Novel Enzymes for Biologics with Hydrolytic Activity Against Thiolactones: Computational, Catalytic and Antimicrobial Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Computational Investigations
2.3. Enzymatic Activity of Enzymes and Their Polymeric Complexes
2.4. Determination of Antimicrobial Action Efficiency
3. Results
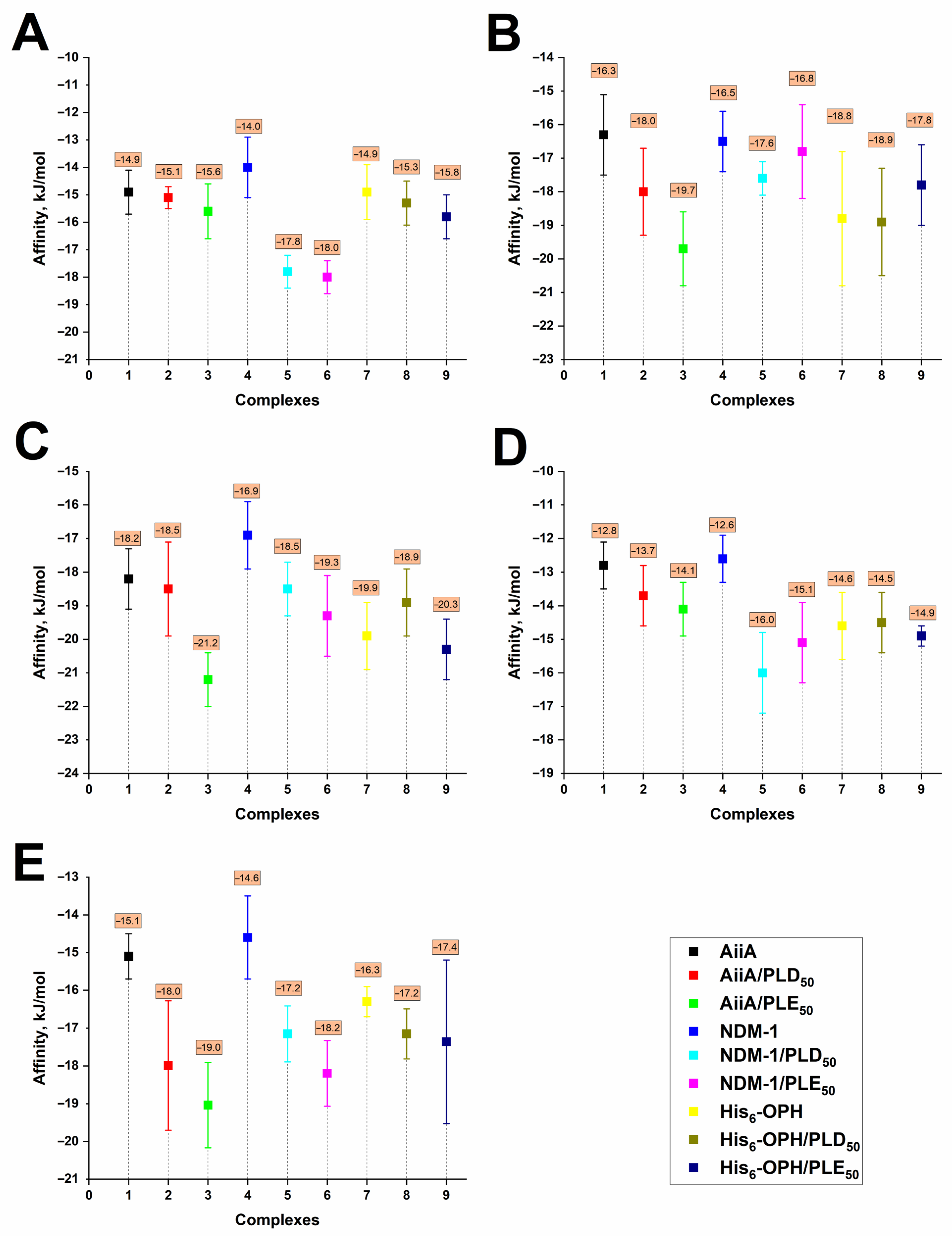
3.1. Computational Modeling of Interaction Between Enzymes and Thiolactones
3.2. Computational Modeling of Interactions of Enzyme/polyAA Complexes with Thiolactones
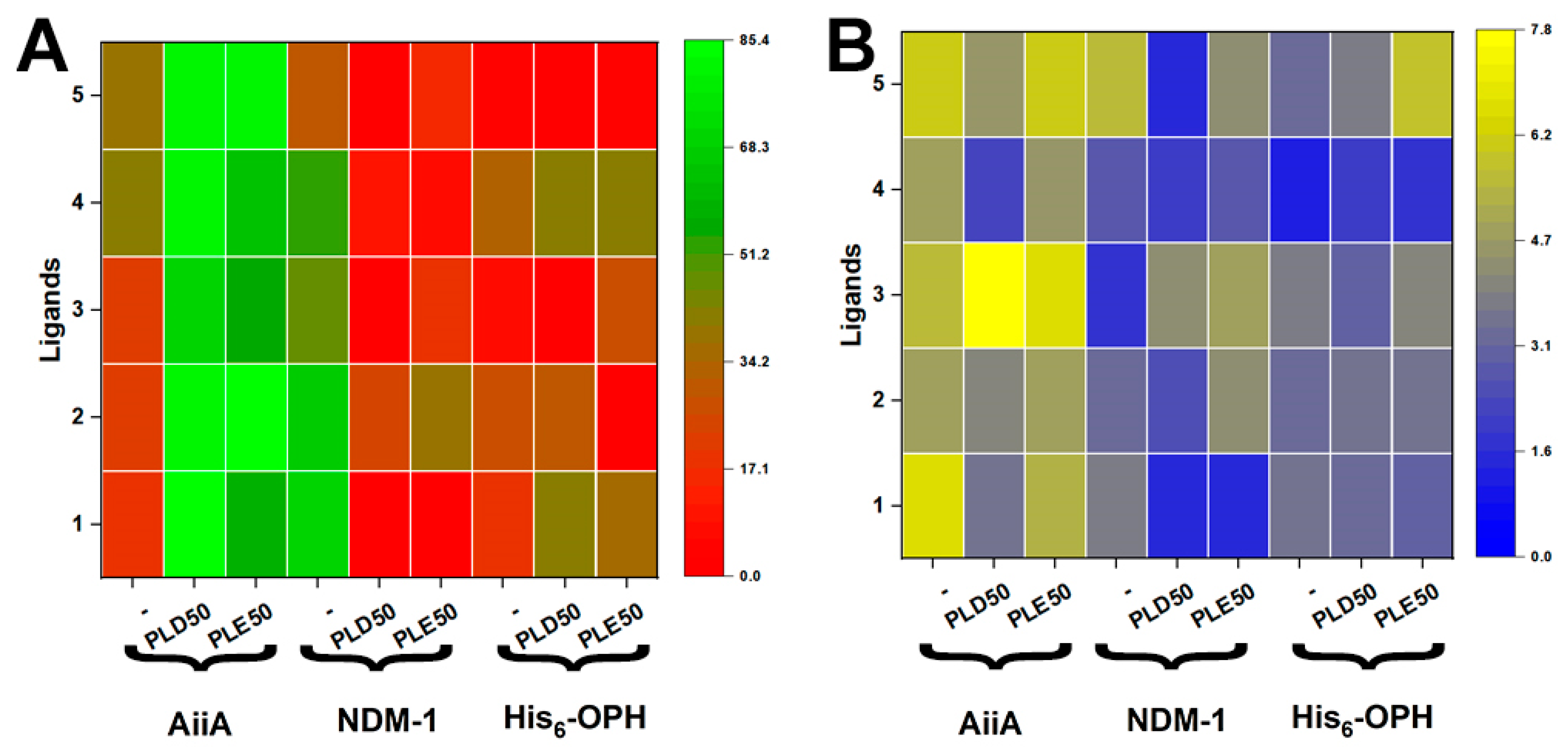
3.3. Catalytic Activity of Enzymes and Their Polyelectrolyte Complexes in Relation to Thiolactones
3.4. Antimicrobial Action of the Enzymes and Their Complexes with polyAA in Combinations with Polymyxin B or Clotrimazole
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| polyAA | Polyamino acids |
| HTL | Homocysteine thiolactone |
| C2–HTL | Ethylhomocysteine thiolactone |
| i–but–HTL | Isobutyrylhomocystein thiolactone |
| PLD50 | Polyaspartic aid |
| PLE50 | Polyglutamic acid |
| His6–OPH | Hexahistidine organophosphate hydrolase |
| AiiA | Lactonase |
| NDM-1 | Metallo-β-lactamase |
| QS | Quorum Sensing |
| ATP | Adenosine triphosphate |
| Pol B | Polymyxin B |
References
- Sartori, S.K.; Diaz, M.A.N.; Diaz, G. Lactones: Classification, synthesis, biological activities, and industrial applications. Tetrahedron 2021, 84, 132001. [Google Scholar] [CrossRef]
- Jakubowski, H. Calcium-dependent human serum homocysteine thiolactone hydrolase. A protective mechanism against protein N-homocysteinylation. J. Biol. Chem. 2000, 275, 3957–3962. [Google Scholar] [CrossRef]
- Alfaraj, R.; Eltayb, E.K.; AlFayez, B.M.; Abohamad, A.; Eltahir, E.; Alenazi, N.A.; Hababah, S.; Alkahtani, H.; Almangour, T.A.; Alqahtani, F.Y.; et al. Studying the anti-virulence activity of meta-bromo-thiolactone against Staphylococcus aureus and MRSA phenotypes. Microbiol. Res. 2023, 14, 1596–1609. [Google Scholar] [CrossRef]
- Boursier, M.E.; Combs, J.B.; Blackwell, H.E. N-Acyl l-Homocysteine thiolactones are potent and stable synthetic modulators of the RhlR Quorum Sensing receptor in Pseudomonas aeruginosa. ACS Chem. Biol. 2019, 14, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Mayville, P.; Ji, G.; Beavis, R.; Yang, H.; Goger, M.; Novick, R.P.; Muir, T.W. Structure-activity analysis of synthetic autoinducing thiolactone peptides from Staphylococcus aureus responsible for virulence. Proc. Natl. Acad. Sci. USA 1999, 96, 1218–1223. [Google Scholar] [CrossRef]
- Rehman, Z.U.; Momin, A.A.; Aldehaiman, A.; Irum, T.; Grünberg, R.; Arold, S.T. The exceptionally efficient quorum quenching enzyme LrsL suppresses Pseudomonas aeruginosa biofilm production. Front. Microbiol. 2022, 22, 977673. [Google Scholar] [CrossRef]
- Zhang, Y.; Brackman, G.; Coenye, T. Pitfalls associated with evaluating enzymatic quorum quenching activity: The case of MomL and its effect on Pseudomonas aeruginosa and Acinetobacter baumannii biofilms. PeerJ 2017, 5, e3251. [Google Scholar] [CrossRef]
- Weiland-Bräuer, N.; Malek, I.; Schmitz, R.A. Metagenomic quorum quenching enzymes affect biofilm formation of Candida albicans and Staphylococcus epidermidis. PLoS ONE 2019, 14, e0211366. [Google Scholar] [CrossRef]
- Sompiyachoke, K.; Bravo, J.; Sikdar, R.; Abdullah, J.; Elias, M.H. A novel screening system to characterize and engineer quorum quenching lactonases. Biotechnol. Bioeng. 2025, 122, 922–935. [Google Scholar] [CrossRef]
- Bergonzi, C.; Schwab, M.; Naik, T.; Naik, T.; Daudé, D.; Chabrière, E.; Elias, M. Structural and biochemical characterization of AaL, a quorum quenching lactonase with unusual kinetic properties. Sci. Rep. 2018, 8, 11262. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Momb, J.; Wang, C.; Liu, D.; Thomas, P.W.; Petsko, G.A.; Guo, H.; Ringe, D.; Fast, W. Mechanism of the quorum-quenching lactonase (AiiA) from Bacillus thuringiensis. 2. Substrate modeling and active site mutations. Biochemistry 2008, 47, 7715–7725. [Google Scholar] [CrossRef]
- Efremenko, E.; Aslanli, A.; Domnin, M.; Stepanov, N.; Senko, O. Enzymes with lactonase activity against fungal quorum molecules as effective antifungals. Biomolecules 2024, 14, 383. [Google Scholar] [CrossRef] [PubMed]
- Hiblot, J.; Gotthard, G.; Elias, M.; Chabriere, E. Differential active site loop conformations mediate promiscuous activities in the lactonase SsoPox. PLoS ONE 2013, 8, e75272. [Google Scholar] [CrossRef] [PubMed]
- Aslanli, A.; Lyagin, I.; Efremenko, E. Novel approach to quorum quenching: Rational design of antibacterials in combination with hexahistidine-tagged organophosphorus hydrolase. Biol. Chem. 2018, 399, 869–879. [Google Scholar] [CrossRef]
- Hwang, J.; Yoo, W.; Shin, S.C.; Kim, K.K.; Kim, H.-W.; Do, H.; Lee, J.H. Structural and biochemical insights into bis(2-hydroxyethyl) terephthalate degrading carboxylesterase isolated from psychrotrophic bacterium Exiguobacterium antarcticum. Int. J. Mol. Sci. 2023, 24, 12022. [Google Scholar] [CrossRef]
- Heng, S.; Tieu, W.; Hautmann, S.; Kuan, K.; Pedersen, D.S.; Pietsch, M.; Gütschow, M.; Abell, A.D. New cholesterol esterase inhibitors based on rhodanine and thiazolidinedione scaffolds. Bioorg Med. Chem. 2011, 19, 7453–7463. [Google Scholar] [CrossRef]
- Birkus, G.; Wang, R.; Liu, X.; Kutty, N.; MacArthur, H.; Cihlar, T.; Gibbs, C.; Swaminathan, S.; Lee, W.; McDermott, M. Cathepsin A is the major hydrolase catalyzing the intracellular hydrolysis of the antiretroviral nucleotide phosphonoamidate prodrugs GS-7340 and GS-9131. Antimicrob. Agents Chemother. 2007, 51, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Riley, M.B.; Strandquist, E.; Weitzel, C.S.; Driskell, J.D. Structure and activity of native and thiolated α-chymotrypsin adsorbed onto gold nanoparticles. Colloids Surf. B Biointerfaces 2022, 220, 112867. [Google Scholar] [CrossRef]
- Azrin, N.A.M.; Ali, M.S.M.; Rahman, R.N.Z.R.A.; Oslan, S.N.; Noor, N.D.M. Versatility of subtilisin: A review on structure, characteristics, and applications. Biotechnol. Appl. Biochem. 2022, 69, 2599–2616. [Google Scholar] [CrossRef]
- Hendrixson, D.R.; Qiu, J.; Shewry, S.C.; Fink, D.L.; Petty, S.; Baker, E.N.; Plaut, A.G.; St Geme, J.W., 3rd. Human milk lactoferrin is a serine protease that cleaves Haemophilus surface proteins at arginine-rich sites. Mol. Microbiol. 2003, 47, 607–617. [Google Scholar] [CrossRef]
- Massucci, M.T.; Giansanti, F.; Di Nino, G.; Turacchio, M.; Giardi, M.F.; Botti, D.; Ippoliti, R.; De Giulio, B.; Siciliano, R.A.; Donnarumma, G.; et al. Proteolytic activity of bovine lactoferrin. Biometals 2004, 17, 249–255. [Google Scholar] [CrossRef]
- Carper, W.R.; Mehra, A.S.; Campbell, D.P.; Levisky, J.A. Gluconolactonase: A zinc containing metalloprotein. Experientia 1982, 38, 1046–1047. [Google Scholar] [CrossRef]
- Jakubowski, H. Proteomic Exploration of Paraoxonase 1 Function in Health and Disease. Int. J. Mol. Sci. 2023, 24, 7764. [Google Scholar] [CrossRef] [PubMed]
- Witucki, Ł.; Jakubowski, H. Depletion of paraoxonase 1 (PON1) dysregulates mTOR, autophagy, and accelerates amyloid beta accumulation in mice. Cells 2023, 12, 746. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, S.; Zhang, Y. Catalytic reaction mechanism of acetylcholinesterase determined by Born−Oppenheimer Ab Initio QM/MM molecular dynamics simulations. J. Phys. Chem. B 2010, 114, 8817–8825. [Google Scholar] [CrossRef] [PubMed]
- Aslanli, A.; Domnin, M.; Stepanov, N.; Senko, O.; Efremenko, E. Action enhancement of antimicrobial peptides by their combination with enzymes hydrolyzing fungal quorum molecules. Int. J. Biol. Macromol. 2024, 280, 136066. [Google Scholar] [CrossRef]
- Lewoń-Mrozek, D.; Kurzynoga, J.; Jędrzejewski, P.; Kędzierska, K.; Partyka, A.; Kuriata-Kordek, M.; Ściskalska, M. Molecular structure of paraoxonase-1 and its modifications in relation to enzyme activity and biological functions—A comprehensive review. Int. J. Mol. Sci. 2024, 25, 13129. [Google Scholar] [CrossRef]
- Mongkhoun, E.; Guégan, P.; Illy, N. γ-Thiobutyrolactone-ethylene carbonate decarboxylative copolymerization, an original pathway to prepare aliphatic oxidizable poly(γ-thioether ester). Polym. Chem. 2023, 14, 3729–3738. [Google Scholar] [CrossRef]
- Suzuki, M.; Makimura, K.; Matsuoka, S.-I. Thiol-mediated controlled ring-opening polymerization of cysteine-derived β-thiolactone and unique features of product polythioester. Biomacromolecules 2016, 17, 1135–1141. [Google Scholar] [CrossRef]
- Sivasankaran, R.P.; Snell, K.; Kunkel, G.; Georgiou, P.; Puente, E.G.; Maynard, H.D. Polymer-mediated protein/peptide therapeutic stabilization: Current progress and future directions. Prog. Polym. Sci. 2024, 156, 101867. [Google Scholar] [CrossRef]
- Lyagin, I.V.; Efremenko, E.N. Biomolecular engineering of biocatalysts hydrolyzing neurotoxic organophosphates. Biochimie 2018, 144, 115–121. [Google Scholar] [CrossRef]
- Hu, Y.; Niu, Y.; Ye, X.; Zhu, C.; Tong, T.; Zhou, Y.; Zhou, X.; Cheng, L.; Ren, B. Staphylococcus aureus synergized with Candida albicans to increase the pathogenesis and drug resistance in cutaneous abscess and peritonitis murine models. Pathogens 2021, 10, 1036. [Google Scholar] [CrossRef]
- Carolus, H.; Van Dyck, K.; Van Dijck, P. Candida albicans and Staphylococcus species: A threatening twosome. Front. Microbiol. 2019, 10, 2162. [Google Scholar] [CrossRef]
- Efremenko, E.; Votchitseva, Y.; Plieva, F.; Galaev, I.; Mattiasson, B. Purification of His6-organophosphate hydrolase using monolithic supermacroporous polyacrylamide cryogels developed for immobilized metal affinity chromatography. Appl. Microbiol. Biotechnol. 2006, 70, 558–563. [Google Scholar] [CrossRef]
- Roy, A.; Kucukural, A.; Zhang, Y. I-TASSER: A unified platform for automated protein structure and function prediction. Nat. Protoc. 2010, 5, 725–738. [Google Scholar] [CrossRef] [PubMed]
- Baker, N.A.; Sept, D.; Joseph, S.; Holst, M.J.; McCammon, J.A. Electrostatics of nanosystems: Application to microtubules and the ribosome. Proc. Natl. Acad. Sci. USA 2001, 98, 10037–10041. [Google Scholar] [CrossRef] [PubMed]
- Dolinsky, T.J.; Czodrowski, P.; Li, H.; Nielsen, J.E.; Jensen, J.H.; Klebe, G.; Baker, N.A. PDB2PQR: Expanding and upgrading automated preparation of biomolecular structures for molecular simulations. Nucleic Acids Res. 2007, 35, W522–W525. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and auto-DockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Ugarova, N.; Koksharov, M.; Lomakina, G. Reagent for Adenosine-5-Triphosphate Determination. Patent RU 2420594, 20 May 2009. [Google Scholar]
- Darvesh, S.; Walsh, R.; Martin, E. Homocysteine thiolactone and human cholinesterases. Cell Mol. Neurobiol. 2007, 27, 33–48. [Google Scholar] [CrossRef]
- Bhattacharya, R.; Kumar, T.; Bansal, A.K.; Verma, R.; Sharma, G.S.; Rahaman, H.; Singh, L.R. Inhibition of Protein N-homocysteinylation by proline: A strategy toward the therapeutic intervention of hyperhomocysteinemia. ACS Omega 2025, 10, 27745–27755. [Google Scholar] [CrossRef] [PubMed]
- Jakubowski, H. Homocysteine thiolactone detoxifying enzymes and Alzheimer’s disease. Int. J. Mol. Sci. 2024, 25, 8095. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.-P.; Song, Y.; Cai, Z.-H.; Lin, Z.-J.; Lin, G.-H.; Wang, Y.; Zhou, J. Anti-quorum sensing activities of selected coral symbiotic bacterial extracts from the south China Sea. Front. Cell. Infect. Microbiol. 2018, 8, 144. [Google Scholar] [CrossRef]
- Borowczyk, K.; Tisonczyk, J.; Jakubowski, H. Metabolism and neurotoxicity of homocysteine thiolactone in mice: Protective role of bleomycin hydrolase. Amino Acids 2012, 43, 1339–1348. [Google Scholar] [CrossRef]
- Suszynska, J.; Tisonczyk, J.; Lee, H.G.; Smith, M.A.; Jakubowski, H. Reduced homocysteine-thiolactonase activity in Alzheimer’s disease. J. Alzheimers Dis. 2010, 19, 1177–1183. [Google Scholar] [CrossRef]
- Santus, P.; Signorello, J.C.; Danzo, F.; Lazzaroni, G.; Saad, M.; Radovanovic, D. Anti-inflammatory and anti-oxidant properties of N-acetylcysteine: A fresh perspective. J. Clin. Med. 2024, 13, 4127. [Google Scholar] [CrossRef]
- Hraiech, S.; Hiblot, J.; Lafleur, J.; Lepidi, H.; Papazian, L.; Rolain, J.M.; Elias, M.; Silby, M.W.; Bzdrenga, J.; Bregeon, F.; et al. Inhaled lactonase reduces Pseudomonas aeruginosa Quorum Sensing and mortality in rat pneumonia. PLoS ONE 2014, 9, e107125. [Google Scholar] [CrossRef] [PubMed]
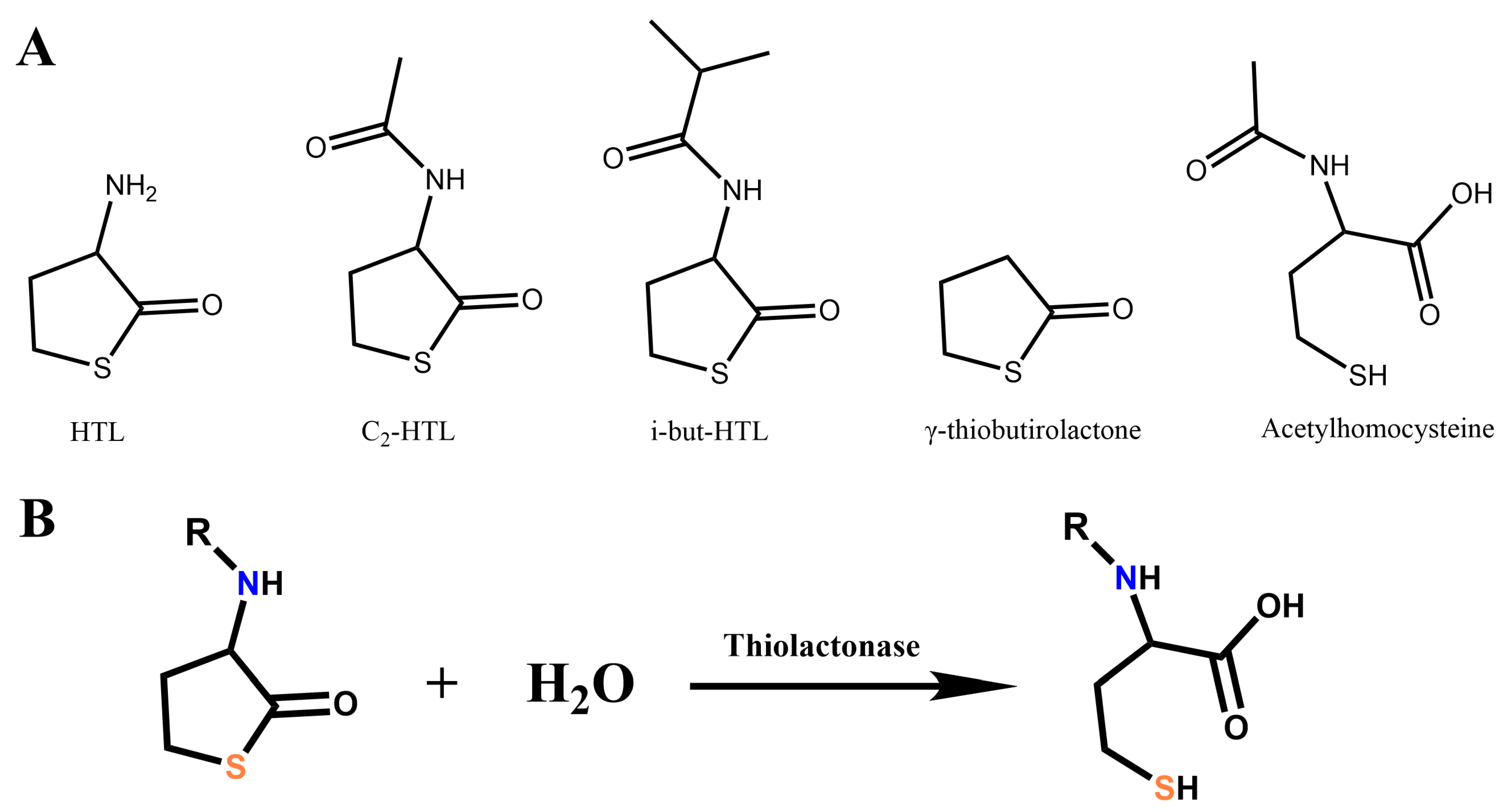
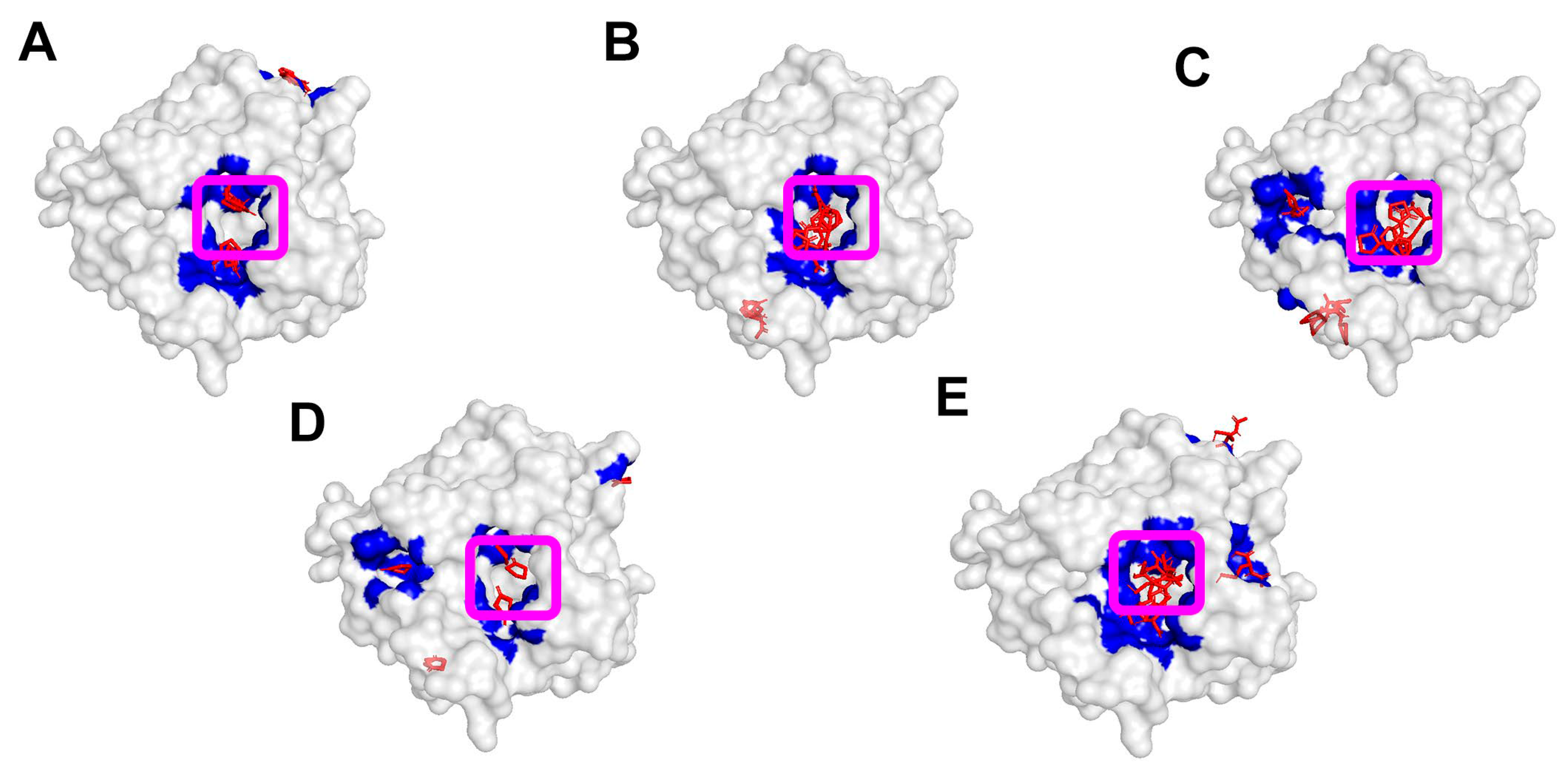
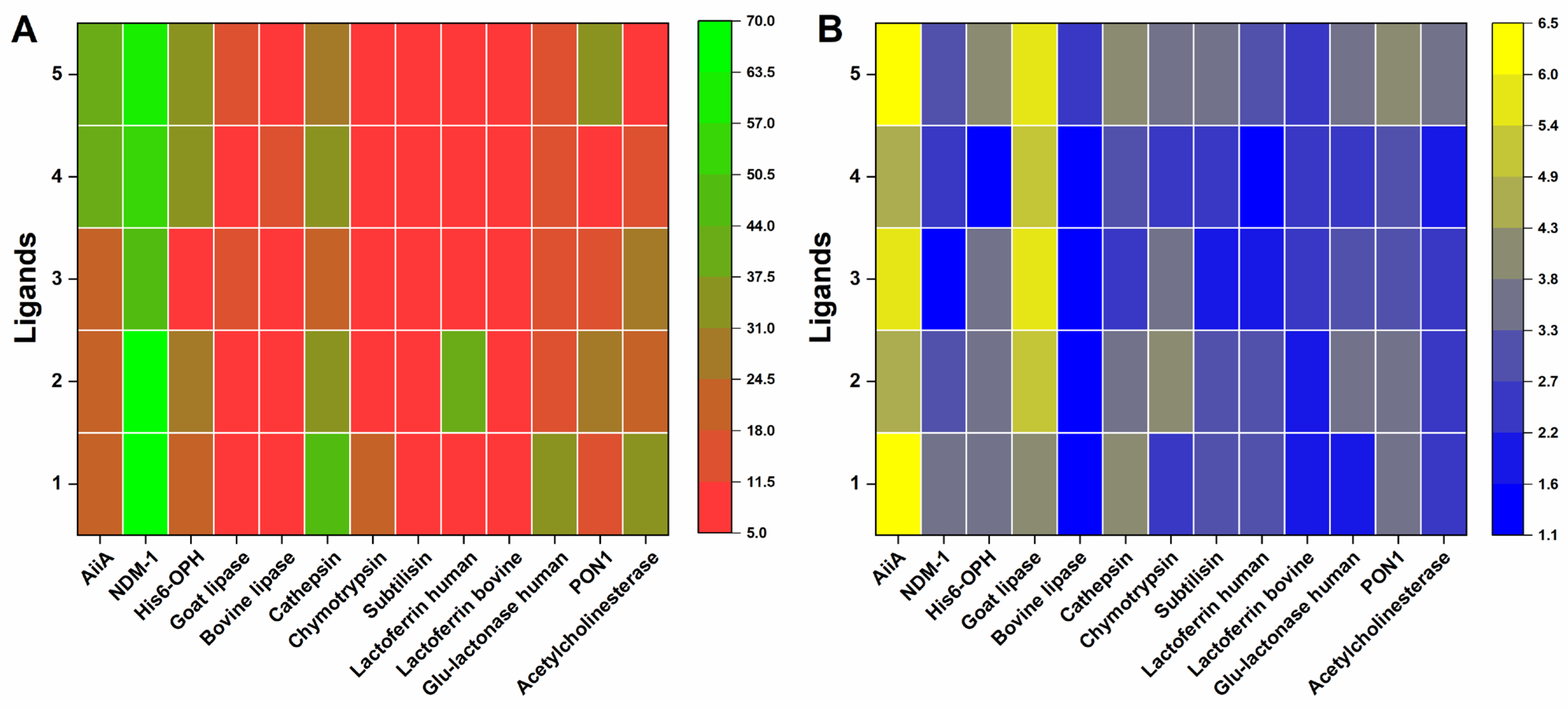
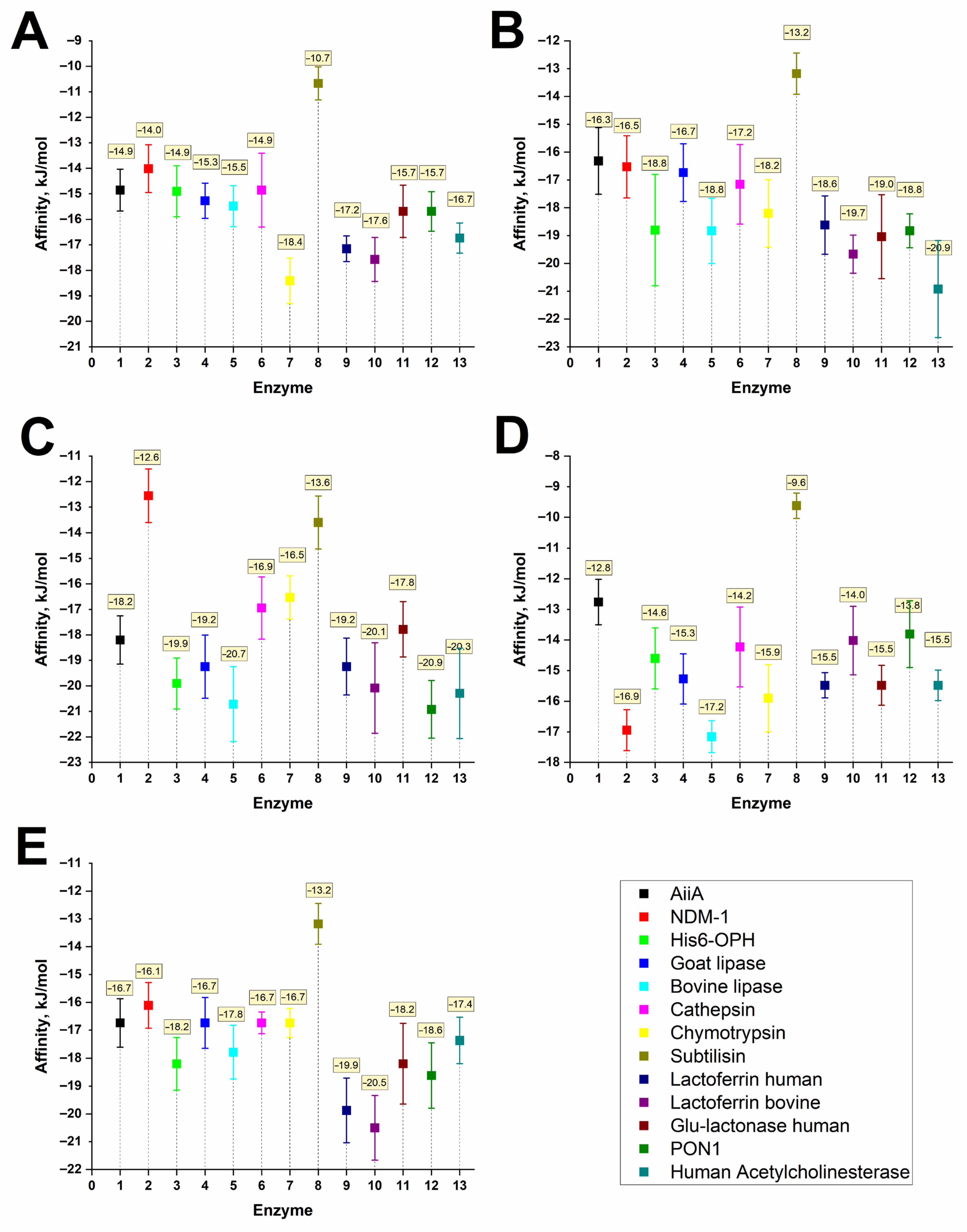
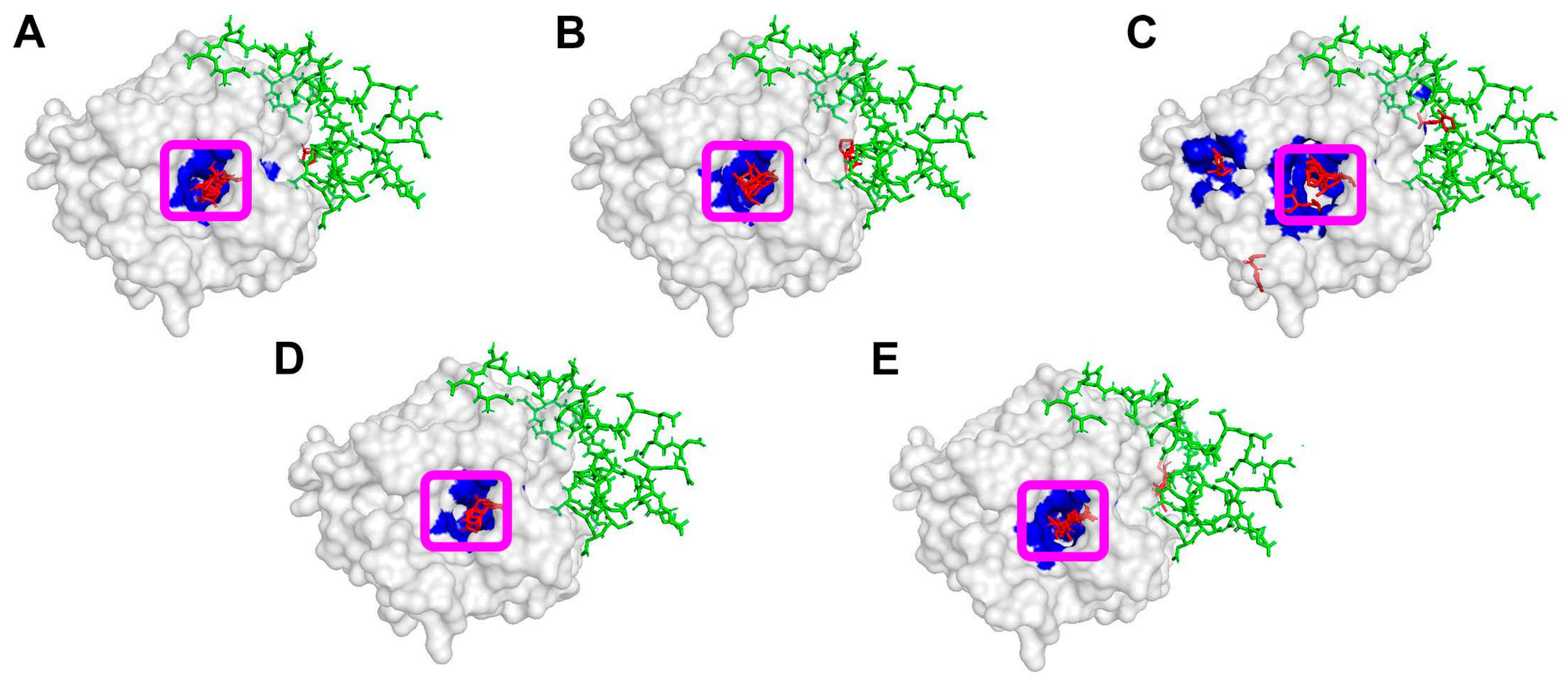

| Group | No. | Enzyme, Origin | PDB ID | Reference |
|---|---|---|---|---|
| Lactonases | 1 | AiiA from Bacillus thuringiensis | 2a7m | [11] |
| 2 | Lactamase NDM-1 from Klebsiella pneumoniae | 5ypi | [13] | |
| 3 | His6-OPH from Pseudomonas diminuta | 1qw7 (OPH) | [14] | |
| Lipases | 4 | Lipase from goat | 6nkg | [15] |
| 5 | Lipase from bovine | 1aql | [16] | |
| Peptidases | 6 | Cathepsin A from human | 4ci9 | [17] |
| 7 | α-chymotrypsin from bovine | 1yph | [18] | |
| 8 | Subtilisin from Bacillus subtilis | 1sbc | [19] | |
| Lactoferrines | 9 | Human lactoferrin | 1lfg | [20] |
| 10 | Lactoferrin from bovine | 1blf | [21] | |
| PON1-like | 11 | Human gluconolactonase | 4gnb | [22] |
| 12 | Human PON1 | 1v04 | [23,24] | |
| Acetylcholinesterase | 13 | Human acetylcholinesterase | 4pqe | [25] |
| Enzyme | Km, mM | Vmax/E0, s−1 | Keff, M−1 s −1 |
|---|---|---|---|
| HTL | |||
| AiiA | na | na | na |
| NDM-1 | 0.26 ± 0.06 | 0.21 ± 0.04 | 826 ± 241 |
| His6–OPH | nd | nd | nd |
| C2–HTL | |||
| AiiA | 0.8 ± 0.3 | 0.11 ± 0.02 | 137 ± 81 |
| NDM-1 | 12 ± 4 | 1.0 ± 0.2 | 80 ± 41 |
| His6–OPH | 3.0 ± 0.5 | 0.010 ± 0.006 | 3.9 ± 0.8 |
| Enzymes and Their Complexes | * Relative Rate of Chemical Reaction, % |
|---|---|
| AiiA | 100 ± 4 |
| AiiA/PLD50 | 111 ± 3 |
| AiiA/PLE50 | 109 ± 4 |
| His6–OPH | 100 ± 3 |
| His6–OPH/PLD50 | 37 ± 5 |
| His6–OPH/PLE50 | 44 ± 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domnin, M.; Sarapina, A.; Aslanli, A.; Senko, O.; Efremenko, E. Novel Enzymes for Biologics with Hydrolytic Activity Against Thiolactones: Computational, Catalytic and Antimicrobial Study. Biologics 2025, 5, 34. https://doi.org/10.3390/biologics5040034
Domnin M, Sarapina A, Aslanli A, Senko O, Efremenko E. Novel Enzymes for Biologics with Hydrolytic Activity Against Thiolactones: Computational, Catalytic and Antimicrobial Study. Biologics. 2025; 5(4):34. https://doi.org/10.3390/biologics5040034
Chicago/Turabian StyleDomnin, Maksim, Anastasia Sarapina, Aysel Aslanli, Olga Senko, and Elena Efremenko. 2025. "Novel Enzymes for Biologics with Hydrolytic Activity Against Thiolactones: Computational, Catalytic and Antimicrobial Study" Biologics 5, no. 4: 34. https://doi.org/10.3390/biologics5040034
APA StyleDomnin, M., Sarapina, A., Aslanli, A., Senko, O., & Efremenko, E. (2025). Novel Enzymes for Biologics with Hydrolytic Activity Against Thiolactones: Computational, Catalytic and Antimicrobial Study. Biologics, 5(4), 34. https://doi.org/10.3390/biologics5040034







