Using the Allen Brain Cell Atlas of the Human Brain to Gain Insights into C-Terminal-Binding Protein 1 (CtBP1)’s Potential Function
Abstract
1. Introduction
2. CtBP Family Proteins and Their Functions
3. CtBP1 and HADDTS
4. ABC Atlas
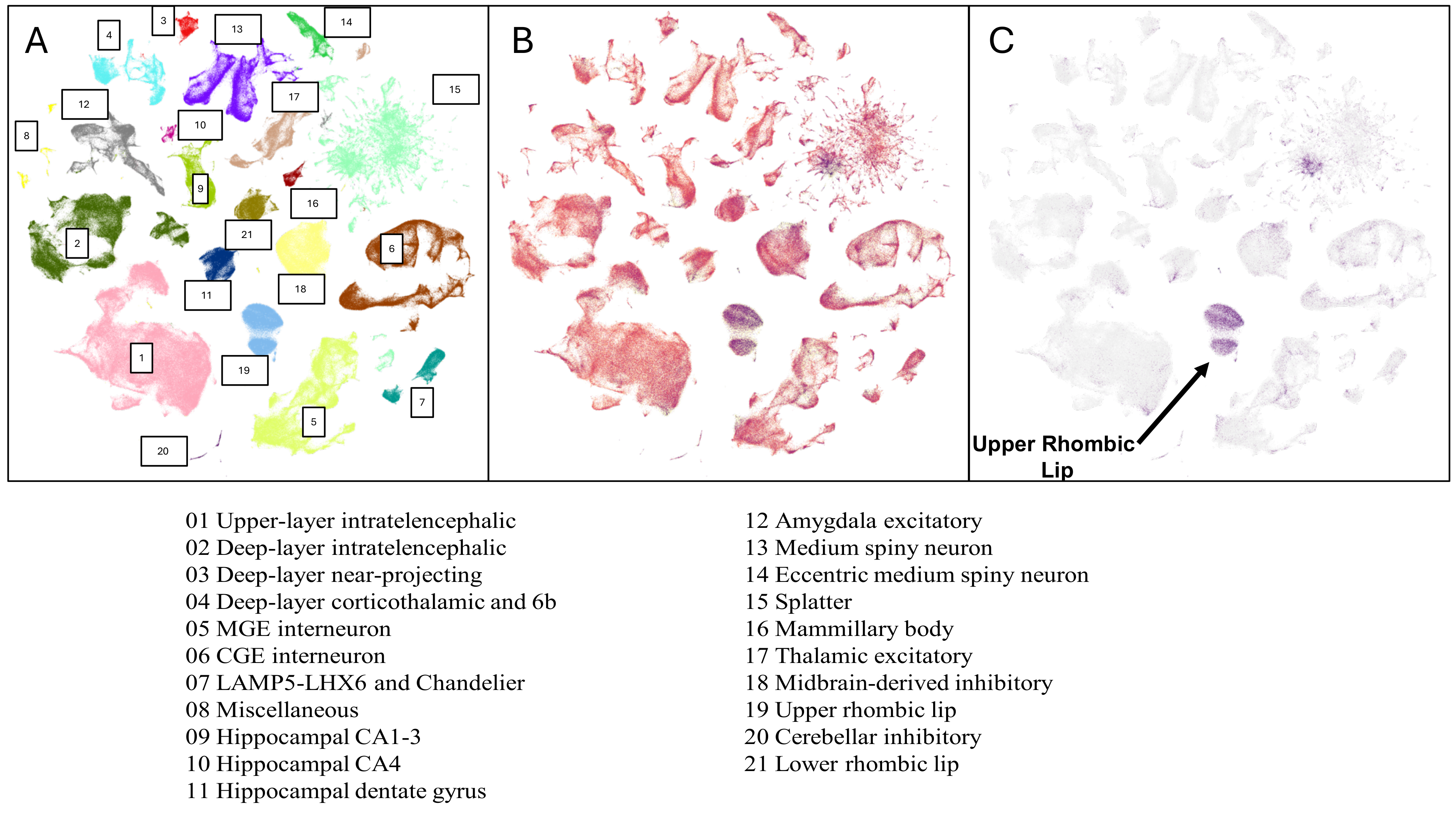
4.1. Step 1: Selection of the Dataset
4.2. Step 2: Identification of Key Cell Properties
4.3. Step 3: Gene Expression Comparison in the URL
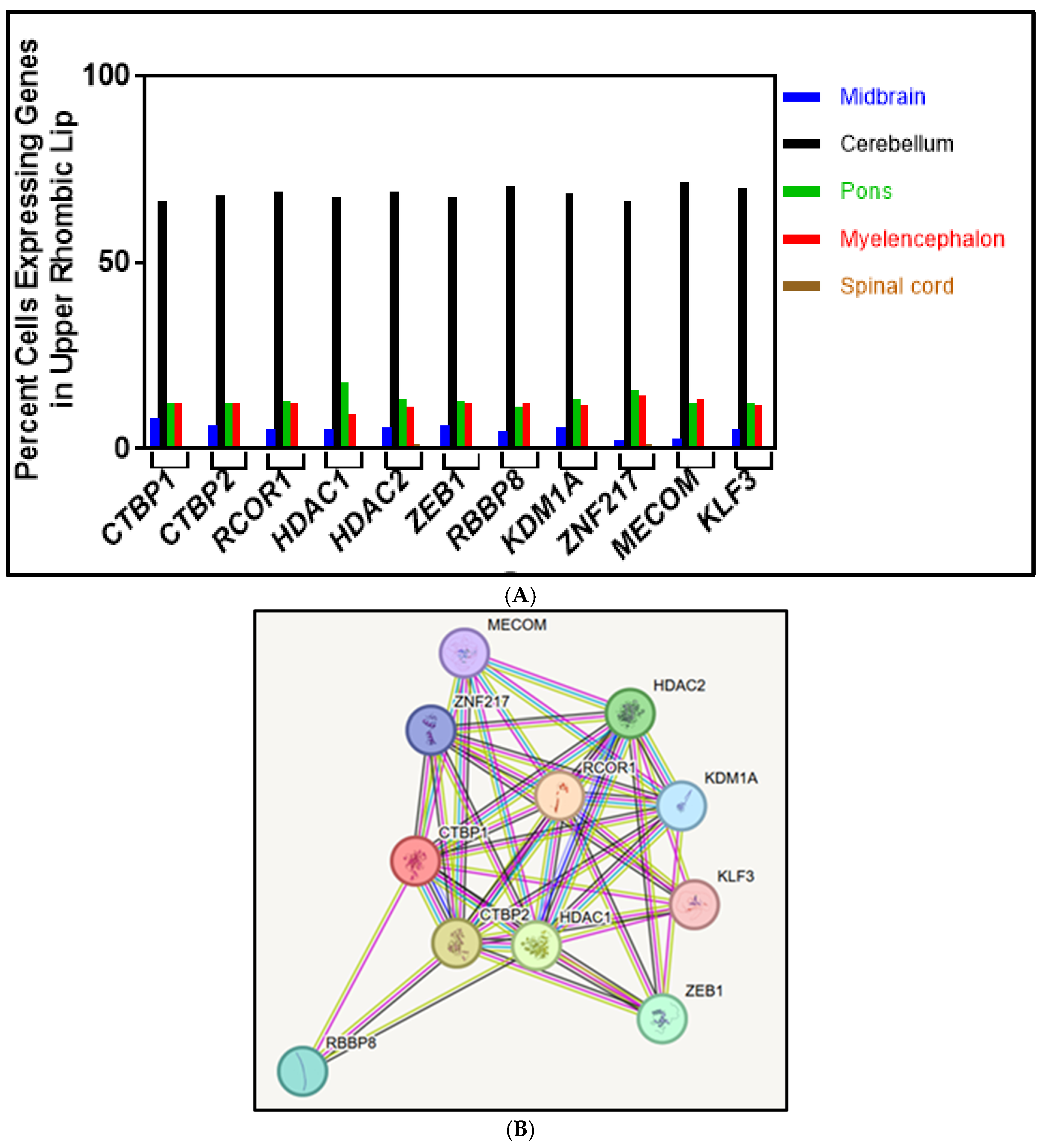
4.4. Step 4: Analysis of CtBP1-Interacting Protein-Coding Genes
5. CtBP1-Interacting Proteins
5.1. CTBP2
5.2. RCOR1
5.3. HDAC1 and HDAC2
5.4. ZEB1
5.5. RBBP8
5.6. KLF3
5.7. KDM1A
5.8. MECOM
5.9. ZNF217
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Beck, D.B.; Cho, M.T.; Millan, F.; Yates, C.; Hannibal, M.; O’Connor, B.; Shinawi, M.; Connolly, A.M.; Waggoner, D.; Halbach, S.; et al. A recurrent de novo CTBP1 mutation is associated with developmental delay, hypotonia, ataxia, and tooth enamel defects. Neurogenetics 2016, 17, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Beck, D.B.; Subramanian, T.; Vijayalingam, S.; Ezekiel, U.R.; Donkervoort, S.; Yang, M.L.; Dubbs, H.A.; Ortiz-Gonzalez, X.R.; Lakhani, S.; Segal, D.; et al. A pathogenic CtBP1 missense mutation causes altered cofactor binding and transcriptional activity. Neurogenetics 2019, 20, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Boyd, J.M.; Subramanian, T.; Schaeper, U.; La Regina, M.; Bayley, S.; Chinnadurai, G. A region in the C-terminus of adenovirus 2/5 E1a protein is required for association with a cellular phosphoprotein and important for the negative modulation of T24-ras mediated transformation, tumorigenesis and metastasis. EMBO J. 1993, 12, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Chinnadurai, G. CtBP, an unconventional transcriptional corepressor in development and oncogenesis. Mol. Cell 2002, 9, 213–224. [Google Scholar] [CrossRef]
- Katsanis, N.; Fisher, E.M. A novel C-terminal binding protein (CTBP2) is closely related to CTBP1, an adenovirus E1A-binding protein, and maps to human chromosome 21q21.3. Genomics 1998, 47, 294–299. [Google Scholar] [CrossRef]
- Blevins, M.A.; Huang, M.; Zhao, R. The Role of CtBP1 in Oncogenic Processes and Its Potential as a Therapeutic Target. Mol. Cancer Ther. 2017, 16, 981–990. [Google Scholar] [CrossRef]
- Fjeld, C.C.; Birdsong, W.T.; Goodman, R.H. Differential binding of NAD+ and NADH allows the transcriptional corepressor carboxyl-terminal binding protein to serve as a metabolic sensor. Proc. Natl. Acad. Sci. USA 2003, 100, 9202–9207. [Google Scholar] [CrossRef]
- Jaiswal, A.; Singh, R. CtBP: A global regulator of balancing acts and homeostases. Biochim. Biophys. Acta Rev. Cancer 2023, 1878, 188886. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Wang, L.; Hao, D.; Li, P.; Su, M.; Zhao, Z.; Liu, T.; Tai, L.; Lu, J.; et al. Metabolic modulation of CtBP dimeric status impacts the repression of DNA damage repair genes and the platinum sensitivity of ovarian cancer. Int. J. Biol. Sci. 2023, 19, 2081–2096. [Google Scholar] [CrossRef]
- Bhambhani, C.; Chang, J.L.; Akey, D.L.; Cadigan, K.M. The oligomeric state of CtBP determines its role as a transcriptional co-activator and co-repressor of Wingless targets. EMBO J. 2011, 30, 2031–2043. [Google Scholar] [CrossRef]
- Hildebrand, J.D.; Soriano, P. Overlapping and unique roles for C-terminal binding protein 1 (CtBP1) and CtBP2 during mouse development. Mol. Cell Biol. 2002, 22, 5296–5307. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, C.; Lopes-Nunes, J.; Esteves, M.; Santos, T.; Vale, A.; Cristovao, A.C.; Ferreira, R.; Bernardino, L. CtBP Neuroprotective Role in Toxin-Based Parkinson’s Disease Models: From Expression Pattern to Dopaminergic Survival. Mol. Neurobiol. 2023, 60, 4246–4260. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Li, Y.; Yu, H.; Hu, Y. CTBP1 Confers Protection for Hippocampal and Cortical Neurons in Rat Models of Alzheimer’s Disease. Neuroimmunomodulation 2019, 26, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Stankiewicz, T.R.; Schroeder, E.K.; Kelsey, N.A.; Bouchard, R.J.; Linseman, D.A. C-terminal binding proteins are essential pro-survival factors that undergo caspase-dependent downregulation during neuronal apoptosis. Mol. Cell Neurosci. 2013, 56, 322–332. [Google Scholar] [CrossRef]
- Karaca, E.; Li, X.; Lewicki, J.; Neofytou, C.; Guerout, N.; Barnabe-Heider, F.; Hermanson, O. The corepressor CtBP2 is required for proper development of the mouse cerebral cortex. Mol. Cell Neurosci. 2020, 104, 103481. [Google Scholar] [CrossRef]
- Hubler, D.; Rankovic, M.; Richter, K.; Lazarevic, V.; Altrock, W.D.; Fischer, K.D.; Gundelfinger, E.D.; Fejtova, A. Differential spatial expression and subcellular localization of CtBP family members in rodent brain. PLoS ONE 2012, 7, e39710. [Google Scholar] [CrossRef]
- Li, H.; Zhang, C.; Yang, C.; Blevins, M.; Norris, D.; Zhao, R.; Huang, M. C-terminal binding proteins 1 and 2 in traumatic brain injury-induced inflammation and their inhibition as an approach for anti-inflammatory treatment. Int. J. Biol. Sci. 2020, 16, 1107–1120. [Google Scholar] [CrossRef]
- Liang, H.; Fekete, D.M.; Andrisani, O.M. CtBP2 downregulation during neural crest specification induces expression of Mitf and REST, resulting in melanocyte differentiation and sympathoadrenal lineage suppression. Mol. Cell Biol. 2011, 31, 955–970. [Google Scholar] [CrossRef]
- Acosta-Baena, N.; Tejada-Moreno, J.A.; Arcos-Burgos, M.; Villegas-Lanau, C.A. CTBP1 and CTBP2 mutations underpinning neurological disorders: A systematic review. Neurogenetics 2022, 23, 231–240. [Google Scholar] [CrossRef]
- Sommerville, E.W.; Alston, C.L.; Pyle, A.; He, L.; Falkous, G.; Naismith, K.; Chinnery, P.F.; McFarland, R.; Taylor, R.W. De novo CTBP1 variant is associated with decreased mitochondrial respiratory chain activities. Neurol. Genet. 2017, 3, e187. [Google Scholar] [CrossRef]
- Jafari Khamirani, H.; Zoghi, S.; Saber Sichani, A.; Dianatpour, M.; Mohammadi, S.; Mohammad Bagher Tabei, S.; Alireza Dastgheib, S. Exome sequencing identified a de novo frameshift pathogenic variant of CTBP1 in an extremely rare case of HADDTS. J. Genet. 2021, 100, 68. [Google Scholar] [CrossRef] [PubMed]
- Allen Institute for Brain Science. Allen Brain Cell Atlas [Transcriptomic Diversity of Cell Types in Adult Human Brain Dataset]. 2011. Available online: https://knowledge.brain-map.org/abcatlas (accessed on 23 December 2024).
- Zaldivar, A.; Krichmar, J.L. Allen Brain Atlas-Driven Visualizations: A web-based gene expression energy visualization tool. Front. Neuroinform. 2014, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Siletti, K.; Hodge, R.; Mossi Albiach, A.; Lee, K.W.; Ding, S.L.; Hu, L.; Lonnerberg, P.; Bakken, T.; Casper, T.; Clark, M.; et al. Transcriptomic diversity of cell types across the adult human brain. Science 2023, 382, eadd7046. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: Protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef]
- Acevedo-Triana, C.A.; Leon, L.A.; Cardenas, F.P. Comparing the Expression of Genes Related to Serotonin (5-HT) in C57BL/6J Mice and Humans Based on Data Available at the Allen Mouse Brain Atlas and Allen Human Brain Atlas. Neurol. Res. Int. 2017, 2017, 7138926. [Google Scholar] [CrossRef]
- Consalez, G.G.; Goldowitz, D.; Casoni, F.; Hawkes, R. Origins, Development, and Compartmentation of the Granule Cells of the Cerebellum. Front. Neural Circuits 2020, 14, 611841. [Google Scholar] [CrossRef]
- Gilthorpe, J.D.; Papantoniou, E.K.; Chedotal, A.; Lumsden, A.; Wingate, R.J. The migration of cerebellar rhombic lip derivatives. Development 2002, 129, 4719–4728. [Google Scholar] [CrossRef]
- Casoni, F.; Croci, L.; Marroni, F.; Demenego, G.; Marullo, C.; Cremona, O.; Codazzi, F.; Consalez, G.G. A spatial-temporal map of glutamatergic neurogenesis in the murine embryonic cerebellar nuclei uncovers a high degree of cellular heterogeneity. J. Anat. 2024, 245, 560–571. [Google Scholar] [CrossRef]
- Oishi, K.; Chotiyanonta, J.; Wu, D.; Miller, M.I.; Mori, S.; Oishi, K.; Pediatric Imaging, N.; Genetics, S. Developmental trajectories of the human embryologic brain regions. Neurosci. Lett. 2019, 708, 134342. [Google Scholar] [CrossRef]
- Hagan, N.; Zervas, M. Wnt1 expression temporally allocates upper rhombic lip progenitors and defines their terminal cell fate in the cerebellum. Mol. Cell Neurosci. 2012, 49, 217–229. [Google Scholar] [CrossRef]
- Vijayalingam, S.; Ezekiel, U.R.; Xu, F.; Subramanian, T.; Geerling, E.; Hoelscher, B.; San, K.; Ganapathy, A.; Pemberton, K.; Tycksen, E.; et al. Human iPSC-Derived Neuronal Cells From CTBP1-Mutated Patients Reveal Altered Expression of Neurodevelopmental Gene Networks. Front. Neurosci. 2020, 14, 562292. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, Q.; Dang, X.; Fan, J.; Song, T.; Li, Z.; Duan, N.; Zhang, W. The CtIP-CtBP1/2-HDAC1-AP1 transcriptional complex is required for the transrepression of DNA damage modulators in the pathogenesis of osteosarcoma. Transl. Oncol. 2022, 21, 101429. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.; Wilson, D.M., 3rd. The involvement of DNA-damage and -repair defects in neurological dysfunction. Am. J. Hum. Genet. 2008, 82, 539–566. [Google Scholar] [CrossRef] [PubMed]
- Pakula, A.; Nagar, S.E.; Sumru Bayin, N.; Christensen, J.B.; Stephen, D.N.; Reid, A.J.; Koche, R.; Joyner, A.L. An increase in reactive oxygen species underlies neonatal cerebellum repair. bioRxiv 2024. [Google Scholar] [CrossRef]
- Takashima, H.; Boerkoel, C.F.; John, J.; Saifi, G.M.; Salih, M.A.; Armstrong, D.; Mao, Y.; Quiocho, F.A.; Roa, B.B.; Nakagawa, M.; et al. Mutation of TDP1, encoding a topoisomerase I-dependent DNA damage repair enzyme, in spinocerebellar ataxia with axonal neuropathy. Nat. Genet. 2002, 32, 267–272. [Google Scholar] [CrossRef]
- Lee, Y.; Katyal, S.; Li, Y.; El-Khamisy, S.F.; Russell, H.R.; Caldecott, K.W.; McKinnon, P.J. The genesis of cerebellar interneurons and the prevention of neural DNA damage require XRCC1. Nat. Neurosci. 2009, 12, 973–980. [Google Scholar] [CrossRef]
- Monestime, C.M.; Taibi, A.; Gates, K.P.; Jiang, K.; Sirotkin, H.I. CoRest1 regulates neurogenesis in a stage-dependent manner. Dev. Dyn. 2019, 248, 918–930. [Google Scholar] [CrossRef]
- Macinkovic, I.; Theofel, I.; Hundertmark, T.; Kovac, K.; Awe, S.; Lenz, J.; Forne, I.; Lamp, B.; Nist, A.; Imhof, A.; et al. Distinct CoREST complexes act in a cell-type-specific manner. Nucleic Acids Res. 2019, 47, 11649–11666. [Google Scholar] [CrossRef]
- Wang, H.; Xiao, Z.; Zheng, J.; Wu, J.; Hu, X.L.; Yang, X.; Shen, Q. ZEB1 Represses Neural Differentiation and Cooperates with CTBP2 to Dynamically Regulate Cell Migration during Neocortex Development. Cell Rep. 2019, 27, 2335–2353.e6. [Google Scholar] [CrossRef]
- Moore, D.L.; Apara, A.; Goldberg, J.L. Kruppel-like transcription factors in the nervous system: Novel players in neurite outgrowth and axon regeneration. Mol. Cell Neurosci. 2011, 47, 233–243. [Google Scholar] [CrossRef]
- Laurent, B.; Ruitu, L.; Murn, J.; Hempel, K.; Ferrao, R.; Xiang, Y.; Liu, S.; Garcia, B.A.; Wu, H.; Wu, F.; et al. A specific LSD1/KDM1A isoform regulates neuronal differentiation through H3K9 demethylation. Mol. Cell 2015, 57, 957–970. [Google Scholar] [CrossRef] [PubMed]
- Katoh, M.; Katoh, M. Notch ligand, JAG1, is evolutionarily conserved target of canonical WNT signaling pathway in progenitor cells. Int. J. Mol. Med. 2006, 17, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Leszczynski, P.; Smiech, M.; Parvanov, E.; Watanabe, C.; Mizutani, K.I.; Taniguchi, H. Emerging Roles of PRDM Factors in Stem Cells and Neuronal System: Cofactor Dependent Regulation of PRDM3/16 and FOG1/2 (Novel PRDM Factors). Cells 2020, 9, 2603. [Google Scholar] [CrossRef] [PubMed]
- Izutsu, K.; Kurokawa, M.; Imai, Y.; Maki, K.; Mitani, K.; Hirai, H. The corepressor CtBP interacts with Evi-1 to repress transforming growth factor beta signaling. Blood 2001, 97, 2815–2822. [Google Scholar] [CrossRef]
- Quinlan, K.G.; Nardini, M.; Verger, A.; Francescato, P.; Yaswen, P.; Corda, D.; Bolognesi, M.; Crossley, M. Specific recognition of ZNF217 and other zinc finger proteins at a surface groove of C-terminal binding proteins. Mol. Cell Biol. 2006, 26, 8159–8172. [Google Scholar] [CrossRef]
- Banck, M.S.; Li, S.; Nishio, H.; Wang, C.; Beutler, A.S.; Walsh, M.J. The ZNF217 oncogene is a candidate organizer of repressive histone modifiers. Epigenet. Off. J. DNA Methylation Soc. 2009, 4, 100–106. [Google Scholar] [CrossRef]
- Cohen, P.A.; Donini, C.F.; Nguyen, N.T.; Lincet, H.; Vendrell, J.A. The dark side of ZNF217, a key regulator of tumorigenesis with powerful biomarker value. Oncotarget 2015, 6, 41566–41581. [Google Scholar] [CrossRef]
- Hamada, N.; Matsuki, T.; Iwamoto, I.; Nishijo, T.; Noda, M.; Tabata, H.; Nakayama, A.; Nagata, K.I. Expression analyses of C-terminal-binding protein 1 (CtBP1) during mouse brain development. Dev. Neurosci. 2023, 46, 262–272. [Google Scholar] [CrossRef]
- Ivanova, D.; Dirks, A.; Montenegro-Venegas, C.; Schone, C.; Altrock, W.D.; Marini, C.; Frischknecht, R.; Schanze, D.; Zenker, M.; Gundelfinger, E.D.; et al. Synaptic activity controls localization and function of CtBP1 via binding to Bassoon and Piccolo. EMBO J. 2015, 34, 1056–1077. [Google Scholar] [CrossRef]
- Ivanova, D.; Imig, C.; Camacho, M.; Reinhold, A.; Guhathakurta, D.; Montenegro-Venegas, C.; Cousin, M.A.; Gundelfinger, E.D.; Rosenmund, C.; Cooper, B.; et al. CtBP1-Mediated Membrane Fission Contributes to Effective Recycling of Synaptic Vesicles. Cell Rep. 2020, 30, 2444–2459.e7. [Google Scholar] [CrossRef]
- Jacobi, H.; Faber, J.; Timmann, D.; Klockgether, T. Update cerebellum and cognition. J. Neurol. 2021, 268, 3921–3925. [Google Scholar] [CrossRef]
- Yuan, Q.; Li, H.; Du, B.; Dang, Q.; Chang, Q.; Zhang, Z.; Zhang, M.; Ding, G.; Lu, C.; Guo, T. The cerebellum and cognition: Further evidence for its role in language control. Cereb. Cortex 2022, 33, 35–49. [Google Scholar] [CrossRef]
| Number of Cells Expressing Genes | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Anatomical Region | CTBP1 | CTBP2 | RCOR1 | HDAC1 | HDAC2 | ZEB1 | RBBB8 | KDM1A | ZNF217 | MECOM | KLF3 |
| Cerebral Cortex | 10 | 20 | 9 | 0 | 13 | 52 | 12 | 17 | 0 | 0 | 6 |
| Hippocampus | 1 | 7 | 1 | 0 | 1 | 10 | 1 | 4 | 0 | 0 | 0 |
| Amygdaloid Complex | 5 | 6 | 2 | 0 | 3 | 5 | 1 | 4 | 0 | 0 | 1 |
| Extended Amygdala | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Basal Nuclei | 18 | 68 | 20 | 0 | 18 | 61 | 4 | 25 | 0 | 0 | 3 |
| Claustrum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Basal Forebrain | 2 | 2 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 |
| Thalamus | 19 | 51 | 15 | 0 | 18 | 58 | 5 | 28 | 0 | 0 | 6 |
| Hypothalamus | 21 | 68 | 30 | 0 | 20 | 76 | 7 | 42 | 0 | 0 | 14 |
| Midbrain | 1610 | 4000 | 1270 | 46 | 1470 | 4490 | 493 | 1810 | 4 | 21 | 476 |
| Cerebellum | 205,000 | 48,600 | 16,500 | 623 | 18,100 | 49,000 | 7040 | 21,700 | 112 | 587 | 6250 |
| Pons | 3420 | 8650 | 3020 | 164 | 3490 | 9470 | 1140 | 4210 | 27 | 99 | 1110 |
| Myelencephalon | 3110 | 8340 | 2950 | 86 | 2970 | 9040 | 1230 | 3720 | 24 | 109 | 1050 |
| Spinal Cord | 134 | 403 | 131 | 3 | 138 | 427 | 59 | 149 | 2 | 4 | 42 |
| Total Cells | 21,335 | 70,216 | 23,949 | 922 | 26,242 | 72,690 | 9992 | 31,709 | 169 | 820 | 8959 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Ezekiel, U.R. Using the Allen Brain Cell Atlas of the Human Brain to Gain Insights into C-Terminal-Binding Protein 1 (CtBP1)’s Potential Function. Biologics 2025, 5, 14. https://doi.org/10.3390/biologics5020014
Lee S, Ezekiel UR. Using the Allen Brain Cell Atlas of the Human Brain to Gain Insights into C-Terminal-Binding Protein 1 (CtBP1)’s Potential Function. Biologics. 2025; 5(2):14. https://doi.org/10.3390/biologics5020014
Chicago/Turabian StyleLee, Suhjin, and Uthayashanker R. Ezekiel. 2025. "Using the Allen Brain Cell Atlas of the Human Brain to Gain Insights into C-Terminal-Binding Protein 1 (CtBP1)’s Potential Function" Biologics 5, no. 2: 14. https://doi.org/10.3390/biologics5020014
APA StyleLee, S., & Ezekiel, U. R. (2025). Using the Allen Brain Cell Atlas of the Human Brain to Gain Insights into C-Terminal-Binding Protein 1 (CtBP1)’s Potential Function. Biologics, 5(2), 14. https://doi.org/10.3390/biologics5020014







