscience. Nat. Rev. Neurosci. 2018, 19, 566–578. [Google Scholar] [CrossRef]
- Holme, P.; Saramäki, J. Temporal networks. Phys. Rep. 2012, 519, 97–125. [Google Scholar] [CrossRef]
- Boccaletti, S.; Bianconi, G.; Criado, R.; del Genio, C.; Gomez-Gardenes, J.; Romance, M.; Sendiña-Nadal, I.; Wang, Z.; Zanin, M. The structure and dynamics of multilayer networks. Phys. Rep. 2014, 544, 1–122. [Google Scholar] [CrossRef]
- Boccaletti, S.; Latora, V.; Moreno, Y.; Chavez, M.; Hwang, D.-U. Complex networks: Structure and dynamics. Phys. Rep. 2006, 424, 175–308. [Google Scholar] [CrossRef]
- Battiston, F.; Amico, E.; Barrat, A.; Bianconi, G.; de Arruda, G.F.; Franceschiello, B.; Iacopini, I.; Kéfi, S.; Latora, V.; Moreno, Y.; et al. The physics of higher-order interactions in complex systems. Nat. Phys. 2021, 17, 1093–1098. [Google Scholar] [CrossRef]
- Friston, K.J. Book review: Brain function, nonlinear coupling, and neuronal transients. Neuroscientist 2001, 7, 406–418. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-L.; Suh, C.S.; Karkoub, M. Impact of Coupling Strength on Reaching Network Consensus. J. Appl. Nonlinear Dyn. 2018, 7, 243–257. [Google Scholar] [CrossRef]
- Robinson, P.A.; Rennie, C.J.; Rowe, D.L.; O’Connor, S.C.; Gordon, E. Multiscale brain modelling. Philos. Trans. R. Soc. B Biol. Sci. 2005, 360, 1043–1050. [Google Scholar] [CrossRef] [PubMed]
- Engel, A.K.; Gerloff, C.; Hilgetag, C.C.; Nolte, G. Intrinsic coupling modes: Multiscale interactions in ongoing brain activity. Neuron 2013, 80, 867–886. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, B. The elusive concept of brain connectivity. NeuroImage 2003, 19, 466–470. [Google Scholar] [CrossRef]
- Jamann, N.; Jordan, M.; Engelhardt, M. Activity-Dependent Axonal Plasticity in Sensory Systems. Neuroscience 2018, 368, 268–282. [Google Scholar] [CrossRef] [PubMed]
- Bechler, M.E.; Swire, M.; Ffrench-Constant, C. Intrinsic and adaptive myelination-A sequential mechanism for smart wiring in the brain. Dev. Neurobiol. 2017, 78, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Jan, Y.-N.; Jan, L. Branching out: Mechanisms of dendritic arborization. Nat. Rev. Neurosci. 2010, 11, 316–328. [Google Scholar] [CrossRef]
- Lippman, J.; Dunaevsky, A. Dendritic spine morphogenesis and plasticity. J. Neurobiol. 2005, 64, 47–57. [Google Scholar] [CrossRef]
- Choquet, D.; Triller, A. The dynamic synapse. Neuron 2013, 80, 691–703. [Google Scholar] [CrossRef] [PubMed]
- Citri, A.; Malenka, R.C. Synaptic plasticity: Multiple forms, functions, and mechanisms. Neuropsychopharmacology 2008, 33, 18–41. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, I. Toward an interpretation of dynamic neural activity in terms of chaotic dynamical systems. Behav. Brain Sci. 2001, 24, 793–810. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Calakos, N. Presynaptic long-term plasticity. Front. Synaptic Neurosci. 2013, 5, 8. [Google Scholar] [CrossRef] [PubMed]
- Lüscher, C.; Nicoll, R.A.; Malenka, R.C.; Muller, D. Synaptic plasticity and dynamic modulation of the postsynaptic membrane. Nat. Neurosci. 2000, 3, 545–550. [Google Scholar] [CrossRef]
- Endo, M. Calcium Ion as a Second Messenger with Special Reference to Excitation-Contraction Coupling. J. Pharmacol. Sci. 2006, 100, 519–524. [Google Scholar] [CrossRef]
- Ramakrishnan, N.A.; Drescher, M.J.; Drescher, D.G. The SNARE complex in neuronal and sensory cells. Mol. Cell. Neurosci. 2012, 50, 58–69. [Google Scholar] [CrossRef]
- Catterall, W.A.; Few, A.P. Calcium Channel Regulation and Presynaptic Plasticity. Neuron 2008, 59, 882–901. [Google Scholar] [CrossRef]
- Felmy, F.; Neher, E.; Schneggenburger, R. Probing the Intracellular Calcium Sensitivity of Transmitter Release during Synaptic Facilitation. Neuron 2003, 37, 801–811. [Google Scholar] [CrossRef]
- Jensen, T.P.; Zheng, K.; Cole, N.; Marvin, J.S.; Looger, L.L.; Rusakov, D.A. Multiplex imaging relates quantal glutamate release to presynaptic Ca2+ homeostasis at multiple synapses in situ. Nat. Commun. 2019, 10, 1414. [Google Scholar] [CrossRef]
- Fioravante, D.; Regehr, W.G. Short-term forms of presynaptic plasticity. Curr. Opin. Neurobiol. 2011, 21, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Swerts, J.-P.; Le van Thai, A.; Vigny, A.; Weber, M.J. Regulation of enzymes responsible for neurotransmitter synthesis and degradation in cultured rat sympathetic neurons: I. Effects of muscle-conditioned medium. Dev. Biol. 1983, 100, 1–11. [Google Scholar] [CrossRef]
- Lesch, K.P.; Bengel, D. Neurotransmitter Reuptake Mechanisms. CNS Drugs 1995, 4, 302–322. [Google Scholar] [CrossRef]
- Amara, S.; Kuhar, M.J. Neurotransmitter Transporters: Recent Progress. Annu. Rev. Neurosci. 1993, 16, 73–93. [Google Scholar] [CrossRef]
- Richerson, G.B.; Wu, Y. Dynamic Equilibrium of Neurotransmitter Transporters: Not Just for Reuptake Anymore. J. Neurophysiol. 2003, 90, 1363–1374. [Google Scholar] [CrossRef]
- Newman, E.A. New roles for astrocytes: Regulation of synaptic transmission. Trends Neurosci. 2003, 26, 536–542. [Google Scholar] [CrossRef]
- Newman, E.A. Glial modulation of synaptic transmission in the retina. Glia 2004, 47, 268–274. [Google Scholar] [CrossRef]
- Fields, R.D.; Stevens-Graham, B. New Insights into Neuron-Glia Communication. Science 2002, 298, 556–562. [Google Scholar] [CrossRef]
- Araque, A.; Carmignoto, G.; Haydon, P.G. Dynamic Signaling Between Astrocytes and Neurons. Annu. Rev. Physiol. 2001, 63, 795–813. [Google Scholar] [CrossRef]
- Edgar, N.; Sibille, E. A putative functional role for oligodendrocytes in mood regulation. Transl. Psychiatry 2012, 2, e109. [Google Scholar] [CrossRef]
- Wu, Y.; Dissing-Olesen, L.; MacVicar, B.A.; Stevens, B. Microglia: Dynamic Mediators of Synapse Development and Plasticity. Trends Immunol. 2015, 36, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Castellani, G.C.; Quinlan, E.M.; Cooper, L.N.; Shouval, H.Z. A biophysical model of bidirectional synaptic plasticity: Dependence on AMPA and NMDA receptors. Proc. Natl. Acad. Sci. USA 2001, 98, 12772–12777. [Google Scholar] [CrossRef] [PubMed]
- Lisman, J.; Yasuda, R.; Raghavachari, S. Mechanisms of CaMKII action in long-term potentiation. Nat. Rev. Neurosci. 2012, 13, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Mulkey, R.M.; Endo, S.; Shenolikar, S.; Malenka, R.C. Involvement of a calcineurin/ inhibitor-1 phosphatase cascade in hippocampal long-term depression. Nature 1994, 369, 486–488. [Google Scholar] [CrossRef]
- Sumi, T.; Harada, K. Mechanism underlying hippocampal long-term potentiation and depression based on competition between endocytosis and exocytosis of AMPA receptors. Sci. Rep. 2020, 10, 14711. [Google Scholar] [CrossRef]
- Chater, T.E.; Goda, Y. The role of AMPA receptors in postsynaptic mechanisms of synaptic plasticity. Front. Cell. Neurosci. 2014, 8, 401. [Google Scholar] [CrossRef]
- Collingridge, G.; Bliss, T. NMDA receptors - their role in long-term potentiation. Trends Neurosci. 1987, 10, 288–293. [Google Scholar] [CrossRef]
- Song, S.; Miller, K.D.; Abbott, L.F. Competitive Hebbian learning through spike-timing-dependent synaptic plasticity. Nat. Neurosci. 2000, 3, 919–926. [Google Scholar] [CrossRef]
- Hebb, D.O. The Organization of Behavior: A Neuropsychological Theory; Psychology Press: East Sussex, UK, 2005. [Google Scholar]
- Caporale, N.; Dan, Y. Spike Timing–Dependent Plasticity: A Hebbian Learning Rule. Annu. Rev. Neurosci. 2008, 31, 25–46. [Google Scholar] [CrossRef]
- Shouval, H.Z.; Wang, S.S.-H.; Wittenberg, G.M. Spike timing dependent plasticity: A consequence of more fundamental learning rules. Front. Comput. Neurosci. 2010, 4, 19. [Google Scholar] [CrossRef]
- Hangen, E.; Cordelières, F.P.; Petersen, J.D.; Choquet, D.; Coussen, F. Neuronal Activity and Intracellular Calcium Levels Regulate Intracellular Transport of Newly Synthesized AMPAR. Cell Rep. 2018, 24, 1001–1012. [Google Scholar] [CrossRef] [PubMed]
- Rose, C.R.; Konnerth, A. Stores Not Just for Storage: Intracellular Calcium Release and Synaptic Plasticity. Neuron 2001, 31, 519–522. [Google Scholar] [CrossRef]
- Kew, J.N.C.; Kemp, J.A. Ionotropic and metabotropic glutamate receptor structure and pharmacology. Psychopharmacology 2005, 179, 4–29. [Google Scholar] [CrossRef] [PubMed]
- Abraham, W.C.; Bear, M.F. Metaplasticity: The plasticity of synaptic plasticity. Trends Neurosci. 1996, 19, 126–130. [Google Scholar] [CrossRef]
- Abbott, L.F.; Nelson, S. Synaptic plasticity: Taming the beast. Nat. Neurosci. 2000, 3, 1178–1183. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.J.; Grimwood, P.D.; Morris, R.G.M. Synaptic Plasticity and Memory: An Evaluation of the Hypothesis. Annu. Rev. Neurosci. 2000, 23, 649–711. [Google Scholar] [CrossRef]
- Bear, M.F.; Malenka, R.C. Synaptic plasticity: LTP and LTD. Curr. Opin. Neurobiol. 1994, 4, 389–399. [Google Scholar] [CrossRef]
- Lumen Learning. Biology for Majors II. Available online: https://courses.lumenlearning.com/wm-biology2/chapter/chemical-and-electrical-synapses/ (accessed on 31 March 2022).
- Lamprecht, R.; E LeDoux, J. Structural plasticity and memory. Nat. Rev. Neurosci. 2004, 5, 45–54. [Google Scholar] [CrossRef]
- Butz, M.; Wörgötter, F.; van Ooyen, A. Activity-dependent structural plasticity. Brain Res. Rev. 2009, 60, 287–305. [Google Scholar] [CrossRef]
- Harris, K.M. Structure, development, and plasticity of dendritic spines. Curr. Opin. Neurobiol. 1999, 9, 343–348. [Google Scholar] [CrossRef]
- Sammons, R.P.; Clopath, C.; Barnes, S.J. Size-Dependent Axonal Bouton Dynamics following Visual Deprivation In Vivo. Cell Rep. 2018, 22, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Tavosanis, G. Dendritic structural plasticity. Dev. Neurobiol. 2011, 72, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Grubb, M.S.; Shu, Y.; Kuba, H.; Rasband, M.N.; Wimmer, V.C.; Bender, K.J. Short- and Long-Term Plasticity at the Axon Initial Segment. J. Neurosci. 2011, 31, 16049–16055. [Google Scholar] [CrossRef] [PubMed]
- Almeida, R.G.; Lyons, D.A. On Myelinated Axon Plasticity and Neuronal Circuit Formation and Function. J. Neurosci. 2017, 37, 10023–10034. [Google Scholar] [CrossRef] [PubMed]
- Designua. (n.d.). Oligodendrocytes Provide Support Axons Produce Myelin Stock Vector (Royalty Free) 235097353. Shutterstock. Available online: https://www.shutterstock.com/image-vector/oligodendrocytes-provide-support-axons-produce-myelin-235097353 (accessed on 27 April 2022).
- Fields, R.D. A new mechanism of nervous system plasticity: Activity-dependent myelination. Nat. Rev. Neurosci. 2015, 16, 756–767. [Google Scholar] [CrossRef] [PubMed]
- Baraban, M.; Mensch, S.; Lyons, D.A. Adaptive myelination from fish to man. Brain Res. 2015, 1641, 149–161. [Google Scholar] [CrossRef]
- Ayala, Y.A.; Pérez-González, D.; Malmierca, M.S. Stimulus-specific adaptation in the inferior colliculus: The role of excitatory, inhibitory and modulatory inputs. Biol. Psychol. 2016, 116, 10–22. [Google Scholar] [CrossRef]
- Biancardi, V.; Saini, J.; Pageni, A.; Prashaad, M.H.; Funk, G.D.; Pagliardini, S. Mapping of the excitatory, inhibitory, and modulatory afferent projections to the anatomically defined active expiratory oscillator in adult male rats. J. Comp. Neurol. 2020, 529, 853–884. [Google Scholar] [CrossRef]
- Friston, K.; Tononi, G.; Sporns, O.; Edelman, G.M. Characterising the complexity of neuronal interactions. Hum. Brain Mapp. 1995, 3, 302–314. [Google Scholar] [CrossRef]
- Babloyantz, A.; Lourenço, C. Brain chaos and computation. Int. J. Neural Syst. 1996, 7, 461–471. [Google Scholar] [CrossRef]
- Vaiana, M.; Muldoon, S.F. Multilayer brain networks. J. Nonlinear Sci. 2020, 30, 2147–2169. [Google Scholar] [CrossRef]
- Betzel, R.F.; Bassett, D.S. Multi-scale brain networks. Neuroimage 2017, 160, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Mountcastle, V.B. The columnar organization of the neocortex. Brain 1997, 120, 701–722. [Google Scholar] [CrossRef] [PubMed]
- Sporns, O.; Betzel, R.F. Modular brain networks. Annu. Rev. Psychol. 2016, 67, 613–640. [Google Scholar] [CrossRef] [PubMed]
- Bullmore, E.; Sporns, O. The economy of brain network organization. Nat. Rev. Neurosci. 2012, 13, 336–349. [Google Scholar] [CrossRef]
- Lee, S.-H.; Dan, Y. Neuromodulation of Brain States. Neuron 2012, 76, 209–222. [Google Scholar] [CrossRef]
- Shettigar, N.; Yang, C.-L.; Suh, C.S. On the Efficacy of Information Transfer in Complex Networks. In Proceedings of the ASME International Mechanical Engineering Congress and Exposition, American Society of Mechanical Engineers, Virtual Conference, 1–5 November 2021; Volume 85628. [Google Scholar]
- Wallenstein, G.V.; Kelso, J.S.; Bressler, S.L. Phase transitions in spatiotemporal patterns of brain activity and behavior. Phys. D Nonlinear Phenom. 1995, 84, 626–634. [Google Scholar] [CrossRef]
- Zeraati, R.; Priesemann, V.; Levina, A. Self-organization toward criticality by synaptic plasticity. Frontiers in Physics 2021, 9, 619661. [Google Scholar] [CrossRef]
- Lorenz, E.N. Deterministic nonperiodic flow. J. Atmos. Sci. 1963, 20, 130–141. [Google Scholar] [CrossRef]
- Sporns, O.; Zwi, J.D. The Small World of the Cerebral Cortex. Neuroinformatics 2004, 2, 145–162. [Google Scholar] [CrossRef]
- Towlson, E.K.; Vertes, P.E.; Ahnert, S.E.; Schafer, W.R.; Bullmore, E.T. The rich club of the C. elegans neuronal connectome. J. Neurosci. 2013, 33, 6380–6387. [Google Scholar] [CrossRef] [PubMed]
- Harriger, L.; Heuvel, M.V.D.; Sporns, O. Rich Club Organization of Macaque Cerebral Cortex and Its Role in Network Communication. PLoS ONE 2012, 7, e46497. [Google Scholar] [CrossRef]
- Bassett, D.S.; Bullmore, E.T. Small-world brain networks revisited. Neuroscientist 2017, 23, 499–516. [Google Scholar] [CrossRef] [PubMed]
- Barardi, A.; Malagarriga, D.; Sancristobal, B.; Garcia-Ojalvo, J.; Pons, A.J. Probing scale interaction in brain dynamics through synchronization. Philos. Trans. R. Soc. B: Biol. Sci. 2014, 369, 1–10. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guevara Erra, R.; Perez Velazquez, J.L.; Rosenblum, M. Neural synchronization from the perspective of non-linear dynamics. Front. Comput. Neurosci. 2017, 11, 98. [Google Scholar] [CrossRef] [PubMed]
- Pikovsky, A.; Rosenblum, M.; Kurths, J.; Hilborn, R.C.; Pikovsky, A.; Rosenblum, M.; Kurths, J.; Hilborn, R.C.; Pikovsky, A.; Rosenblum, M.; et al. Synchronization: A Universal Concept in Nonlinear Science No 12; Cambridge University Press: Cambridge, UK, 2003. [Google Scholar]
- Kuramoto, Y.; Battogtokh, D. Coexistence of coherence and incoherence in nonlocally coupled phase oscillators. arXiv 2020, arXiv:cond-mat/0210694. [Google Scholar]
- Oliveira, H.M.; Melo, L.V. Huygens synchronization of two clocks. Sci. Rep. 2015, 5, 1–12. [Google Scholar] [CrossRef]
- Winfree, A.T. Biological rhythms and the behavior of populations of coupled oscillators. J. Theor. Biol. 1967, 16, 15–42. [Google Scholar] [CrossRef]
- Arenas, A.; Diaz-Guilera, A.; Kurths, J.; Moreno, Y.; Zhou, C. Synchronization in complex networks. Phys. Rep. 2008, 469, 93–153. [Google Scholar] [CrossRef]
- Li, Z.; Duan, Z.; Chen, G.; Huang, L. Consensus of Multiagent Systems and Synchronization of Complex Networks: A Unified Viewpoint. IEEE Trans. Circuits Syst. I Regul. Pap. 2009, 57, 213–224. [Google Scholar]
- Kolb, B.; Whishaw, I.Q. Brain plasticity and behavior. Annu. Rev. Psychol. 1998, 49, 43–64. [Google Scholar] [CrossRef] [PubMed]
- Kitzbichler, M.G.; Smith, M.L.; Christensen, S.R.; Bullmore, E. Broadband criticality of human brain network synchronization. PLoS Comput. Biol. 2009, 5, e1000314. [Google Scholar] [CrossRef]
- Fell, J.; Axmacher, N. The role of phase synchronization in memory processes. Nat. Rev. Neurosci. 2011, 12, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Reimann, M.W.; Nolte, M.; Scolamiero, M.; Turner, K.; Perin, R.; Chindemi, G.; Dlotko, P.; Levi, R.; Hess, K.; Markram, H.; et al. Cliques of Neurons Bound into Cavities Provide a Missing Link between Structure and Function. Front. Comput. Neurosci. 2017, 11, 48. [Google Scholar] [CrossRef]
- Northoff, G.; Huang, Z. How do the brain’s time and space mediate consciousness and its different dimensions? Temporo-spatial theory of consciousness (TTC). Neurosci. Biobehav. Rev. 2017, 80, 630–645. [Google Scholar] [CrossRef]
- Kraikivski, P. Systems of Oscillators Designed for a Specific Conscious Percept. New Math. Nat. Comput. 2020, 16, 73–88. [Google Scholar] [CrossRef]
- Kraikivski, P. A Dynamic Mechanistic Model of Perceptual Binding. Mathematics 2022, 10, 1135. [Google Scholar] [CrossRef]
- Naumann, R.K.; Ondracek, J.M.; Reiter, S.; Shein-Idelson, M.; Tosches, M.A.; Tamawaki, T.M.; Laurent, G. The reptilian brain. Curr. Biol. 2015, 25, R317–R321. [Google Scholar] [CrossRef] [PubMed]
- Rikhye, R.V.; Wimmer, R.D.; Halassa, M.M. Toward an Integrative Theory of Thalamic Function. Annu. Rev. Neurosci. 2018, 41, 163–183. [Google Scholar] [CrossRef]
- Lui, J.H.; Hansen, D.V.; Kriegstein, A.R. Development and Evolution of the Human Neocortex. Cell 2011, 146, 18–36. [Google Scholar] [CrossRef]
- Lodi, M.; Della Rossa, F.; Sorrentino, F.; Storace, M. Analyzing synchronized clusters in neuron networks. Sci. Rep. 2020, 10, 16336. [Google Scholar] [CrossRef] [PubMed]
- Russo, R.; Herrmann, H.J.; de Arcangelis, L. Brain modularity controls the critical behavior of spontaneous activity. Sci. Rep. 2014, 4, 4312. [Google Scholar] [CrossRef] [PubMed]
- Deco, G.; Buehlmann, A.; Masquelier, T.; Hugues, E. The role of rhythmic neural synchronization in rest and task conditions. Front. Hum. Neurosci. 2011, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Melloni, L.; Molina, C.; Pena, M.; Torres, D.; Singer, W.; Rodriguez, E. Synchronization of neural activity across cortical areas correlates with conscious perception. J. Neurosci. 2007, 27, 2858–2865. [Google Scholar] [CrossRef] [PubMed]
- Bliss, T.V.P.; Sam, F.C. Long-term potentiation and long-term depression: A clinical perspective. Clinics 2011, 66, 3–17. [Google Scholar] [CrossRef]
- Timofeev, I.; Bazhenov, M.; Seigneur, J.; Sejnowski, T. Neuronal synchronization and thalamocortical rhythms in sleep, wake and epilepsy. In Jasper’s Basic Mechanisms of the Epilepsies, 4th ed.; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- Babloyantz, A.; Salazar, J.M.; Nicolis, C. Evidence of chaotic dynamics of brain activity during the sleep cycle. Phys. Lett. A 1985, 111, 152–156. [Google Scholar] [CrossRef]
- Coombes, S.; Lord, G.J.; Owen, M.R. Waves and bumps in neuronal networks with axo-dendritic synaptic interactions. Phys. D Nonlinear Phenom. 2003, 178, 219–241. [Google Scholar] [CrossRef]
- Nunez, P.L. The brain wave equation: A model for the EEG. Math. Biosci. 1974, 21, 279–297. [Google Scholar] [CrossRef]
- Camazine, S.; Deneubourg, J.L.; Franks, N.R.; Sneyd, J.; Theraula, G.; Bonabeau, E. Self-Organization in Biological Systems; Princeton University Press: Princeton, NJ, USA, 2020. [Google Scholar]
- Eigen, M.; Schuster, P. A principle of natural self-organization. Naturwissenschaften 1977, 64, 541–565. [Google Scholar] [CrossRef]
- Tzelepi, A.; Bezerianos, T.; Bodis-Wollner, I. Functional properties of sub-bands of oscillatory brain waves to pattern visual stimulation in man. Clin. Neurophysiol. 2000, 111, 259–269. [Google Scholar] [CrossRef]
- Voytek, B.; Knight, R.T. Dynamic network communication as a unifying neural basis for cognition, development, aging, and disease. Biol. Psychiatry 2015, 77, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Hyafil, A.; Giraud, A.L.; Fontolan, L.; Gutkin, B. Neural cross-frequency coupling: Connecting architectures, mechanisms, and functions. Trends Neurosci. 2015, 38, 725–740. [Google Scholar] [CrossRef]
- Röhr, V.; Berner, R.; Lameu, E.L.; Popovych, O.V.; Yanchuk, S. Frequency cluster formation and slow oscillations in neural populations with plasticity. PLoS ONE 2019, 14, e0225094. [Google Scholar] [CrossRef] [PubMed]
- Klapoetke, N.C.; Murata, Y.; Kim, S.S.; Pulver, S.R.; Birdsey-Benson, A.; Cho, Y.K.; Morimoto, T.K.; Chuong, A.S.; Carpenter, E.J.; Tian, Z.; et al. Independent optical excitation of distinct neural populations. Nat. Methods 2014, 11, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Harper, N.; McAlpine, D. Optimal neural population coding of an auditory spatial cue. Nature 2004, 430, 682–686. [Google Scholar] [CrossRef]
- Dean, I.; Harper, N.S.; McAlpine, D. Neural population coding of sound level adapts to stimulus statistics. Nat. Neurosci. 2005, 8, 1684–1689. [Google Scholar] [CrossRef]
- Gu, B.-M.; van Rijn, H.; Meck, W.H. Oscillatory multiplexing of neural population codes for interval timing and working memory. Neurosci. Biobehav. Rev. 2015, 48, 160–185. [Google Scholar] [CrossRef]
- Bonnefond, M.; Kastner, S.; Jensen, O. Communication between brain areas based on nested oscillations. eNeuro 2017, 4, 1–14. [Google Scholar] [CrossRef]
- Hughes, T.W.; Williamson, I.A.; Minkov, M.; Fan, S. Wave physics as an analog recurrent neural network. Sci. Adv. 2019, 5, eaay6946. [Google Scholar] [CrossRef]
- Palla, G.; Derenyi, I.; Farkas, I.; Vicsek, T. Uncovering the overlapping community structure of complex networks in nature and society. Nature 2005, 435, 814–818. [Google Scholar] [CrossRef]
- Krnjević, K. Chemical nature of synaptic transmission in vertebrates. Physiol. Rev. 1974, 54, 418–540. [Google Scholar] [CrossRef]
- Kreher, S.A.; Mathew, D.; Kim, J.; Carlson, J.R. Translation of sensory input into behavioral output via an olfactory system. Neuron 2008, 59, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Iyer, R.; Menon, V.; Buice, M.; Koch, C.; Mihalas, S. The influence of synaptic weight distribution on neuronal population dynamics. PLOS Comput. Biol. 2013, 9, e1003248. [Google Scholar] [CrossRef]
- Barbour, B.; Brunel, N.; Hakim, V.; Nadal, J.P. What can we learn from synaptic weight distributions? TRENDS Neurosci. 2007, 30, 622–629. [Google Scholar] [CrossRef]
- Blakemore, C.; Nachmias, J.; Sutton, P. The perceived spatial frequency shift: Evidence for frequency-selective neurones in the human brain. J. Physiol. 1970, 210, 727–750. [Google Scholar] [CrossRef]
- Steriade, M. Grouping of brain rhythms in corticothalamic systems. Neuroscience 2006, 137, 1087–1106. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.A.; Hinton, G.E. Models of information processing in the brain. In Parallel Models of Associative Memory; Psychology Press: East Sussex, UK, 2014; pp. 33–74. [Google Scholar]
- Teyler, T.J.; Rudy, J.W. The hippocampal indexing theory and episodic memory: Updating the index. Hippocampus 2007, 17, 1158–1169. [Google Scholar] [CrossRef]
- Hermann, A.; Bieber, A.; Keck, T.; Vaitl, D.; Stark, R. Brain structural basis of cognitive reappraisal and expressive suppression. Soc. Cogn. Affect. Neurosci. 2014, 9, 1435–1442. [Google Scholar] [CrossRef] [PubMed]
- Bassett, D.S.; Meyer-Lindenber, A.; Achard, S.; Duke, T.; Bullmore, E. Adaptive reconfiguration of fractal small-world human brain functional networks. Proc. Natl. Acad. Sci. USA 2006, 103, 19518–19523. [Google Scholar] [CrossRef]
- Meunier, D.; Lambiotte, R.; Fornito, A.; Ersche, K.; Bullmore, E.T. Hierarchical modularity in human brain functional networks. Front. Neuroinformatics 2009, 3, 37. [Google Scholar] [CrossRef]
- La Rocca, D.; Zilber, N.; Abry, P.; van Wassenhove, V.; Ciuciu, P. Self-similarity and multifractality in human brain activity: A wavelet-based analysis of scale-free brain dynamics. J. Neurosci. Methods 2018, 309, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Lutzenberger, W.; Presissl, H.; Pulvermuller, F. Fractal dimension of electroencephalographic time series and underlying brain processes. Biol.Cybern. 1995, 73, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Hilgetag, C.C.; Goulas, A. ‘Hierarchy’ in the organization of brain networks. Philos. Trans. R. Soc. B 2020. [Google Scholar] [CrossRef] [PubMed]
- Meunier, D.; Lambiotte, R.; Bullmore, E.T. Modular and hierarchically modular organization of brain networks. Front. Neurosci. 2010, 4, 200. [Google Scholar] [CrossRef] [PubMed]
- Comstock, D.C.; Hove, M.J.; Balasubramaniam, R. Sensorimotor synchronization with auditory and visual modalities: Behavioral and neural differences. Front. Comput. Neurosci. 2018, 12, 53. [Google Scholar] [CrossRef]
- He, Y.; Wang, J.; Wang, L.; Chen, Z.J.; Yan, C.; Yang, H.; Tang, H.; Zhu, C.; Gong, Q.; Zang, Y.; et al. Uncovering intrinsic modular organization of spontaneous brain activity in humans. PLoS ONE 2009, 4, e5226. [Google Scholar] [CrossRef] [PubMed]
- Casanova, M.F.; Casanova, E.L. The modular organization of the cerebral cortex: Evolutionary significance and possible links to neurodevelopmental conditions. J. Comp. Neurol. 2019, 527, 1720–1730. [Google Scholar] [CrossRef]
- Wei, Y.; Scholtens, L.H.; Turk, E.; Van Den Heuvel, M.P. Multiscale examination of cytoarchitectonic similarity and human brain connectivity. Netw. Neurosci. 2018, 3, 124–137. [Google Scholar] [CrossRef]
- Douglas, R.; Martin, K. Neocortex. In The Synaptic Organization of the Brain; Shepherd, G.M., Ed.; Oxford University Press: Oxford, UK, 1998; pp. 459–509. [Google Scholar]
- Mogan, H.; Verhoog, M.B.; Doreswamy, K.K.; Eyal, G.; Aardse, R.; Groot, C.; van der Sluis, S. Dendritic and axonal architecture of individual pyramidal neurons across layers of adult human neocortex. Cereb. Cortex. 2015, 25, 4839–4853. [Google Scholar]
- Sauseng, P.; Klimesch, W. What does phase information of oscillatory brain activity tell us about cognitive processes? Neurosci. Biobehav. Rev. 2008, 32, 1001–1013. [Google Scholar] [CrossRef]
- Sauseng, P.; Klimesch, W.; Gruber, W.R.; Birbaumer, N. Cross-frequency phase synchronization: A brain mechanism of memory matching and attention. Neuroimage 2008, 40, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Singer, W. Synchronization of cortical activity and its putative role in information processing and learning. Annu. Rev. Physiol. 1993, 55, 349–374. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, G.M.G.; Yamawaki, Y. Untangling the cortico-thalamo-cortical loop: Cellular pieces of a knotty circuit puzzle. Nat. Rev. Neurosci. 2021, 22, 389–406. [Google Scholar] [CrossRef] [PubMed]
- Huguenard, J.R.; McCormick, D.A. Thalamic synchrony and dynamic regulation of global forebrain oscillations. Trends Neurosci. 2007, 30, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Kober, H.; Barrett, L.F.; Joseph, J.; Bliss-Moreau, E.; Lindquist, K.; Wager, T.D. Functional grouping and cortical–subcortical interactions in emotion: A meta-analysis of neuroimaging studies. Neuroimage 2008, 42, 998–1031. [Google Scholar] [CrossRef]
- Neubert, F.X.; Mars, R.B.; Buch, E.R.; Olivier, E.; Rushworth, M.F. Cortical and subcortical interactions during action reprogramming and their related white matter pathways. Proc. Natl. Acad. Sci. USA 2010, 107, 13240–13245. [Google Scholar] [CrossRef]
- Lumer, E.D.; Edelman, G.M.; Tononi, G. Neural dynamics in a model of the thalamocortical system. I. Layers, loops and the emergence of fast synchronous rhythms. Cereb. Cortex (New York, NY: 1991) 1997, 7, 207–227. [Google Scholar] [CrossRef]
- Kilgard, M.P.; Vazquez, J.L.; Engineer, N.D.; Pandya, P.K. Experience dependent plasticity alters cortical synchronization. Hear. Res. 2007, 229, 171–179. [Google Scholar] [CrossRef][Green Version]
- Verbeke, P.; Verguts, T. Learning to synchronize: How biological agents can couple neural task modules for dealing with the stability-plasticity dilemma. PLoS Comput. Biol. 2019, 15, e1006604. [Google Scholar] [CrossRef]
- Kahana, M.J. The cognitive correlates of human brain oscillations. J. Neurosci. 2006, 26, 1669–1672. [Google Scholar] [CrossRef]
- Briand, L.A.; Gritton, H.; Howe, W.M.; Young, D.A.; Sarter, M. Modulators in concert for cognition: Modulator interactions in the prefrontal cortex. Prog. Neurobiol. 2007, 83, 69–91. [Google Scholar] [CrossRef] [PubMed]
- Bolkan, S.S.; Stujenske, J.M.; Parnaudeau, S.; Spellman, T.J.; Rauffenbart, C.; Abbas, A.I.; Harris, A.Z.; Gordon, J.A.; Kellendonk, C. Thalamic projections sustain prefrontal activity during working memory maintenance. Nat. Neurosci. 2017, 20, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Hultman, R.; Ulrich, K.; Sachs, B.D.; Blount, C.; Carlson, D.E.; Ndubuizu, N.; Bagot, R.C.; Parise, E.M.; Vu, M.A.T.; Gallagher, N.M.; et al. Brain-wide electrical spatiotemporal dynamics encode depression vulnerability. Cell 2018, 173, 166–180. [Google Scholar] [CrossRef] [PubMed]
- Price, C.J.; Friston, K.J. Degeneracy and cognitive anatomy. Trends Cogn. Sci. 2002, 6, 416–421. [Google Scholar] [CrossRef]
- Edelman, G.M.; Gally, J.A. Degeneracy and complexity in biological systems. Proc. Natl. Acad. Sci. USA 2001, 98, 13763–13768. [Google Scholar] [CrossRef]
- Ward, L.M. Synchronous neural oscillations and cognitive processes. Trends Cogn. Sci. 2003, 7, 553–559. [Google Scholar] [CrossRef]
- Murphy, B.K.; Miller, K.D. Balanced amplification: A new mechanism of selective amplification of neural activity patterns. Neuron 2009, 61, 635–648. [Google Scholar] [CrossRef]
- Oby, E.R.; Golub, M.D.; Hennig, J.A.; Degenhart, A.D.; Tyler-Kabara, E.C.; Byron, M.Y.; Chase, S.M.; Batista, A.P. New neural activity patterns emerge with long-term learning. Proc. Natl. Acad. Sci. USA 2019, 116, 15210–15215. [Google Scholar] [CrossRef]
- Patterson, R.D.; Holdsworth, J. A functional model of neural activity patterns and auditory images. Adv. Speech Hear. Lang. Processing 1996, 3 Part B, 547–563. [Google Scholar]
- Ackman, J.B.; Crair, M.C. Role of emergent neural activity in visual map development. Curr. Opin. Neurobiol. 2014, 24, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Mesulam, M. Representation, inference, and transcendent encoding in neurocognitive networks of the human brain. Ann. Neurol. 2008, 64, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Friston, K. The free-energy principle: A unified brain theory? Nat. Rev. Neurosci. 2010, 11, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Rathour, R.K.; Narayanan, R. Degeneracy in hippocampal physiology and plasticity. Hippocampus 2019, 29, 980–1022. [Google Scholar] [CrossRef] [PubMed]
- Morgane, P.J.; Galler, J.R.; Mokler, D.J. A review of systems and networks of the limbic forebrain/limbic midbrain. Prog. Neurobiol. 2005, 75, 143–160. [Google Scholar] [CrossRef] [PubMed]
- Hariri, A.R. Modulating Emotional Responses: Effects of a Neocortical Network on the Limbic System. Ph.D. Thesis, University of California, Los Angeles, CA, USA, 2001. [Google Scholar]
- Woods, D.L.; Wyma, J.M.; Yund, E.W.; Herron, T.J.; Reed, B. Factors influencing the latency of simple reaction time. Front. Hum. Neurosci. 2015, 9, 131. [Google Scholar] [CrossRef]
- Bavelier, D.; Neville, H.J. Cross-modal plasticity: Where and how? Nat. Rev. Neurosci. 2002, 3, 443–452. [Google Scholar] [CrossRef]
- Calvert, G.A. Crossmodal processing in the human brain: Insights from functional neuroimaging studies. Cereb. Cortex 2001, 11, 1110–1123. [Google Scholar] [CrossRef]
- Chouzouris, T.; Omelchenko, I.; Zakharova, A.; Hlinka, J.; Jiruska, P.; Scholl, E. Chimera states in brain networks: Empirical neural vs. modular fractal connectivity. Chaos Interdiscip. J. Nonlinear Sci. 2018, 28, 045112. [Google Scholar] [CrossRef]
- Beggs, J.M.; Plenz, D. Neuronal avalanches in neocortical circuits. J. Neurosci. 2003, 23, 11167–11177. [Google Scholar] [CrossRef]
- Wang, Z.; Zonghua, L. A brief review of chimera state in empirical brain networks. Front. Physiol. 2020, 11, 724. [Google Scholar] [CrossRef]
- Majhi, S.; Bera, B.K.; Ghosh, D.; Perc, M. Chimera states in neuronal networks: A review. Phys. Life Rev. 2019, 28, 100–121. [Google Scholar] [CrossRef] [PubMed]
- Tadić, B.; Melnik, R. Self-organised critical dynamics as a key to fundamental features of complexity in physical, biological, and social networks. Dynamics 2021, 1, 181–197. [Google Scholar] [CrossRef]
- Mora, T.; Bialek, W. Are biological systems poised at criticality? J. Stat. Phys. 2011, 144, 268–302. [Google Scholar] [CrossRef]
- Zimmern, V. Why brain criticality is clinically relevant: A scoping review. Front. Neural Circuits 2020, 14, 54. [Google Scholar] [CrossRef] [PubMed]
- Beggs, J.M.; Timme, N. Being critical of criticality in the brain. Front. Physiol. 2012, 3, 163. [Google Scholar] [CrossRef] [PubMed]
- Meisel, C.; Storch, A.; Hallmeyer-Elgner, S.; Bullmore, E.; Gross, T. Failure of adaptive self-organized criticality during epileptic seizure attacks. PLoS Comput. Biol. 2012, 8, e1002312. [Google Scholar] [CrossRef] [PubMed]
- Faure, P.; Korn, H. Is there chaos in the brain? I. Concepts of nonlinear dynamics and methods of investigation. Comptes Rendus l’Académie Sci. Ser. III Sci. Vie 2001, 324, 773–793. [Google Scholar] [CrossRef]
- Abry, P.; Wendt, H.; Jaffard, S.; dider, G. Multivariate scale-free temporal dynamics: From spectral (Fourier) to fractal (wavelet) analysis. Comptes Rendus Phys. 2019, 20, 489–501. [Google Scholar] [CrossRef]
- Smith, J.H.; Rowland, C.; Harland, B.; Moslehi, S.; Montgomery, R.D.; Schobert, K.; Watterson, W.J.; Dalrymple-Alford, J.; Taylor, R.P. How neurons exploit fractal geometry to optimize their network connectivity. Sci. Rep. 2021, 11, 1–13. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shettigar, N.; Yang, C.-L.; Tu, K.-C.; Suh, C.S. On The Biophysical Complexity of Brain Dynamics: An Outlook. Dynamics 2022, 2, 114-148. https://doi.org/10.3390/dynamics2020006
Shettigar N, Yang C-L, Tu K-C, Suh CS. On The Biophysical Complexity of Brain Dynamics: An Outlook. Dynamics. 2022; 2(2):114-148. https://doi.org/10.3390/dynamics2020006
Chicago/Turabian StyleShettigar, Nandan, Chun-Lin Yang, Kuang-Chung Tu, and C. Steve Suh. 2022. "On The Biophysical Complexity of Brain Dynamics: An Outlook" Dynamics 2, no. 2: 114-148. https://doi.org/10.3390/dynamics2020006
APA StyleShettigar, N., Yang, C.-L., Tu, K.-C., & Suh, C. S. (2022). On The Biophysical Complexity of Brain Dynamics: An Outlook. Dynamics, 2(2), 114-148. https://doi.org/10.3390/dynamics2020006






