Traumatic Brain Injury and Secondary Neurodegenerative Disease
Abstract
1. Introduction
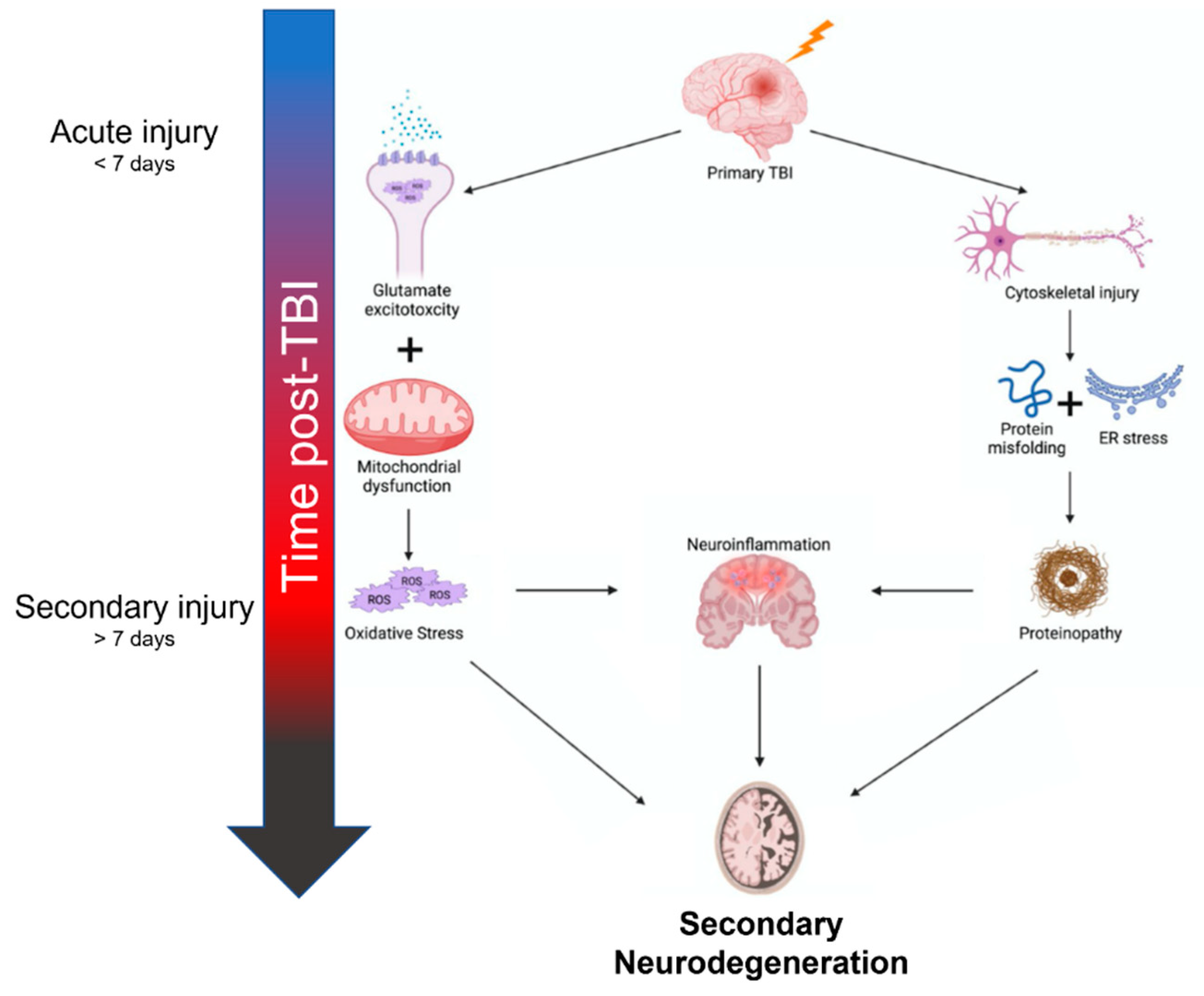
2. Mechanisms of Neurodegeneration after TBI
3. Imaging
4. Cognitive and Behavioral Manifestations
5. Therapeutic Targets
5.1. Oxidative Stress
5.2. Cell-Based Therapies
5.3. Aggregation-Prone Proteins
5.4. Neuroinflammation
| Therapeutic Target | Outcomes & Mechanism of Action | References |
|---|---|---|
| Oxidative Stress | ||
| PEG-SOD | Catalyzes the degradation of superoxide radicals; combined with PEG molecules to increase in vivo half-life | [64] |
| Polyphenols & Flavonoids | Water-soluble antioxidants; directly react with ROS in addition to stimulating the Nrf2-ARE pathway | [65,66,67,68] |
| Mitoquinone | Acts as a renewable antioxidant to reduce mitochondrial ROS | [70] |
| Cell-based Therapies | ||
| Neural stem cells | Improves motor recovery and cognition by replacing neurons lost to neurodegeneration | [73,74,75] |
| Mesenchymal stromal stem cells | Reduces proinflammatory mediators and improved functional recovery. IL-10 overexpression alters microglial polarization in favor of anti-inflammatory processes | [76,77] |
| Aggregation-prone Proteins | ||
| Tau | Preventing pathologic accumulation via immunization against phosphorylated tau improves neurocognitive outcomes | [84,85,86] |
| Amyloid-beta protein | Aβ may be targeted through enhanced clearance (immunization), decreased production (α-secretase overexpression), or decreased aggregation (Aβ binding molecules). | [87,88,89,90] |
| Neuroinflammation | ||
| Minocycline | Attenuates microglial activation and improves functional outcomes | [92,93,94] |
| Doxycycline | Decreases MMP-9 activity and preserves blood-brain-barrier integrity | [95] |
| Hydroxychloroquine/chloroquine | Attenuates microglial activation and preserves blood-brain-barrier integrity | [96,97] |
| PPAR agonists | Attenuates microglial activation and mitochondrial dysfunction, decreases TBI lesion sizes | [98,99,100] |
| Cannabinoid 2 receptor agonist | Prevents neuronal degeneration and preserves blood-brain-barrier integrity | [101] |
| Inflammasomes | Decreases pro-inflammatory mediators such as caspase-1, IL-18, & IL-1β. Can also reduce pyroptotic cell death by inhibiting gasdermin D cleavage. | [102,103,104] |
| Interferon-beta | Attenuates neuroinflammation, decreases lesion volume, and improves long-term functional outcomes | [105] |
6. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Fesharaki-Zadeh, A. Chronic Traumatic Encephalopathy: A Brief Overview. Front. Neurol. 2019, 10, 713. [Google Scholar] [CrossRef] [PubMed]
- Raymont, V.; Thayanandan, T. What do we know about the risks of developing dementia after traumatic brain injury? Minerva Med. 2021, 112, 288–297. [Google Scholar] [CrossRef]
- Crane, P.K.; Gibbons, L.E.; Dams-O’Connor, K.; Trittschuh, E.; Leverenz, J.B.; Keene, C.D.; Sonnen, J.; Montine, T.J.; Bennett, D.A.; Leurgans, S.; et al. Association between Traumatic Brain Injury and Late Life Neurodegenerative Conditions and Neuropathological Findings. JAMA Neurol. 2016, 73, 1062–1069. [Google Scholar] [CrossRef]
- Delic, V.; Beck, K.D.; Pang, K.C.H.; Citron, B.A. Biological links between traumatic brain injury and Parkinson’s disease. Acta Neuropathol. Commun. 2020, 8, 45. [Google Scholar] [CrossRef]
- Ramos-Cejudo, J.; Wisniewski, T.; Marmar, C.; Zetterberg, H.; Blennow, K.; de Leon, M.J.; Fossati, S. Traumatic Brain Injury and Alzheimer’s Disease: The Cerebrovascular Link. eBioMedicine 2018, 28, 21–30. [Google Scholar] [CrossRef]
- VanItallie, T.B. Traumatic brain injury (TBI) in collision sports: Possible mechanisms of transformation into chronic traumatic encephalopathy (CTE). Metabolism 2019, 100, 153943. [Google Scholar] [CrossRef]
- Werner, C.; Engelhard, K. Pathophysiology of traumatic brain injury. Br. J. Anaesth. 2007, 99, 4–9. [Google Scholar] [CrossRef]
- Gaetz, M. The neurophysiology of brain injury. Clin. Neurophysiol. 2004, 115, 4–18. [Google Scholar] [CrossRef]
- Johnstone, V.P.; Shultz, S.R.; Yan, E.B.; O’Brien, T.J.; Rajan, R. The acute phase of mild traumatic brain injury is characterized by a distance-dependent neuronal hypoactivity. J. Neurotrauma. 2014, 31, 1881–1895. [Google Scholar] [CrossRef]
- Risbrough, V.B.; Vaughn, M.N.; Friend, S.F. Role of Inflammation in Traumatic Brain Injury–Associated Risk for Neuropsychiatric Disorders: State of the Evidence and Where Do We Go from Here. Biol. Psychiatry 2022, 91, 438–448. [Google Scholar] [CrossRef]
- Mayer, A.R.; Quinn, D.K.; Master, C.L. The spectrum of mild traumatic brain injury: A review. Neurology 2017, 89, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Hetz, C.; Saxena, S. ER stress and the unfolded protein response in neurodegeneration. Nat. Rev. Neurol. 2017, 13, 477–491. [Google Scholar] [CrossRef]
- Freire, M.A.M. Pathophysiology of neurodegeneration following traumatic brain injury. West Indian Med. J. 2012, 61, 751–755. [Google Scholar]
- Cruz-Haces, M.; Tang, J.; Acosta, G.; Fernandez, J.; Shi, R. Pathological correlations between traumatic brain injury and chronic neurodegenerative diseases. Transl. Neurodegener. 2017, 6, 20. [Google Scholar] [CrossRef]
- Khatri, N.; Thakur, M.; Pareek, V.; Kumar, S.; Sharma, S.; Datusalia, A.K. Oxidative Stress: Major Threat in Traumatic Brain Injury. CNS Neurol. Disord. Drug Targets 2018, 17, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.-I.; Jou, M.-J. Oxidative stress caused by mitochondrial calcium overload. Ann. N. Y. Acad. Sci. 2010, 1201, 183–188. [Google Scholar] [CrossRef]
- Ma, Q. Role of Nrf2 in Oxidative Stress and Toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, H. Targeting the NF-E2-Related Factor 2 Pathway: A Novel Strategy for Traumatic Brain Injury. Mol. Neurobiol. 2018, 55, 1773–1785. [Google Scholar] [CrossRef]
- Egawa, J.; Pearn, M.L.; Lemkuil, B.P.; Patel, P.M.; Head, B.P. Membrane lipid rafts and neurobiology: Age-related changes in membrane lipids and loss of neuronal function. J. Physiol. 2016, 594, 4565–4579. [Google Scholar] [CrossRef] [PubMed]
- Head, B.P.; Peart, J.N.; Panneerselvam, M.; Yokoyama, T.; Pearn, M.L.; Niesman, I.R.; Bonds, J.A.; Schilling, J.M.; Miyanohara, A.; Headrick, J.; et al. Loss of Caveolin-1 Accelerates Neurodegeneration and Aging. PLoS ONE 2010, 5, e15697. [Google Scholar] [CrossRef]
- Webster, K.M.; Wright, D.; Sun, M.; Semple, B.D.; Ozturk, E.; Stein, D.G.; O’Brien, T.; Shultz, S.R. Progesterone treatment reduces neuroinflammation, oxidative stress and brain damage and improves long-term outcomes in a rat model of repeated mild traumatic brain injury. J. Neuroinflamm. 2015, 12, 238. [Google Scholar] [CrossRef]
- Johnson, V.E.; Stewart, W.; Smith, D.H. Axonal pathology in traumatic brain injury. Exp. Neurol. 2013, 246, 35–43. [Google Scholar] [CrossRef]
- Jang, S.H. Diagnostic Problems in Diffuse Axonal Injury. Diagnostics 2020, 10, 117. [Google Scholar] [CrossRef]
- Lozano, D.; Schimmel, S.J.; Acosta, S. Neuroinflammation in traumatic brain injury: A chronic response to an acute injury. Brain Circ. 2017, 3, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Pearn, M.L.; Niesman, I.R.; Egawa, J.; Sawada, A.; Almenar-Queralt, A.; Shah, S.B.; Duckworth, J.L.; Head, B.P. Pathophysiology Associated with Traumatic Brain Injury: Current Treatments and Potential Novel Therapeutics. Cell. Mol. Neurobiol. 2017, 37, 571–585. [Google Scholar] [CrossRef]
- O’Brien, W.T.; Pham, L.; Symons, G.F.; Monif, M.; Shultz, S.R.; McDonald, S.J. The NLRP3 inflammasome in traumatic brain injury: Potential as a biomarker and therapeutic target. J. Neuroinflamm. 2020, 17, 104–112. [Google Scholar] [CrossRef]
- Gerakis, Y.; Hetz, C. Emerging roles of ER stress in the etiology and pathogenesis of Alzheimer’s disease. FEBS J. 2018, 285, 995–1011. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.Z.A.; Zhao, D.; Khan, S.H.; Yang, L. Unfolded Protein Response Pathways in Neurodegenerative Diseases. J. Mol. Neurosci. 2015, 57, 529–537. [Google Scholar] [CrossRef]
- Washington, P.M.; Villapol, S.; Burns, M.P. Polypathology and Dementia After Brain Trauma: Does Brain Injury Trigger Distinct Neurodegenerative Diseases, or Should They Be Classified Together as Traumatic Encephalopathy? Exp. Neurol. 2016, 275 Pt 3, 381–388. [Google Scholar] [CrossRef]
- Uryu, K.; Chen, X.-H.; Martinez, D.; Browne, K.D.; Johnson, V.E.; Graham, D.I.; Lee, V.M.-Y.; Trojanowski, J.Q.; Smith, D.H. Multiple proteins implicated in neurodegenerative diseases accumulate in axons after brain trauma in humans. Exp. Neurol. 2007, 208, 185–192. [Google Scholar] [CrossRef]
- Johnson, V.E.; Stewart, W.; Smith, D.H. Widespread τ and amyloid-β pathology many years after a single traumatic brain injury in humans. Brain Pathol. 2012, 22, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, W.L.; MacKinnon, M.-A.; Stewart, J.E.; Graham, D.I. Stereology of cerebral cortex after traumatic brain injury matched to the Glasgow Outcome Score. Brain 2010, 133, 139–160. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.H.; Doyle, D.; Ford, I.; Gennarelli, T.A.; Graham, D.I.; McLellan, D.R. Diffuse axonal injury in head injury: Definition, diagnosis and grading. Histopathology 1989, 15, 49–59. [Google Scholar] [CrossRef]
- Hofman, P.A.; Verhey, F.R.; Wilmink, J.T.; Rozendaal, N.; Jolles, J. Brain lesions in patients visiting a memory clinic with postconcussional sequelae after mild to moderate brain injury. J. Neuropsychiatry Clin. Neurosci. 2002, 14, 176–184. [Google Scholar] [CrossRef]
- Bigler, E.D.; Tate, D.F. Brain volume, intracranial volume, and dementia. Investig. Radiol. 2001, 36, 539–546. [Google Scholar] [CrossRef]
- Mamere, A.E.; Saraiva, L.A.L.; Matos, A.L.M.; Carneiro, A.A.O.; Santos, A.C. Evaluation of Delayed Neuronal and Axonal Damage Secondary to Moderate and Severe Traumatic Brain Injury Using Quantitative MR Imaging Techniques. Am. J. Neuroradiol. 2009, 30, 947–952. [Google Scholar] [CrossRef] [PubMed]
- Graham, N.S.; Sharp, D.J. Understanding neurodegeneration after traumatic brain injury: From mechanisms to clinical trials in dementia. J. Neurol. Neurosurg. Psychiatry 2019, 90, 1221–1233. [Google Scholar] [CrossRef]
- Bigler, E.D. Anterior and middle cranial fossa in traumatic brain injury: Relevant neuroanatomy and neuropathology in the study of neuropsychological outcome. Neuropsychology 2007, 21, 515–531. [Google Scholar] [CrossRef]
- De La Plata, C.D.M.; Garces, J.; Kojori, E.S.; Grinnan, J.; Krishnan, K.; Pidikiti, R.; Spence, J.; Devous, M.D.; Moore, C.; McColl, R.; et al. Deficits in Functional Connectivity of Hippocampal and Frontal Lobe Circuits After Traumatic Axonal Injury. Arch. Neurol. 2011, 68, 74–84. [Google Scholar]
- Veeramuthu, V.; Narayanan, V.; Kuo, T.L.; Delano-Wood, L.; Chinna, K.; Bondi, M.W.; Waran, V.; Ganesan, D.; Ramli, N. Diffusion Tensor Imaging Parameters in Mild Traumatic Brain Injury and Its Correlation with Early Neuropsychological Impairment: A Longitudinal Study. J. Neurotrauma 2015, 32, 1497–1509. [Google Scholar] [CrossRef]
- Farbota, K.D.; Bendlin, B.B.; Alexander, A.L.; Rowley, H.A.; Dempsey, R.J.; Johnson, S.C. Longitudinal diffusion tensor imaging and neuropsychological correlates in traumatic brain injury patients. Front. Hum. Neurosci. 2012, 6, 160. [Google Scholar] [CrossRef] [PubMed]
- Hellyer, P.J.; Leech, R.; Ham, T.E.; Bonnelle, V.; Sharp, D.J. Individual prediction of white matter injury following traumatic brain injury. Ann. Neurol. 2013, 73, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Holleran, L.; Kim, J.H.; Gangolli, M.; Stein, T.; Alvarez, V.; McKee, A.; Brody, D.L. Axonal disruption in white matter underlying cortical sulcus tau pathology in chronic traumatic encephalopathy. Acta Neuropathol. 2017, 133, 367–380. [Google Scholar] [CrossRef]
- Palacios, E.M.; Sala-Llonch, R.; Junque, C.; Roig, T.; Tormos, J.M.; Bargallo, N.; Vendrell, P. White matter integrity related to functional working memory networks in traumatic brain injury. Neurology 2012, 78, 852–860. [Google Scholar] [CrossRef]
- Wang, J.Y.; Bakhadirov, K.; Abdi, H.; Devous, M.D.; De La Plata, C.D.M.; Moore, C.; Madden, C.J.; Diaz-Arrastia, R. Longitudinal changes of structural connectivity in traumatic axonal injury. Neurology 2011, 77, 818–826. [Google Scholar] [CrossRef] [PubMed]
- Sidaros, A.; Engberg, A.W.; Sidaros, K.; Liptrot, M.G.; Herning, M.; Petersen, P.; Paulson, O.B.; Jernigan, T.L.; Rostrup, E. Diffusion tensor imaging during recovery from severe traumatic brain injury and relation to clinical outcome: A longitudinal study. Brain 2008, 131, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Stern, R.A.; Adler, C.H.; Chen, K.; Navitsky, M.; Luo, J.; Dodick, D.W.; Alosco, M.L.; Tripodis, Y.; Goradia, D.D.; Martin, B.; et al. Tau Positron-Emission Tomography in Former National Football League Players. N. Engl. J. Med. 2019, 380, 1716–1725. [Google Scholar] [CrossRef]
- Hong, Y.T.; Veenith, T.; Dewar, D.; Outtrim, J.G.; Mani, V.; Williams, C.; Pimlott, S.; Hutchinson, P.J.; Tavares, A.; Canales, R.; et al. Amyloid imaging with carbon 11-labeled Pittsburgh compound B for traumatic brain injury. JAMA Neurol. 2014, 71, 23–31. [Google Scholar] [CrossRef]
- Mielke, M.M.; Savica, R.; Wiste, H.J.; Weigand, S.D.; Vemuri, P.; Knopman, D.S.; Lowe, V.J.; Roberts, R.O.; Machulda, M.M.; Geda, Y.E.; et al. Head trauma and in vivo measures of amyloid and neurodegeneration in a population-based study. Neurology 2014, 82, 70–76. [Google Scholar] [CrossRef]
- Scott, G.; Ramlackhansingh, A.F.; Edison, P.; Hellyer, P.; Cole, J.; Veronese, M.; Leech, R.; Greenwood, R.J.; Turkheimer, F.E.; Gentleman, S.M.; et al. Amyloid pathology and axonal injury after brain trauma. Neurology 2016, 86, 821–828. [Google Scholar] [CrossRef]
- Chen, S.T.; Siddarth, P.; Merrill, D.A.; Martinez, J.; Emerson, N.D.; Liu, J.; Wong, K.-P.; Satyamurthy, N.; Giza, C.C.; Huang, S.-C.; et al. FDDNP-PET Tau Brain Protein Binding Patterns in Military Personnel with Suspected Chronic Traumatic Encephalopathy1. J. Alzheimer’s Dis. 2018, 65, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Leavitt, M.J.; Bernick, C.B.; Leger, G.C.; Rabinovici, G.; Banks, S.J. A Systematic Review of Positron Emission Tomography of Tau, Amyloid Beta, and Neuroinflammation in Chronic Traumatic Encephalopathy: The Evidence to Date. J. Neurotrauma 2018, 35, 2015–2024. [Google Scholar] [CrossRef]
- Pierre, K.; Dyson, K.; Dagra, A.; Williams, E.; Porche, K.; Lucke-Wold, B. Chronic Traumatic Encephalopathy: Update on Current Clinical Diagnosis and Management. Biomedicines 2021, 9, 415. [Google Scholar] [CrossRef] [PubMed]
- Barrio, J.R.; Small, G.W.; Wong, K.P.; Huang, S.C.; Liu, J.; Merrill, D.A.; Giza, C.C.; Fitzsimmons, R.P.; Omalu, B.; Bailes, J.; et al. In Vivo characterization of chronic traumatic encephalopathy using [F-18]FDDNP PET brain imaging. Proc. Natl. Acad. Sci. USA 2015, 112, E2039–E2047. [Google Scholar] [CrossRef] [PubMed]
- Olsen, A.; Babikian, T.; Bigler, E.D.; Caeyenberghs, K.; Conde, V.; Dams-O’Connor, K.; Dobryakova, E.; Genova, H.; Grafman, J.; Håberg, A.K.; et al. Toward a global and reproducible science for brain imaging in neurotrauma: The ENIGMA adult moderate/severe traumatic brain injury working group. Brain Imaging Behav. 2021, 15, 526–554. [Google Scholar] [CrossRef] [PubMed]
- Amyot, F.; Arciniegas, D.B.; Brazaitis, M.P.; Curley, K.C.; Diaz-Arrastia, R.; Gandjbakhche, A.; Herscovitch, P.; Hinds, S.R., 2nd; Manley, G.T.; Pacifico, A.; et al. A Review of the Effectiveness of Neuroimaging Modalities for the Detection of Traumatic Brain Injury. J. Neurotrauma 2015, 32, 1693–1721. [Google Scholar] [CrossRef]
- Christensen, J.; Wright, D.K.; Yamakawa, G.R.; Shultz, S.R.; Mychasiuk, R. Repetitive Mild Traumatic Brain Injury Alters Glymphatic Clearance Rates in Limbic Structures of Adolescent Female Rats. Sci. Rep. 2020, 10, 6254. [Google Scholar]
- Piantino, J.; Schwartz, D.L.; Luther, M.; Newgard, C.; Silbert, L.; Raskind, M.; Pagulayan, K.; Kleinhans, N.; Iliff, J.; Peskind, E. Link between Mild Traumatic Brain Injury, Poor Sleep, and Magnetic Resonance Imaging: Visible Perivascular Spaces in Veterans. J. Neurotrauma 2021, 38, 2391–2399. [Google Scholar] [CrossRef]
- Kaur, J.; Davoodi-Bojd, E.; Fahmy, L.M.; Zhang, L.; Ding, G.; Hu, J.; Zhang, Z.; Chopp, M.; Jiang, Q. Magnetic Resonance Imaging and Modeling of the Glymphatic System. Diagnostics 2020, 10, 344. [Google Scholar] [CrossRef]
- Taoka, T.; Naganawa, S. Glymphatic imaging using MRI. J. Magn. Reson. Imaging 2020, 51, 11–24. [Google Scholar] [CrossRef]
- Gupta, R.K.; Prasad, S. Age-Dependent Alterations in the Interactions of NF-κB and N-myc with GLT-1/EAAT2 Promoter in the Pericontusional Cortex of Mice Subjected to Traumatic Brain Injury. Mol. Neurobiol. 2016, 53, 3377–3388. [Google Scholar] [CrossRef] [PubMed]
- MacFarlane, M.P.; Glenn, T.C. Neurochemical cascade of concussion. Brain Inj. 2015, 29, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Esopenko, C.; Levine, B. Aging, Neurodegenerative Disease, and Traumatic Brain Injury: The Role of Neuroimaging. J. Neurotrauma 2015, 32, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Sen, N. Traumatic brain injury: A risk factor for neurodegenerative diseases. Rev. Neurosci. 2016, 27, 93–100. [Google Scholar] [CrossRef]
- Borgen, I.M.; Røe, C.; Brunborg, C.; Tenovuo, O.; Azouvi, P.; Dawes, H.; Majdan, M.; Ranta, J.; Rusnak, M.; Wiegers, E.J.; et al. Care transitions in the first 6 months following traumatic brain injury: Lessons from the CENTER-TBI study. Ann. Phys. Rehabil. Med. 2021, 64, 101458. [Google Scholar] [CrossRef]
- Abdelmalik, P.A.; Draghic, N.; Ling, G.S.F. Management of moderate and severe traumatic brain injury. Transfusion 2019, 59, 1529–1538. [Google Scholar] [CrossRef]
- Kemper, B.; Von Wild, K. Neuropsychological fields in early neurotrauma rehabilitation. Zentralbl. Neurochir. 1999, 60, 168–171. [Google Scholar]
- Prince, C.; Bruhns, M.E. Evaluation and Treatment of Mild Traumatic Brain Injury: The Role of Neuropsychology. Brain Sci. 2017, 7, 105. [Google Scholar] [CrossRef]
- Sheinerman, K.S.; Umansky, S.R. Early detection of neurodegenerative diseases: Circulating brain-enriched microRNA. Cell Cycle. 2013, 12, 1–2. [Google Scholar] [CrossRef]
- Tartaglia, M.C.; Hazrati, L.-N.; Davis, K.D.; Green, R.E.A.; Wennberg, R.; Mikulis, D.; Ezerins, L.J.; Keightley, M.; Tator, C. Chronic traumatic encephalopathy and other neurodegenerative proteinopathies. Front. Hum. Neurosci. 2014, 8, 30. [Google Scholar] [CrossRef]
- McKee, A.C.; Stein, T.; Nowinski, C.J.; Stern, R.; Daneshvar, D.; Alvarez, V.E.; Lee, H.-S.; Hall, G.; Wojtowicz, S.M.; Baugh, C.; et al. The spectrum of disease in chronic traumatic encephalopathy. Brain 2013, 136, 43–64. [Google Scholar] [CrossRef] [PubMed]
- McKee, A.C.; Cantu, R.C.; Nowinski, C.J.; Hedley-Whyte, E.T.; Gavett, B.; Budson, A.E.; Santini, V.E.; Lee, H.-S.; Kubilus, C.A.; Stern, R. Chronic Traumatic Encephalopathy in Athletes: Progressive Tauopathy After Repetitive Head Injury. J. Neuropathol. Exp. Neurol. 2009, 68, 709–735. [Google Scholar] [CrossRef]
- Deb, S.; Burns, J. Neuropsychiatric consequences of traumatic brain injury: A comparison between two age groups. Brain Inj. 2007, 21, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.; Rosenberg, P.; Miles, Q.S.; Patadia, D.; Treiber, K.; Bertrand, M.; Norton, M.; Steinberg, M.; Tschanz, J.; Lyketsos, C. Neuropsychiatric Symptoms in Dementia Patients with and without a History of Traumatic Brain Injury. J. Neuropsychiatry Clin. Neurosci. 2010, 22, 166–172. [Google Scholar] [CrossRef]
- Jordan, B.D. The clinical spectrum of sport-related traumatic brain injury. Nat. Rev. Neurol. 2013, 9, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Bray, M.J.C.; Richey, L.N.; Bryant, B.R.; Krieg, A.; Jahed, S.; Tobolowsky, W.; LoBue, C.; Peters, M.E. Traumatic brain injury alters neuropsychiatric symptomatology in all-cause dementia. Alzheimer’s Dement. 2021, 17, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Padmakumar, S.; Kulkarni, P.; Ferris, C.F.; Bleier, B.S.; Amiji, M.M. Traumatic brain injury and the development of parkinsonism: Understanding pathophysiology, animal models, and therapeutic targets. Biomed. Pharmacother. 2022, 149, 112812. [Google Scholar] [CrossRef]
- Anderson, K.E. Behavioral disturbances in Parkinson’s disease. Dialog. Clin. Neurosci. 2004, 6, 323–332. [Google Scholar] [CrossRef]
- Srinivasan, G.; Brafman, D.A. The Emergence of Model Systems to Investigate the Link Between Traumatic Brain Injury and Alzheimer’s Disease. Front. Aging Neurosci. 2021, 13, 813544. [Google Scholar] [CrossRef]
- Bature, F.; Guinn, B.; Pang, D.; Pappas, Y. Signs and symptoms preceding the diagnosis of Alzheimer’s disease: A systematic scoping review of literature from 1937 to 2016. BMJ Open 2017, 7, e015746. [Google Scholar] [CrossRef] [PubMed]
- Muizelaar, J.P.; Marmarou, A.; Young, H.F.; Choi, S.C.; Wolf, A.; Schneider, R.L.; Kontos, H.A. Improving the outcome of severe head injury with the oxygen radical scavenger polyethylene glycol-conjugated superoxide dismutase: A Phase II trial. J. Neurosurg. 1993, 78, 375–382. [Google Scholar] [CrossRef]
- Fang, J.; Wang, H.; Zhou, J.; Dai, W.; Zhu, Y.; Zhou, Y.; Wang, X.; Zhou, M.-L. Baicalin provides neuroprotection in traumatic brain injury mice model through Akt/Nrf2 pathway. Drug Des. Dev. Ther. 2018, 12, 2497–2508. [Google Scholar] [CrossRef]
- Shi, Z.; Qiu, W.; Xiao, G.; Cheng, J.; Zhang, N. Resveratrol Attenuates Cognitive Deficits of Traumatic Brain Injury by Activating p38 Signaling in the Brain. Med Sci. Monit. 2018, 24, 1097–1103. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, C.; Peng, W.; Xia, Z.; Gan, P.; Huang, W.; Shi, Y.; Fan, R. Hydroxysafflor yellow A exerts antioxidant effects in a rat model of traumatic brain injury. Mol. Med. Rep. 2016, 14, 3690–3696. [Google Scholar] [CrossRef]
- Xu, J.; Wang, H.; Ding, K.; Zhang, L.; Wang, C.; Li, T.; Wei, W.; Lu, X. Luteolin provides neuroprotection in models of traumatic brain injury via the Nrf2–ARE pathway. Free Radic. Biol. Med. 2014, 71, 186–195. [Google Scholar] [CrossRef]
- Ismail, H.; Shakkour, Z.; Tabet, M.; Abdelhady, S.; Kobaisi, A.; Abedi, R.; Nasrallah, L.; Pintus, G.; Al-Dhaheri, Y.; Mondello, S.; et al. Traumatic Brain Injury: Oxidative Stress and Novel Anti-Oxidants Such as Mitoquinone and Edaravone. Antioxidants 2020, 9, 943. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, H.; Shen, R.; Fang, J.; Yang, Y.; Dai, W.; Zhu, Y.; Zhou, M. Mitochondrial-targeted antioxidant MitoQ provides neuroprotection and reduces neuronal apoptosis in experimental traumatic brain injury possibly via the Nrf2-ARE pathway. Am. J. Transl. Res. 2018, 10, 1887–1899. [Google Scholar]
- Bonilla, C.; Zurita, M. Cell-Based Therapies for Traumatic Brain Injury: Therapeutic Treatments and Clinical Trials. Biomedicines 2021, 9, 669. [Google Scholar] [CrossRef]
- Xiong, Y.; Mahmood, A.; Chopp, M. Current understanding of neuroinflammation after traumatic brain injury and cell-based therapeutic opportunities. Chin. J. Traumatol. 2018, 21, 137–151. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Acosta, S.; Tuazon, J.P.; Xu, K.; Nguyen, H.; Lippert, T.; Liska, M.G.; Semechkin, A.; Garitaonandia, I.; Gonzalez, R.; et al. Human parthenogenetic neural stem cell grafts promote multiple regenerative processes in a traumatic brain injury model. Theranostics 2019, 9, 1029–1046. [Google Scholar] [CrossRef]
- Xiong, L.-L.; Hu, Y.; Zhang, P.; Zhang, Z.; Li, L.-H.; Gao, G.-D.; Zhou, X.-F.; Wang, T.-H. Neural Stem Cell Transplantation Promotes Functional Recovery from Traumatic Brain Injury via Brain Derived Neurotrophic Factor-Mediated Neuroplasticity. Mol. Neurobiol. 2018, 55, 2696–2711. [Google Scholar] [CrossRef]
- Haus, D.L.; López-Velázquez, L.; Gold, E.M.; Cunningham, K.M.; Perez, H.; Anderson, A.J.; Cummings, B.J. Transplantation of human neural stem cells restores cognition in an immunodeficient rodent model of traumatic brain injury. Exp. Neurol. 2016, 281, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Liu, Y.; Yan, K.; Chen, L.; Chen, X.-R.; Li, P.; Chen, F.-F.; Jiang, X.-D. Anti-inflammatory and immunomodulatory mechanisms of mesenchymal stem cell transplantation in experimental traumatic brain injury. J. Neuroinflamm. 2013, 10, 571. [Google Scholar] [CrossRef]
- Peruzzaro, S.T.; Andrews, M.M.M.; Al-Gharaibeh, A.; Pupiec, O.; Resk, M.; Story, D.; Maiti, P.; Rossignol, J.; Dunbar, G.L. Transplantation of mesenchymal stem cells genetically engineered to overexpress interleukin-10 promotes alternative inflammatory response in rat model of traumatic brain injury. J. Neuroinflamm. 2019, 16, 2. [Google Scholar] [CrossRef]
- Bird, S.M.; Sohrabi, H.R.; Sutton, T.A.; Weinborn, M.; Rainey-Smith, S.R.; Brown, B.; Patterson, L.; Taddei, K.; Gupta, V.; Carruthers, M.; et al. Cerebral amyloid-β accumulation and deposition following traumatic brain injury—A narrative review and meta-analysis of animal studies. Neurosci. Biobehav. Rev. 2016, 64, 215–228. [Google Scholar] [CrossRef]
- Ojo, J.O.; Mouzon, B.; Algamal, M.; Leary, P.; Lynch, C.; Abdullah, L.; Evans, J.; Mullan, M.; Bachmeier, C.; Stewart, W.; et al. Chronic Repetitive Mild Traumatic Brain Injury Results in Reduced Cerebral Blood Flow, Axonal Injury, Gliosis, and Increased T-Tau and Tau Oligomers. J. Neuropathol. Exp. Neurol. 2016, 75, 636–655. [Google Scholar] [CrossRef]
- Ikonomovic, M.D.; Abrahamson, E.E.; Carlson, S.W.; Graham, S.H.; Dixon, C.E. Novel therapies for combating chronic neuropathological sequelae of TBI. Neuropharmacology 2019, 145, 160–176. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, H.; Zeng, J.; Pluimer, B.; Dong, S.; Xie, X.; Guo, X.; Ge, T.; Liang, X.; Feng, S.; et al. Mild traumatic brain injury induces microvascular injury and accelerates Alzheimer-like pathogenesis in mice. Acta Neuropathol. Commun. 2021, 9, 74. [Google Scholar] [CrossRef]
- Shin, M.-K.; Vázquez-Rosa, E.; Koh, Y.; Dhar, M.; Chaubey, K.; Cintrón-Pérez, C.J.; Barker, S.; Miller, E.; Franke, K.; Noterman, M.F.; et al. Reducing acetylated tau is neuroprotective in brain injury. Cell 2021, 184, 2715–2732.e23. [Google Scholar] [CrossRef] [PubMed]
- Albayram, O.; Kondo, A.; Mannix, R.; Smith, C.; Tsai, C.-Y.; Colin, S.; Herbert, M.K.; Qiu, J.; Monuteaux, M.; Driver, J.; et al. Cis P-tau is induced in clinical and preclinical brain injury and contributes to post-injury sequelae. Nat. Commun. 2017, 8, 1000. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.-L.; Hu, W.; Tung, Y.C.; Liu, F.; Gong, C.-X.; Iqbal, K. Tau passive immunization blocks seeding and spread of Alzheimer hyperphosphorylated Tau-induced pathology in 3 × Tg-AD mice. Alzheimer’s Res. Ther. 2018, 10, 13. [Google Scholar] [CrossRef]
- Braczynski, A.K.; Schulz, J.B.; Bach, J.-P. Vaccination strategies in tauopathies and synucleinopathies. J. Neurochem. 2017, 143, 467–488. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.P.; Kondo, A.; Albayram, O.; Herbert, M.K.; Liu, H.; Zhou, X.Z. Potential of the Antibody Against cis-Phosphorylated Tau in the Early Diagnosis, Treatment, and Prevention of Alzheimer Disease and Brain Injury. JAMA Neurol. 2016, 73, 1356–1362. [Google Scholar] [CrossRef]
- Mockett, B.G.; Richter, M.; Abraham, W.C.; Müller, U.C. Therapeutic Potential of Secreted Amyloid Precursor Protein APPsα. Front. Mol. Neurosci. 2017, 10, 30. [Google Scholar] [CrossRef]
- Corrigan, F.; Vink, R.; Blumbergs, P.C.; Masters, C.L.; Cappai, R.; Heuvel, C.V.D. Evaluation of the effects of treatment with sAPPα on functional and histological outcome following controlled cortical impact injury in mice. Neurosci. Lett. 2012, 515, 50–54. [Google Scholar] [CrossRef]
- Corrigan, F.; Vink, R.; Blumbergs, P.C.; Masters, C.L.; Cappai, R.; Heuvel, C.V.D. sAPPα rescues deficits in amyloid precursor protein knockout mice following focal traumatic brain injury. J. Neurochem. 2012, 122, 208–220. [Google Scholar] [CrossRef]
- Cohen, A.D.; Ikonomovic, M.D.; Abrahamson, E.E.; Paljug, W.R.; DeKosky, S.T.; Lefterov, I.M.; Koldamova, R.P.; Shao, L.; Debnath, M.L.; Mason, N.S.; et al. Anti-Amyloid Effects of Small Molecule Aβ-Binding Agents in PS1/APP Mice. Lett. Drug Des. Discov. 2009, 6, 437. [Google Scholar] [CrossRef]
- Celorrio, M.; Shumilov, K.; Payne, C.; Vadivelu, S.; Friess, S.H. Acute minocycline administration reduces brain injury and improves long-term functional outcomes after delayed hypoxemia following traumatic brain injury. Acta Neuropathol. Commun. 2022, 10, 10. [Google Scholar] [CrossRef]
- Whitney, K.; Nikulina, E.; Rahman, S.N.; Alexis, A.; Bergold, P.J. Delayed dosing of minocycline plus N-acetylcysteine reduces neurodegeneration in distal brain regions and restores spatial memory after experimental traumatic brain injury. Exp. Neurol. 2021, 345, 113816. [Google Scholar] [CrossRef]
- Bye, N.; Habgood, M.D.; Callaway, J.K.; Malakooti, N.; Potter, A.; Kossmann, T.; Morganti-Kossmann, M.C. Transient neuroprotection by minocycline following traumatic brain injury is associated with attenuated microglial activation but no changes in cell apoptosis or neutrophil infiltration. Exp. Neurol. 2007, 204, 220–233. [Google Scholar] [CrossRef]
- Robinson, B.D.; Isbell, C.L.; Melge, A.R.; Lomas, A.M.; Shaji, C.A.; Mohan, C.G.; Huang, J.H.; Tharakan, B. Doxycycline prevents blood–brain barrier dysfunction and microvascular hyperpermeability after traumatic brain injury. Sci. Rep. 2022, 12, 5415. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wang, X.; Chen, X.; Fang, Y.; Chen, K.; Peng, W.; Wang, Z.; Guo, K.; Tan, X.; Liang, F.; et al. Hydroxychloroquine attenuates neuroinflammation following traumatic brain injury by regulating the TLR4/NF-κB signaling pathway. J. Neuroinflamm. 2022, 19, 71. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Li, R.; Cui, C.-M.; Gao, J.-L.; Sun, L.-Q.; Wang, Y.-C.; Wang, K.-J.; Tian, Y.-X.; Cui, J.-Z. Chloroquine exerts neuroprotection following traumatic brain injury via suppression of inflammation and neuronal autophagic death. Mol. Med. Rep. 2015, 12, 2323–2328. [Google Scholar] [CrossRef]
- Yonutas, H.M.; Sullivan, P.G. Targeting PPAR isoforms following CNS injury. Curr. Drug Targets 2013, 14, 733–742. [Google Scholar] [CrossRef]
- Strosznajder, A.K.; Wójtowicz, S.; Jeżyna, M.J.; Sun, G.Y.; Strosznajder, J.B. Recent Insights on the Role of PPAR-β/δ in Neuroinflammation and Neurodegeneration, and Its Potential Target for Therapy. Neuromol. Med. 2021, 23, 86–98. [Google Scholar] [CrossRef]
- Sauerbeck, A.; Gao, J.; Readnower, R.; Liu, M.; Pauly, J.R.; Bing, G.; Sullivan, P.G. Pioglitazone attenuates mitochondrial dysfunction, cognitive impairment, cortical tissue loss, and inflammation following traumatic brain injury. Exp. Neurol. 2011, 227, 128–135. [Google Scholar] [CrossRef]
- Amenta, P.S.; Jallo, J.I.; Tuma, R.F.; Elliott, M.B. A cannabinoid type 2 receptor agonist attenuates blood-brain barrier damage and neurodegeneration in a murine model of traumatic brain injury. J. Neurosci. Res. 2012, 90, 2293–2305. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Zhong, W.; Gu, Y.; Li, Y. Emerging Mechanisms and Targeted Therapy of Pyroptosis in Central Nervous System Trauma. Front. Cell Dev. Biol. 2022, 10, 832114. [Google Scholar] [CrossRef]
- Irrera, N.; Russo, M.; Pallio, G.; Bitto, A.; Mannino, F.; Minutoli, L.; Altavilla, D.; Squadrito, F. The Role of NLRP3 Inflammasome in the Pathogenesis of Traumatic Brain Injury. Int. J. Mol. Sci. 2020, 21, 6204. [Google Scholar] [CrossRef]
- Ismael, S.; Nasoohi, S.; Ishrat, T. MCC950, the Selective Inhibitor of Nucleotide Oligomerization Domain-Like Receptor Protein-3 Inflammasome, Protects Mice against Traumatic Brain Injury. J. Neurotrauma 2018, 35, 1294–1303. [Google Scholar] [CrossRef]
- Barrett, J.P.; Henry, R.; Shirey, K.A.; Doran, S.J.; Makarevich, O.D.; Ritzel, R.; Meadows, V.A.; Vogel, S.N.; Faden, A.I.; Stoica, B.A.; et al. Interferon-β Plays a Detrimental Role in Experimental Traumatic Brain Injury by Enhancing Neuroinflammation That Drives Chronic Neurodegeneration. J. Neurosci. 2020, 40, 2357–2370. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zheng, J.; Xu, S.; Fang, Y.; Wu, Y.; Zeng, J.; Shao, A.; Shi, L.; Lu, J.; Mei, S.; et al. Mer regulates microglial/macrophage M1/M2 polarization and alleviates neuroinflammation following traumatic brain injury. J. Neuroinflamm. 2021, 18, 2. [Google Scholar] [CrossRef] [PubMed]
- Nathalie, M.; Polineni, S.P.; Chin, C.N.; Fawcett, D.; Clervius, H.; Maria, Q.S.; Legnay, F.; Rego, L.; Mahavadi, A.K.; Jermakowicz, W.J.; et al. Targeting Microglial Polarization to Improve TBI Outcomes. CNS Neurol. Disord. Drug Targets 2021, 20, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, C.; Fan, S.; Wu, S.; Yang, F.; Fang, Z.; Fu, H.; Li, Y. Omega-3 polyunsaturated fatty acid attenuates the inflammatory response by modulating microglia polarization through SIRT1-mediated deacetylation of the HMGB1/NF-κB pathway following experimental traumatic brain injury. J. Neuroinflamm. 2018, 15, 116. [Google Scholar] [CrossRef]
- Chio, C.-C.; Lin, M.-T.; Chang, C.-P. Microglial activation as a compelling target for treating acute traumatic brain injury. Curr. Med. Chem. 2015, 22, 759–770. [Google Scholar] [CrossRef] [PubMed]
| Neurotrauma-Related Disease | Key Behavioral Features | References |
|---|---|---|
| Chronic traumatic encephalopathy | Paranoia, mood swings, apathy, impulsivity, depression, and suicidality | [70,71,72] |
| Unclassified dementia | Anxiety, apathy, and possibly agitation/disinhibition | [73,74,75,76] |
| Parkinson’s disease | Motivational decline and slowed thinking | [3,77,78] |
| Alzheimer’s disease | Depression, cognitive impairment, memory loss | [79,80] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dodd, W.S.; Panther, E.J.; Pierre, K.; Hernandez, J.S.; Patel, D.; Lucke-Wold, B. Traumatic Brain Injury and Secondary Neurodegenerative Disease. Trauma Care 2022, 2, 510-522. https://doi.org/10.3390/traumacare2040042
Dodd WS, Panther EJ, Pierre K, Hernandez JS, Patel D, Lucke-Wold B. Traumatic Brain Injury and Secondary Neurodegenerative Disease. Trauma Care. 2022; 2(4):510-522. https://doi.org/10.3390/traumacare2040042
Chicago/Turabian StyleDodd, William S., Eric J. Panther, Kevin Pierre, Jairo S. Hernandez, Devan Patel, and Brandon Lucke-Wold. 2022. "Traumatic Brain Injury and Secondary Neurodegenerative Disease" Trauma Care 2, no. 4: 510-522. https://doi.org/10.3390/traumacare2040042
APA StyleDodd, W. S., Panther, E. J., Pierre, K., Hernandez, J. S., Patel, D., & Lucke-Wold, B. (2022). Traumatic Brain Injury and Secondary Neurodegenerative Disease. Trauma Care, 2(4), 510-522. https://doi.org/10.3390/traumacare2040042








