Abstract
Background. Although biosimilars have been increasingly used over recent years, some concerns about a potential loss of efficacy and altered safety profile when switching from an originator to a biosimilar still exist. Interchangeability can be a challenge for dermatologists too. An extensive systematic review of published switching studies among originators and biosimilars was carried out in order to provide evidence regarding the effects derived from the switch in terms of efficacy and safety outcomes in real-life contexts. Results. Thirty-seven articles were included in this systematic review (14 studies related to adalimumab, 10 to etanercept, 12 to infliximab, and 1 each to adalimumab, etanercept, and infliximab). Studies were mainly carried out among European countries. Most of them were observational studies or register-based studies. The majority of studies enrolled patients diagnosed with psoriasis or psoriatic arthritis who underwent a single switch from the originator to the biosimilar. Overall, the studies’ results demonstrated that switching between adalimumab, etanercept, and infliximab originators and biosimilars is safe and effective in a real-life setting of patients with dermatological conditions. Only a few studies highlighted an increase in the risk of loss of efficacy as well as an increased rate of AEs, both of which were identified as the main causes of biosimilar discontinuation, probably associated with the well-known phenomenon of the nocebo effect. Conclusion. Switching from a biologic originator to its biosimilar is safe and effective. Only a few studies have evaluated the switch among biosimilars; thus, no firm conclusion can be drawn for this type of switch in terms of the efficacy and safety outcomes. Based on our results, we believe that biosimilars can be considered interchangeable with their reference products and that no additional switch studies are necessary to support switching among originators and biosimilars in clinical practice. However, the continuous monitoring of all biologics (both originators and biosimilars) in routine clinical practice is strongly needed given their peculiar safety profile.
1. Introduction
Across the European Union (EU) countries, biosimilars started to receive the marketing authorization in 2006. Since that date and up to January 2023, 86 biosimilar medicines received the marketing approval by the European Commission (EC). At the beginning, biosimilars were mainly represented by copies of small therapeutic proteins, such as hormones (somatropin) and growth factors (filgrastim and epoetin). Instead, in recent years in the European market, there has been a gradual increase in the access to more complex biosimilars (monoclonal antibodies—mAbs—and fusion proteins) used for the treatment of rheumatic, dermatological, gastroenterological, and hematological disorders (including neoplastic diseases) [1]. Biosimilars currently approved for the treatment of dermatologic disorders (psoriasis, psoriatic arthritis—PsA, hidradenitis suppurativa—HS, and pemphigus vulgaris) include those bearing etanercept, adalimumab, infliximab, and rituximab [2]. Apart from etanercept, which is a fusion protein that blocks the tumor necrosis factor alpha (TNF-α), the other biosimilars are mAbs. In particular, infliximab is a chimeric mAb that blocks TNF-α, rituximab is a chimeric mAb acting against CD-20, while adalimumab is a human mAb that blocks TNF-α [3,4]. TNFα is a pro-inflammatory cytokine that promotes inflammation, through the increase of inflammatory cells entry into lesional skin, the stimulation of keratinocyte production, and the activation of dermal macrophages and dendritic cells [5]. This inflammatory pathway explains the complex alterations in epidermal growth of patients affected by psoriasis who have high levels of TNF-α in both blood and lesional skin [6].
In EU countries, the development of biosimilars for regulatory purposes follows a well-defined step-wise approach, the so-called comparability exercise, which assess and compare the Quality, Nonclinical and Clinical aspects of new biosimilars with their respective reference products [7,8,9]. This process consists of three main phases. The first phase aims to demonstrate the comparability between the biosimilar and the reference product in terms of quality, through the analysis of potential differences in the structure, physicochemical properties, impurities, and variability from batch to batch. In this phase, the manufacturing process, features related to protein binding, signal transduction, and functional activity are assessed as well. It is not expected that the biosimilar and the reference product will be identical in terms of all quality attributes, but if differences are detected, the marketing authorization holder should demonstrate that they have no impact on the clinical performance of the biosimilar [10]. The second phase of the comparability exercise aims to evaluate pharmacokinetics, pharmacodynamics, and toxicological aspects of the new biosimilar. These studies include the conduction of immunogenicity tests. Lastly, the clinical comparability is assessed in the last phase when phase I and III clinical studies are performed [8,9]. Thus, according to the European regulatory framework on biosimilars, the conduction of switching studies demonstrating the interchangeability before the marketing authorization of biosimilars is not required. As recently reported in a joint statement released by the European Medicines Agency (EMA) and Heads of Medicines Agencies (HMA) in September 2022, based on the experience gained in the clinical practice over the past years on biosimilars and their reference products, they should be considered as interchangeable considering that they are comparable in terms of efficacy, safety, and immunogenicity [11]. Even though biosimilars have been increasingly used over recent years, patients and certain clinicians are still concerned about a potential loss of efficacy and altered safety profile when switching to a biosimilar [12]. Concerns are mainly related to the non-medical switch that could be performed for situations not related to the drug’s efficacy, safety, or other medical reasons. Interchangeability is a major challenge for dermatologists too, who are dealing with the choice to switch from an originator to a biosimilar in patients with an established treatment.
Here, an extensive systematic review of published switching studies was carried out in order to provide evidence regarding the effects derived from the switch between originators and biosimilars in terms of the efficacy and safety outcomes.
2. Results
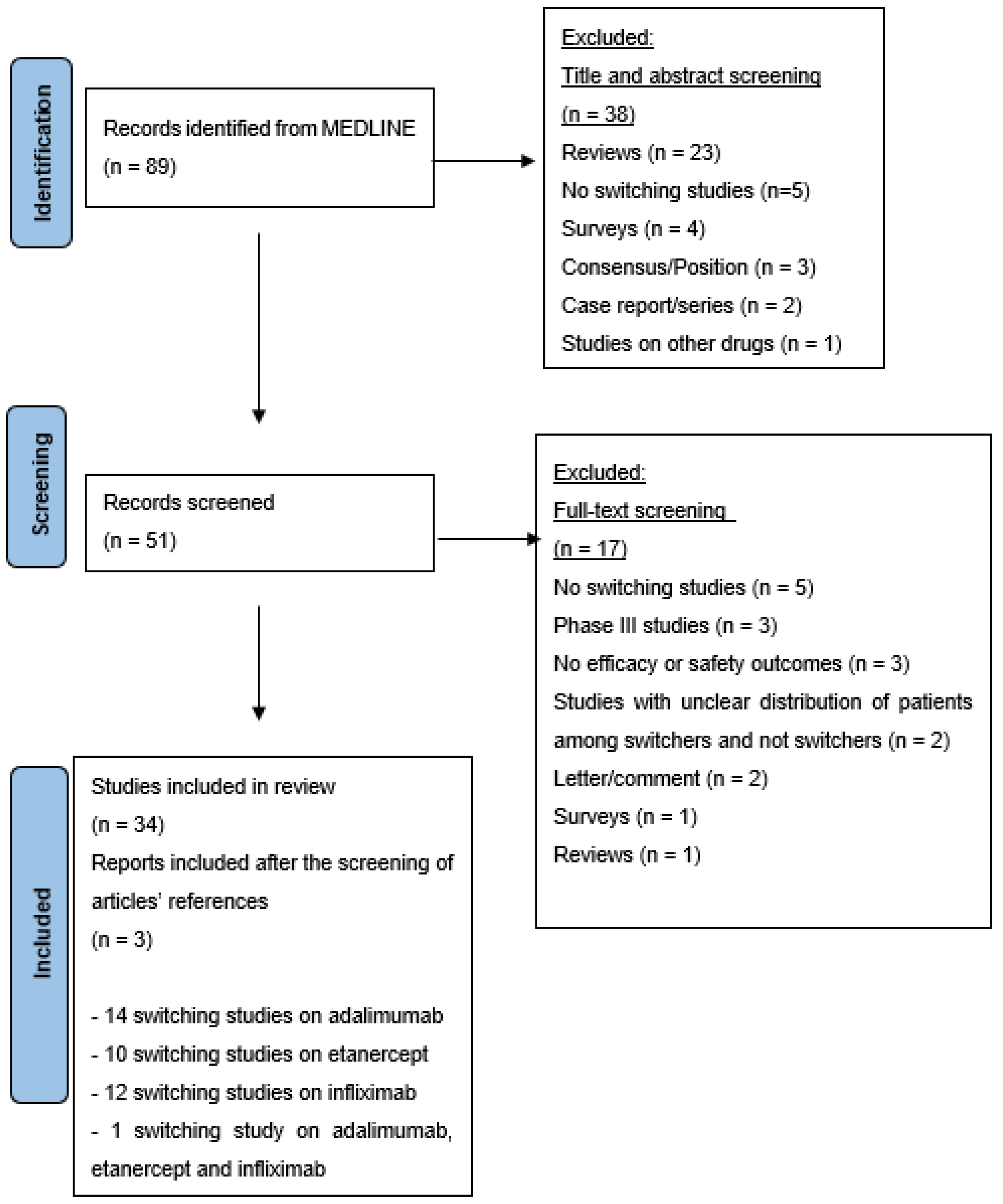
A systematic search yielded 89 citations. Following an initial screening of the title and abstract, 50 records were chosen, and a more thorough examination yielded 34 citations [13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46]. In addition, both authors (MMN and CS) agreed regarding the inclusion of 3 additional publications based on the screening of the articles’ references [47,48,49]. Overall, 37 articles were included in this systematic review (14 studies related to adalimumab, 10 to etanercept, 12 to infliximab, and 1 each to adalimumab, etanercept and infliximab) (Figure 1). No switching studies among rituximab products in dermatology were found.
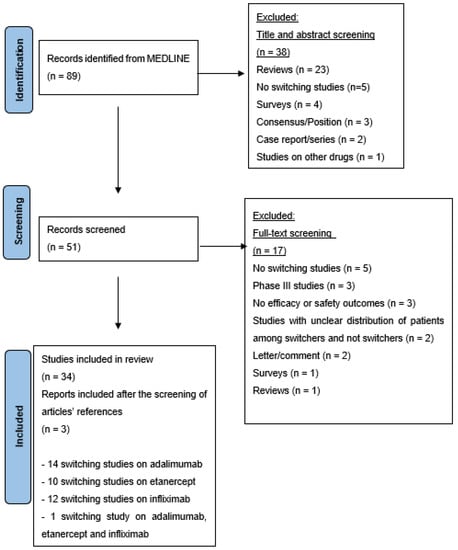
Figure 1.
PRISMA flow chart illustrating literature search outcomes.
The main characteristics of switching studies included in this review are reported in Table 1. In Table 2, an overview of the outcomes and results of the included studies has been reported.

Table 1.
Overview of switching studies included in the systematic review.

Table 2.
Outcome and main results of switching studies included in the systematic review.
2.1. Adalimumab Switching Studies
Fourteen studies [13,14,15,16,17,18,19,20,21,22,23,24,25,49] evaluated the effects in terms of the efficacy and safety derived from the switch among adalimumab products. All studies were carried out in Europe (10/14 in Italy). Taken together, the included studies enrolled 3791 patients, of whom 1504 suffered from dermatologic disorders [PsA and HS (5 studies each) and psoriasis (4 studies)]. The follow-up duration of included studies ranged from 12 weeks to 1 year.
Overall, the majority of studies evaluating the switch among adalimumab products indicated a similarity in their efficacy and safety profiles. For instance, in the observational retrospective study BIRRA (BIologics Retention Rate Assessment) [13], which enrolled 1046 patients treated with adalimumab (193 patients switched from the originator to the biosimilar), authors found that switchers were more persistent than patients receiving the originator and naïve patients receiving the biosimilar. Similarly, Bruni C et al. [14] reported no difference in the control of disease activity, everyday life disability, and safety after switching from the adalimumab originator to SB5 in patients with PsA. Another recent study carried out by the same researchers [15] on a population of 172 patients (59 with PsA) showed that patients who had switched from the adalimumab originator to SB5 maintained a satisfactory level of persistence in treatment. Among patients who switched back to the originator, there were 3 patients with PsA who achieved disease control once switched back. Di Cesare A et al. [49], who described the effects of adalimumab switch among 20 patients with psoriasis, reported no changes in the psoriasis area severity index (PASI) score in 90% of patients who were switched from the originator. In 2 patients, the loss of efficacy on cutaneous symptoms led to biosimilar discontinuation and shift to anti-IL-12/23 (one case) and to adjuvant methotrexate (one case). No significant differences in PASI and the Visual Analogue Scale (VAS) scores before and after the switch were reported by Gallo G et al. who enrolled 73 patients with psoriasis who had received the adalimumab originator for a median time of 34 months [16]. Other studies that enrolled patients with psoriasis [17,18] and HS [19] reported similar conclusions. Finally, using the DANBIO registry, Nabi H et al. [20] evaluated the effects of a switch from the adalimumab originator to biosimilars among 321 with PsA (148 to GP2017 and 173 to SB5). Patients who switched to GP2017 had higher disease activity and had received more number of biologic treatments previously compared to those who switched to SB5. No significant changes in disease activity were found 6 months before and after the switch. At 1 year, treatment withdrawal occurred in 8.5% of patients treated with GP2017 and 12.9% of patients receiving SB5. The main reasons of treatment discontinuation were loss of efficacy and the occurrence of AEs.
Some of the included studies reported a higher discontinuation rate among biosimilar users mainly due to AEs or loss of efficacy. For instance, the results of the retrospective study carried out by Burlando M et al. [21] on 326 HS patients reported a greater loss of effectiveness among patients allocated in the biosimilar group vs. those in the originator group (IRR = 2.2; 95% CI 1.5–3.2, p < 0.001). The registry-based study carried out by Kirsten N et al. [22] among 94 HS patients highlighted that 33% of them experienced AEs or loss of efficacy after switching, and that significant differences at the time of switch and 12–14 weeks after the switch were identified for the IHS4 score (p < 0.001) in both groups of patients (with and without loss of efficacy). Similarly, Montero-Vilchez T et al. [23] reported that, out of 17 HS patients who switched from the adalimumab originator to the biosimilar for no medical reasons, 10 patients reported severe pain at the injection site and loss of HiSCR response. Finally, Roccuzzo G et al. [24] reported high discontinuation rates among biosimilar users due to severe pain at the injection site. Only Scrivo et al. [25] evaluated the effects of the switch among adalimumab biosimilars. They carried out a prospective cohort study among 150 patients, of whom 110 (40 with PsA) switched from the adalimumab originator to the SB5 biosimilar and 40 (25 with PsA) switched from the ABP501 biosimilar to the SB5 biosimilar. In both groups of patients at month 4, no differences in disease activity scores for rheumatic diseases were reported, even though PsA patients belonging to the first group experienced a worsening as detected through the VAS (p = 0.02) and the Health Assessment Questionnaire (HAQ) (p = 0.03).
2.2. Etanercept Switching Studies
Ten studies [26,27,28,29,30,31,32,33,34,35] evaluated the effects in terms of efficacy and safety deriving from the switch among etanercept products. All studies were carried out in Europe (6/10 in Italy). Taken together, the included studies enrolled 3596 patients, of which 915 suffered from dermatologic disorders [PsA (8 studies) and psoriasis (2 studies)]. The duration of the included studies ranged from 24 weeks to 24 months. Out of 10 studies, 2 [26,27] evaluated the efficacy and safety of cross-switching from etanercept originator to the biosimilar SB4 and subsequently to the biosimilar GP2015. In particular, Kiltz U et al. [26] carried out a retrospective chart review of 100 patients (19 with PsA) who underwent a cross-switching from the etanercept originator to SB4 and subsequently to GP2015. The mean time of treatment with the originator ETN prior to the first switch was 3.3 years. According to the DAS-28 score, at the first-switch visit, PsA patients had a moderate-to-high disease activity. After the second switch, the retention rate at 6 months was 89%. Six patients discontinued due to inefficacy; of them, 4 patients were switched back to the etanercept originator reaching their former state of low disease activity during follow-up, while 2 patients received another biologic. Piaserico S et al. [27] carried out a multi-center, prospective, observational cohort study among 76 patients with moderate-to-severe psoriasis. At the beginning of the study, the median PASI was 10.5, while it decreased to 2 after the first switch and to 0.5–1 after the second switch (follow-up at 12 months after the second switch). Based on the overall results, authors concluded that switching between two etanercept biosimilars (from SB4 to GP2015) is safe and effective. A similar conclusion was made by Pescitelli L et al. [28] who evaluated the effects of switching among 44 patients with psoriasis, of whom 32 underwent a switch to SB4. Over a follow-up period of 24 weeks, clinical remission was confirmed in 92% of patients with psoriasis and 64% of patients with psoriatic arthritis.
Ditto MC et al. [29] carried out a prospective study among 87 patients who accepted the switch from the originator (median duration of treatment 7 years) to the biosimilar. The biosimilar was discontinued in 11/85 patients (5 with PsA) mainly due to loss of efficacy, subjective reasons or the occurrence of AEs. Switching from the originator to the biosimilar was associated with the occurrence AEs or loss of efficacy in the study of Felis-Giemza A et al. too [30]. Indeed, the authors reported 24 switch-back to the originator. The loss of efficacy was confirmed in 9 patients using clinical scores, while 9 patients reported subjective loss of efficacy. In the retrospective study of Bruni C et al. [31], out of 200 enrolled patients (81 PsA; originator treatment duration 7 ± 4 years), 30 experienced SB4 withdrawals due to the occurrence of AEs (n = 11) or loss of efficacy (n = 19).
The effects of switching were evaluated by Bonifati C et al. [32] one year after the switch from the reference etanercept to SB4 among 87 patients with PsA. Almost 90% of patients maintained a clinical Disease Activity Index for Psoriatic Arthritis (cDAPSA) ≤ 13, suggesting that SB4 is effective in maintaining a state of low disease activity in the majority of patients switched from the originator. Using the DANBIO registry, Glintborg B et al. [33] evaluated the effects of switch from etanercept originator to SB4 among 407 with PsA, reporting similar disease activity 3 months before and after the switch. During the follow-up, 120 patients switched back to the etanercept originator mainly due to subjective reasons. Overall, the results of switcher studies highlighted a crude persistence on treatment at 1 year of 82% vs. 88% in an ETN historic cohort and 70% in the non-switched population. Tweehuysen L et al. [34] carried out a prospective study among 635 patients who switched from the originator to SB4 (128 with PsA). At 6 months, the crude treatment persistence rate for SB4 and etanercept originator was 90% and 92%, respectively. Lastly, Benucci M et al. [35] evaluated the effects of non-medical switching also in terms of immunogenicity through the detection of etanercept and levels of anti-drug antibodies (ADA). The activity disease status (through the DAPSA for PsA patients) and TNF-α levels were evaluated as well. Out of 79 patients who switched to SB4, 26 patients (33%) withdrew the treatment due to loss of efficacy (n = 19) or AEs (n = 7). The results of this study highlighted a difference at 6 and 12 months between switchers and naïve in terms of the HAQ and CRP, which decreased at 6 months in the naïve cohort, while HAQ decreased later (between 6 and 12 months) in the switcher cohort. In addition, among non-responder switcher patients, there was a progressive reduction in the circulating drug level at 6 and 12 months. Lastly, TNF-α levels were higher among switcher responders than in non-responders at 6 and 12 months. These data seem to be not a proof of SB4′s immunogenicity, since no anti-SB4 antibodies have been detected neither in the naïve group nor in the switcher group and, as reported by the authors, etanercept is in general associated with a low risk of immunogenicity in switching studies.
2.3. Infliximab Switching Studies
Twelve studies [36,37,38,39,40,41,42,43,44,45,47,48] evaluated the effects in terms of the efficacy and safety derived from the switch among infliximab products. Eleven studies were carried out in Europe, while one study was conducted in Japan. Overall, the included studies enrolled 3125 patients, of which 639 suffered from dermatologic disorders [PsA (9 studies) and psoriasis (5 studies)]. The duration of included studies ranged from 23 weeks to 78 weeks. Apart from Nabi H et al. [36], who evaluated the effects of switching among infliximab biosimilars, all included studies analyzed the effects associated with the switch from the originator to one of infliximab biosimilars. Specifically, using data from the DANBIO registry, Nabi H et al. [36] carried out a cohort study among 434 patients (70 with PsA) with the aim to evaluate the retention rates one year after the switch between infliximab biosimilars CT-P13 and GP1111 and factors associated with GP1111 withdrawal. Retention rates at 1 year were 83% and 92% for the originator-naïve and originator-experienced, respectively. GP1111 retention rates were higher in originator-experienced patients compared to originator-naïve patients with PsA [HR = 0.2 (95% CI: 0.1 to 0.8)]. No significant changes in disease activity were found before and after the switch.
The NOR-SWITCH [37] study was a randomized, non-inferiority, double-blind, phase 4 trial with 52 weeks of follow-up evaluating the effects of the switch to the infliximab biosimilar among 241 patients (30 with PsA and 35 with psoriasis) on stable treatment with the infliximab originator (at least 6 months of treatment). Disease worsening occurred in 53 patients in the infliximab originator group and in 61 patients in the CT-P13 group (adjusted treatment difference −4.4%, 95% CI −12.7 to 3.9). The frequency of AEs was similar between the groups. Goll GL et al. [38] carried out the extension study of the NOR-SWITCH study among 380 patients (20 with PsA and 31 with psoriasis), demonstrating no differences in disease worsening between groups (patients switched at main study baseline and those switched at extension study baseline). Gisondi et al. assessed the efficacy and safety of switching from the infliximab originator to SB2 in 96 patients with chronic plaque psoriasis, highlighting that the severity of psoriasis remained stable also after the switch as confirmed using the PASI at 6 months. Out of 96 patients, 10 experienced losses of efficacy or acute infusion reactions, leading to treatment withdrawal [47]. Similarly, the results of the prospective study carried out by Dapavo P et al. [39] in 35 patients with psoriasis (of whom, 30 underwent a switch from the infliximab originator to the biosimilar, while 5 were biologic naïve and started the treatment with the biosimilar) revealed no differences in the PASI and VAS scores before and after the switch to the biosimilar.
Similar results were reported by Lauret et al. [48], who carried out a 3-year observational study in 140 PsA patients who underwent to a switch from the innovator infliximab to an infliximab biosimilar (CT-P13), followed by a switch to a second infliximab biosimilar (SB2). The study’s results reported that the risk of treatment discontinuation was significantly higher in patients with positive ADA at the baseline visit or during follow-up compared to patients without ADA (Hazard Ratio 2.37, 95% CI 1.38–4.05, p = 0.002). Moreover, patients who discontinued infliximab (n = 60) were more likely to have positive ADA (p = 0.002). ADA were detected in 12 patients out of 26 who discontinued infliximab because of primary or secondary loss of efficacy and in 4 patients who discontinued due to infusion-related reaction.
Scherlinger M et al. [40] enrolled 89 patients, of whom 12 with PsA underwent a switch from an infliximab originator to a biosimilar. After a median follow-up of 33 weeks, 72% of patients were still treated with CT-P13. Almost half of the patients requesting to re-switch to the originator reported a negative perception of the new treatment without any worsening of their disease activity score, suggesting a possible reluctance of patients to switch. Boone NW et al. [41] carried out a pragmatic trial among 125 patients, of whom 5 with PsA were switched from the originator (mean treatment duration 2.9 ± 1.2 years). The authors reported a good response in switchers with PsA, and no neutralizing antibody against infliximab was observed. Positive results were reported by Glintborg B et al. [42] who used data from the DANBIO registry to evaluate the efficacy and safety of switching from the infliximab originator among 802 patients (120 with PsA with a prior infliximab treatment duration of 6.3 years). No difference was found in disease activities 3 months before and after the switch. Among patients with PsA, 16 (11 women) withdrew the treatment. Tweehuysen L et al. [43] carried out the BIO-SWITCH study, a multicenter prospective cohort study among 192 patients (50 with PsA). The results revealed that the DAS28-CRP remained stable at month 6 and that the levels of CRP, infliximab, and ADA did not change. AEs more commonly reported were arthralgia, fatigue, pruritus, and myalgia. Valido A et al. [44] evaluated the effects of a non-medical switch at 1 year among 60 patients (8 with PsA with a mean treatment duration with the infliximab originator of 9.6 years), demonstrating that disease activity remained stable over the observation period and similar to the values observed with the infliximab originator. For 42 patients (2 with PsA), blood samples were collected before and after the switch. The analysis of these samples revealed no major changes in the levels of ADA and infliximab before and after the switch.
Lastly, the only study performed outside European countries (the study location was Japan) was the one carried out by Morita A et al. [45]. The authors carried out a prospective post-marketing surveillance with the aim to evaluate the safety, efficacy, and drug survival after non-medical switching from the infliximab originator among 165 patients with psoriasis (105 switchers). At the follow-up of 1 year, AEs occurred in 29 patients (infusion reaction was the most frequently reported AE), of whom 2 had serious AEs (acute cholecystitis and interstitial pneumonia). Among switchers, 57% of patients had already reached PASI < 1 owing to pretreatment with infliximab and CT-P13 maintained this status.
Finally, only one study, the one carried out by Provenzano G et al. [46], evaluated the effects of non-medical switching in patients receiving adalimumab, etanercept, or infliximab. One hundred forty-five patients (45 diagnosed with PsA) who had been treated for at least 2 years with these drugs and who had achieved stable clinical remission or low disease activity for at least 6 months were switched to the corresponding biosimilars. After a median of 17.5 months, almost 85% were stable on clinical remission or had low disease activity while on therapy with biosimilars.
3. Materials and Methods
3.1. Literature Search
Two authors (MMN and CS) independently performed a literature search of all publications up to January 2023 on the Medline database using the following keywords: (rituximab OR etanercept OR adalimumab OR infliximab OR biosimilar) AND originator AND (switch OR switching OR transitioning) AND (dermatology OR psoriasis OR psoriatic arthritis OR Hidradenitis Suppurativa OR pemphigus vulgaris). In addition, linked references in all relevant articles were searched.
3.2. Study Selection
Publications were included if they were clinical studies carried out after the marketing approval of each biosimilar (both randomized clinical trials and observational studies that provide real-world evidence) and described the switch from originators to biosimilars or from one biosimilar to another in terms of the efficacy or safety (occurrence of adverse events—AEs) outcomes. Non-English publications, non-human research, phase I–III clinical studies, editorials, comments, and surveys were excluded. Phase I–III clinical studies were excluded because we aimed to provide an overview of the effects of switching in real-life conditions, thus reporting data from clinical studies carried out after the marketing approval of biosimilars. Screening was based on reading the titles and abstracts of the publications and included all clinical studies that mentioned switching between originators and biosimilars.
3.3. Data Extraction, Quality Assessment, and Analysis
Data on selected articles were imported into MS Excel. Data were collected from the full-text publications relevant to originator-to-biosimilar and biosimilar-to-biosimilar switch, including study design and duration, country, patient demographics, and efficacy and safety outcomes. All endpoints were reported in a descriptive manner; no meta-analysis was performed because of varying study designs, endpoints, and statistical methodologies used in the selected studies.
At the end of study selection and analysis, three authors (CP, AC, and AA) carried out a further analysis on the extracted data to check whether information were missed or not.
Due to variability in individual studies, a cross-study quality assessment was not feasible.
4. Discussion
In this systematic review, an overview of switching clinical studies among biologic originators and biosimilars used to treat dermatologic conditions and carried out in real-life settings has been provided. Studies included in this review enrolled patients who received infliximab, adalimumab, and etanercept, all TNF-α-blocking agents, mainly used to treat psoriasis and PsA (32 out of 37 studies). Only a limited number of studies enrolled patients diagnosed with HS (5 out of 37 studies). In our opinion, this difference in disease distribution in included studies could be explained by the different prevalence of psoriasis, PsA, and HS. Indeed, the prevalence of PsA is estimated to be 2–3% (among patients with psoriasis the prevalence of PsA varies from 6% to 42%), while the prevalence of HS seems to be much lower (0.40%) [50,51].
We found that the 36 out of 37 included studies were carried out in Europe and mainly in Italy (only 1 study was carried out in Japan). This can be related to the regulatory policies that were issued across EU countries in recent years in order to increase the prescription of biosimilars by physicians. For instance, across Italian regions, biosimilar prescribing and switching are recommended and there are prescribing quotas in some regions [52]. As reported by Ditto et al. [29], in a region of northern Italy (Piedmont), SB4/Benepali won the auction vs. Enbrel in 2017 with a significant price difference. Thus, in this Region, a commission including members of the Regional Pharmaceutical Service and rheumatologists was established in order to produce a regional guidance on prescribing drugs for naïve patients and in case of a switch. Similarly, policies to encourage the use and prescription of biosimilars were also approved in Denmark and in Germany [53].
Out of 37 included studies, only 1, the NOR-SWITCH trial [37], was designed as a randomized controlled trial. This was one of the first studies to be carried out with the specific aim to evaluate the effects deriving from switching among infliximab products. It was designed as an independent study sponsored by the Norwegian government that is characterized by few limitations, such as the choice to enroll patients with different diseases, the baseline characteristics in relation to disease activity (not all patients were in clinical remission at the time of enrollment), and the concomitant treatment with immunosuppressants that was higher in the biosimilar group (this may have led to better results with the biosimilar) [54]. Therefore, although the NOR-SWITCH study concluded that switching from the infliximab originator to the biosimilar is non-inferior to continued treatment, its results should be interpreted with caution due to its limitations. In order to provide the highest level of evidence, a switching study should have the following characteristics: study design randomized and controlled; at least one switch from the originator to the biosimilar; assessment of the effects of the switch on the immunogenicity profile; a sufficient washout period between switches; a sufficient study power to assess efficacy and safety; and a sufficient follow-up period [55]. Based on these recommendations, the majority of studies included in this systematic review do not fulfill the criteria to provide high level of evidence on switching among biologic products. Apart from the limitations of the NOR-SWITCH study, many other weaknesses have been identified in the other included studies. Indeed, many of them have an unclear methodology, such as for example the studies by Ditto et al. [29] and Felis-Giemza et al. [30] that seem to be trials even though nothing is reported in the methodology regarding patient randomization and distribution among groups.
Overall, the results of the included studies demonstrated a good efficacy and safety profile for all biologics after the switch and no unexpected AEs were reported in any of the included studies. Where evaluated, no differences in AEs or immunogenicity before and after switching were reported, even though Lauret et al. [48] found that the risk of treatment discontinuation with the infliximab biosimilar was higher in patients with positive ADA and that patients who discontinued infliximab were more likely to have positive ADA. According to literature data, ADA tend to appear within the first 6 months of therapy with adalimumab and infliximab [56,57,58]. The development of ADA, mainly observed with murine and chimeric mAbs, represents the main sign of immunogenicity, which consists of a tendency to trigger an unwanted immune response against self-antigens [59]. Immunogenicity may have important consequences both in terms of efficacy and safety (reduction of therapeutic efficacy, alterations in drug exposure and plasma concentrations, and hypersensitivity reactions), which could explain the association between positive ADA and treatment discontinuation found in the study by Lauret et al. [48].
When a switch, especially multiple switches, is performed for non-medical reasons (as it happened in many of clinical studies included in this review), it can be frequently related to the so-called nocebo effect. This is a combination of psychological and physiological phenomena associated with actual or perceived harm that occur as a consequence of patients’ negative expectancies (and not because of the pharmacologic actions of treatment) [60] and that can negatively affect the benefit/risk ratio of the drug [61]. Indeed, as reported by Felis-Giemza [30], the subjective loss of efficacy is defined as a disease’ worsening perceptible only by the patient without objective measurements. Many risk factors for the occurrence of nocebo effects have been identified, including those related to the physician (expression of uncertainty, extensive reference to potential biosimilar-induced AEs), patient characteristics (female gender, personal belief, mental disorders), drug-related factors (route of administration, negative publicity), features of the healthcare setting (interaction with the staff and other patients), and factors related to the disease process (status, duration, and therapeutic history) [62]. For instance, a systematic review of original articles addressing physicians’ perceptions on biosimilars’ uptake reported different knowledge and attitudes towards biosimilars. Indeed, even though physicians had positive attitudes towards biosimilars, prescribing was limited. Thus, authors suggested that education programs are needed to support the uptake of biosimilars [63]. As observed in the BIO-SWITCH study [43], a Dutch registry of patients with long-term stable disease who switched from the infliximab originator to the biosimilar following a government mandate (thus, a non-medical switch), the discontinuation rate at month 6 was 24% and 78% of AEs were subjective events (mood disturbances, fatigue). Additionally, among those who discontinued the infliximab biosimilar due to a perceived lack of efficacy, scores on objective measures of disease (swollen joint count and C-reactive protein) from baseline were stable. Recently, Petit J et al. [64] evaluated an intervention aimed to reduce the nocebo effect associated with switching between the infliximab products. The study included 45 patients with inflammatory rheumatic disease and healthcare professionals who were trained on the switch. Authors evaluated the retention rate at 34 weeks and the discontinuation rates due to a possible nocebo effect (with a comparison with a historical cohort of patients). Results revealed that the retention rate after 34 weeks was 91.2%. At 12 months, the retention rate with SB2 was 84.5% vs. 88.4% for the historical cohort (p = 0.52). The biosimilar discontinuation due to a possible nocebo effect was 6.6% after 12 months. The results of this study suggest that a tailored training of healthcare professionals and communication to patients might reduce the risk of nocebo effects in non-medical switches.
5. Strengths and Limitations
This systematic review has several limitations. First of all, the search was made using a single database. In addition, the majority of the included studies were observational, which are themselves characterized by well-known limitations, such as the lack of appropriate controls or the absence of long-term effects of switching. Indeed, many studies were characterized by a relatively short follow-up (≤6 months in 10 studies out of 36) and a limited sample size. In addition, only a few studies analyzed the effects of cross-switching or switch among biosimilars. Thus, conclusions about this type of switch should be made with caution. Lastly, the study was limited by a high heterogeneity, both clinical and statistical, across studies that could be explained by different populations, heterogeneous clinical samples, and diagnostic criteria. Finally, another limitation lies in the disease activity indices used in the included studies, such as the HAQ, PASI, CRP, DAS28 and DAS28-CRP, DAPSA, Psoriatic Arthritis Response Criteria (PsARC), and Minimal Disease Activity (MDA). Indeed, even though these tools have a good validity, there are still contrasting data in the classification of patients according to the disease activity levels [65]. In addition, in some cases, the loss of efficacy was inferred on the basis of the patients’ subjective evaluations.
On the other hand, our study has many strengths. First of all, the literature search was done by two authors already experienced in performing this type of study. Secondly, we decided to include only clinical studies carried out after the marketing approval of each biosimilar. Indeed, even though randomized controlled trials represent the gold standard for determining the efficacy and safety of healthcare interventions, their intrinsic bars limit the results’ generalizability [66]. Real-world studies, that include those based on the collection of primary or secondary data from healthcare registries, add new knowledge since they allow to investigate a more diverse group of patients than those included in clinical trials, such as patients with comorbid conditions, those taking multiple medications, geriatric patients, and other vulnerable populations normally excluded by clinical trials. Third, we provided an updated analysis to January 2023 about the effects deriving from switching for three biologic drugs that are among the most used worldwide [67,68].
6. Conclusions
Data from the literature provided in this systematic review suggest that switching between adalimumab, etanercept, and infliximab originators and biosimilars is safe and effective in a real-life setting of patients suffering from dermatological conditions. Only few studies have highlighted an increase in the risk of loss of efficacy or increased rate of AEs, both of which have been identified as main causes of biosimilar discontinuation. In our opinion, this observation could be related to the nocebo effect, which is a common phenomenon especially among patients receiving biologic therapies (given their administration route—intravenous—and the negative perceptions that persist among certain physicians and that, in turn, are able to influence patients’ perceptions). Lastly, only few studies evaluated the switch among biosimilars; therefore, no conclusion can be drawn for this type of switch in terms of the efficacy and safety outcomes. Based on our results and given the clinical experience gained with biosimilars during last 15 years, in line with what was already stated by other authors [69] and with the latest joint statement released by the EMA and HMA, we believe that biosimilars approved by the EC according to the EMA’s stringent regulatory requirements can be considered interchangeable with their reference products and that no additional switch studies are necessary to support switching among originators and biosimilars in clinical practice. Of course, the continuous monitoring of originators and biosimilars in real-life contexts is still necessary considering that biologic drugs, especially mAbs, have peculiar safety profiles. Thus, ongoing pharmacovigilance monitoring [70,71] is essential to detect uncommon AEs and unanticipated changes in efficacy or safety profiles that may arise as a result of modifications in the manufacturing process. Running ad hoc pharmacovigilance studies may improve the public trust in biosimilars, increasing their adoption that ultimately delivers savings and value-added services to support patient care.
Author Contributions
M.M.N. and C.S. wrote the original draft. M.M.N., C.P., A.C., A.A. and C.S. were responsible for the conceptualization, data curation, methodology, project administration, writing—review & editing, resources, and investigation. E.C. and D.R. were responsible for data curation, writing—review & editing and investigation. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
This is a systematic review. No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
Correction Statement
This article has been republished with a minor correction to the readability of Figure 1. This change does not affect the scientific content of the article.
References
- Available online: https://www.ema.europa.eu/en/human-regulatory/overview/biosimilar-medicines-overview (accessed on 20 January 2023).
- Lebwohl, M. Biosimilars in Dermatology. JAMA Dermatol. 2021, 157, 641–642. [Google Scholar] [CrossRef]
- Scavone, C.; Sportiello, L.; Sullo, M.G.; Ferrajolo, C.; Ruggiero, R.; Sessa, M.; Berrino, P.M.; di Mauro, G.; Berrino, L.; Rossi, F.; et al. Safety Profile of Anticancer and Immune-Modulating Biotech Drugs Used in a Real World Setting in Campania Region (Italy): BIO-Cam Observational Study. Front. Pharmacol. 2017, 8, 607. [Google Scholar] [CrossRef] [PubMed]
- Scavone, C.; Sessa, M.; Clementi, E.; Corrao, G.; Leone, R.; Mugelli, A.; Rossi, F.; Spina, E.; Capuano, A. Real World Data on the Utilization Pattern and Safety Profile of Infliximab Originator Versus Biosimilars in Italy: A Multiregional Study. BioDrugs 2018, 32, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, A.B.; Chamian, F.; Masud, S.; Cardinale, I.; Abello, M.V.; Lowes, M.A.; Chen, F.; Magliocco, M.; Krueger, J.G. TNF inhibition rapidly down-regulates multiple proinflammatory pathways in psoriasis plaques. J. Immunol. 2005, 75, 2721–2729. [Google Scholar] [CrossRef]
- Zaba, L.C.; Cardinale, I.; Gilleaudeau, P.; Sullivan-Whalen, M.; Suárez-Fariñas, M.; Fuentes-Duculan, J.; Novitskaya, I.; Khatcherian, A.; Bluth, M.J.; Lowes, M.A.; et al. Amelioration of epidermal hyperplasia by TNF inhibition is associated with reduced Th17 responses. J. Exp. Med. 2007, 204, 3183–3194. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.ema.europa.eu/en/documents/leaflet/biosimilars-eu-information-guide-healthcare-professionals_en.pdf (accessed on 20 January 2023).
- Scavone, C.; Rafaniello, C.; Berrino, L.; Rossi, F.; Capuano, A. Strengths, weaknesses and future challenges of biosimilars’ development. An opinion on how to improve the knowledge and use of biosimilars in clinical practice. Pharm. Res. 2017, 126, 138–142. [Google Scholar] [CrossRef]
- Scavone, C.; Sportiello, L.; Berrino, L.; Rossi, F.; Capuano, A. Biosimilars in the European Union from comparability exercise to real world experience: What we achieved and what we still need to achieve. Pharm. Res. 2017, 119, 265–271. [Google Scholar] [CrossRef]
- Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-similar-biological-medicinal-products-containing-biotechnology-derived-proteins-active_en-0.pdf (accessed on 28 February 2023).
- Available online: https://www.ema.europa.eu/en/news/biosimilar-medicines-can-be-interchanged (accessed on 20 January 2023).
- Cohen, H.P.; Blauvelt, A.; Rifkin, R.M.; Danese, S.; Gokhale, S.B.; Woollett, G. Switching Reference Medicines to Biosimilars: A Systematic Literature Review of Clinical Outcomes. Drugs 2018, 78, 463–478. [Google Scholar] [CrossRef]
- Becciolini, A.; Parisi, S.; Caccavale, R.; Bravi, E.; Lumetti, F.; Andracco, R.; Volpe, A.; Gardelli, L.; Girelli, F.; Di Donato, E.; et al. Adalimumab and ABP 501 in the Treatment of a Large Cohort of Patients with Inflammatory Arthritis: A Real Life Retrospective Analysis. J. Pers. Med. 2022, 12, 335. [Google Scholar] [CrossRef]
- Bruni, C.; Bitti, R.; Nacci, F.; Cometi, L.; Tofani, L.; Bartoli, F.; Fiori, G.; Matucci-Cerinic, M. Efficacy and safety of switching from reference adalimumab to SB5 in a real-life cohort of inflammatory rheumatic joint diseases. Clin. Rheumatol. 2021, 40, 85–91. [Google Scholar] [CrossRef]
- Bruni, C.; Gentileschi, S.; Pacini, G.; Bardelli, M.; Tofani, L.; Bartoli, F.; Baldi, C.; Cometi, L.; Fiori, G.; Nacci, F.; et al. Switching from originator adalimumab to biosimilar SB5 in a rheumatology cohort: Persistence on treatment, predictors of drug interruption and safety analysis. Ther. Adv. Musculoskelet. Dis. 2021, 13, 1759720X211033679. [Google Scholar] [CrossRef] [PubMed]
- Gallo, G.; Rostagno, E.; Siliquini, N.; Stroppiana, E.; Verrone, A.; Ortoncelli, M.; Quaglino, P.; Dapavo, P.; Ribero, S. Efficacy of switching from adalimumab originator to adalimumab biosimilar in moderate to severe psoriasis patients: A Real-life experience in a tertiary referral centre. Australas. J. Dermatol. 2021, 62, e431–e432. [Google Scholar] [CrossRef] [PubMed]
- Giunta, A.; Zangrilli, A.; Bavetta, M.; Manfreda, V.; Pensa, C.; Bianchi, L. A single-centre, observational, retrospective, real-life study evaluating adalimumab biosimilar ABP 501 in the treatment of plaque-type psoriasis and psoriatic arthritis in originator-naïve patients and in patients undergoing non-medical switch from originator. Curr. Med. Res. Opin. 2021, 37, 1099–1102. [Google Scholar] [CrossRef] [PubMed]
- Loft, N.; Egeberg, A.; Rasmussen, M.K.; Bryld, L.E.; Nissen, C.V.; Dam, T.N.; Ajgeiy, K.K.; Iversen, L.; Skov, L. Outcomes following a Mandatory Nonmedical Switch from Adalimumab Originator to Adalimumab Biosimilars in Patients with Psoriasis. JAMA Dermatol. 2021, 157, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Ricceri, F.; Rosi, E.; Di Cesare, A.; Pescitelli, L.; Fastame, M.T.; Prignano, F. Clinical experience with adalimumab biosimilar imraldi in hidradenitis suppurativa. Derm. Ther. 2020, 33, e14387. [Google Scholar] [CrossRef]
- Nabi, H.; Georgiadis, S.; Loft, A.G.; Hendricks, O.; Jensen, D.V.; Andersen, M.; Chrysidis, S.; Colic, A.; Danebod, K.; Hussein, M.R.; et al. Comparative effectiveness of two adalimumab biosimilars in 1318 real-world patients with inflammatory rheumatic disease mandated to switch from originator adalimumab: Nationwide observational study emulating a randomised clinical trial. Ann. Rheum. Dis. 2021, 80, 1400–1409. [Google Scholar] [CrossRef]
- Burlando, M.; Fabbrocini, G.; Marasca, C.; Dapavo, P.; Chiricozzi, A.; Malvaso, D.; Dini, V.; Campanati, A.; Offidani, A.; Dattola, A.; et al. Adalimumab Originator vs. Biosimilar in Hidradenitis Suppurativa: A Multicentric Retrospective Study. Biomedicines 2022, 10, 2522. [Google Scholar] [CrossRef] [PubMed]
- Kirsten, N.; Ohm, F.; Gehrdau, K.; Girbig, G.; Stephan, B.; Ben-Anaya, N.; Pinter, A.; Bechara, F.G.; Presser, D.; Zouboulis, C.C.; et al. Switching from Adalimumab Originator to Biosimilar in Patients with Hidradenitis Suppurativa Results in Losses of Response-Data from the German HS Registry HSBest. Life 2022, 12, 1518. [Google Scholar] [CrossRef]
- Montero-Vilchez, T.; Cuenca-Barrales, C.; Rodriguez-Tejero, A.; Martinez-Lopez, A.; Arias-Santiago, S.; Molina-Leyva, A. Switching from Adalimumab Originator to Biosimilar: Clinical Experience in Patients with Hidradenitis Suppurativa. J. Clin. Med. 2022, 11, 1007. [Google Scholar] [CrossRef]
- Roccuzzo, G.; Rozzo, G.; Burzi, L.; Repetto, F.; Dapavo, P.; Ribero, S.; Quaglino, P. Switching from adalimumab originator to biosimilars in hidradenitis suppurativa: What’s beyond cost-effectiveness? Derm. Ther. 2022, 35, e15803. [Google Scholar] [CrossRef]
- Scrivo, R.; Castellani, C.; Mancuso, S.; Sciarra, G.; Giardina, F.; Bevignani, G.; Ceccarelli, F.; Spinelli, F.R.; Alessandri, C.; Di Franco, M.; et al. Effectiveness of non-medical switch from adalimumab bio-originator to SB5 biosimilar and from ABP501 adalimumab biosimilar to SB5 biosimilar in patients with chronic inflammatory arthropathies: A monocentric observational study. Clin. Exp. Rheumatol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Kiltz, U.; Tsiami, S.; Baraliakos, X.; Andreica, I.; Kiefer, D.; Braun, J. Effectiveness and safety of a biosimilar-to-biosimilar switch of the TNF inhibitor etanercept in patients with chronic inflammatory rheumatic diseases. Ther. Adv. Musculoskelet. Dis. 2022, 14, 1759720X221119593. [Google Scholar] [CrossRef]
- Piaserico, S.; Conti, A.; Messina, F.; Meneguzzo, A.; Odorici, G.; Bellinato, F.; Gisondi, P. Cross-Switch from Etanercept Originator to Biosimilar SB4 and to GP2015 in Patients with Chronic Plaque Psoriasis. BioDrugs 2021, 35, 469–471. [Google Scholar] [CrossRef]
- Pescitelli, L.; Lazzeri, L.; Di Cesare, A.; Tripo, L.; Ricceri, F.; Prignano, F. Clinical experience with the etanercept biosimilar SB4 in psoriatic patients. Int. J. Clin. Pharm. 2019, 41, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Ditto, M.C.; Parisi, S.; Priora, M.; Sanna, S.; Peroni, C.L.; Laganà, A.; D’Avolio, A.; Fusaro, E. Efficacy and safety of a single switch from etanercept originator to etanercept biosimilar in a cohort of inflammatory arthritis. Sci. Rep. 2020, 10, 16178. [Google Scholar] [CrossRef] [PubMed]
- Felis-Giemza, A.; Chmurzyńska, K.; Nałęcz-Janik, J.; Romanowska-Próchnicka, K.; Świerkocka, K.; Wudarski, M.; Olesińska, M. Observational study of inflammatory arthritis treatment by etanercept originator switched to an etanercept biosimilar. Reumatologia. 2019, 57, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Bruni, C.; Gentileschi, S.; Pacini, G.; Baldi, C.; Capassoni, M.; Tofani, L.; Bardelli, M.; Cometi, L.; Cantarini, L.; Nacci, F.; et al. The switch from etanercept originator to SB4: Data from a real-life experience on tolerability and persistence on treatment in joint inflammatory diseases. Ther. Adv. Musculoskelet. Dis. 2020, 12, 1759720X20964031. [Google Scholar] [CrossRef]
- Bonifati, C.; De Felice, C.; Lora, V.; Morrone, A.; Graceffa, D. Effectiveness of etanercept biosimilar SB4 in maintaining low disease activity in patients with psoriatic arthritis switched from etanercept originator: An open-label one year study. J. Dermatol. Treat. 2020, 31, 687–691. [Google Scholar] [CrossRef]
- Glintborg, B.; Loft, A.G.; Omerovic, E.; Hendricks, O.; Linauskas, A.; Espesen, J.; Danebod, K.; Jensen, D.V.; Nordin, H.; Dalgaard, E.B.; et al. To switch or not to switch: Results of a nationwide guideline of mandatory switching from originator to biosimilar etanercept. One-year treatment outcomes in 2061 patients with inflammatory arthritis from the DANBIO registry. Ann. Rheum. Dis. 2019, 78, 192–200. [Google Scholar] [CrossRef]
- Tweehuysen, L.; Huiskes, V.J.B.; van den Bemt, B.J.F.; Vriezekolk, J.E.; Teerenstra, S.; van den Hoogen, F.H.J.; van den Ende, C.H.; den Broeder, A.A. Open-Label, Non-Mandatory Transitioning from Originator Etanercept to Biosimilar SB4: Six-Month Results from a Controlled Cohort Study. Arthritis Rheumatol. 2018, 70, 1408–1418. [Google Scholar] [CrossRef]
- Benucci, M.; Damiani, A.; Russo, E.; Li Gobbi, F.; Grossi, V.; Amedei, A.; Infantino, M.; Manfredi, M. Predicting Loss of Efficacy after Non-Medical Switching: Correlation between Circulating TNF-α Levels and SB4 in Etanercept to SB4 Switchers and Naïve Patients with Rheumatic Disease. J. Pers. Med. 2022, 12, 1174. [Google Scholar] [CrossRef] [PubMed]
- Nabi, H.; Hendricks, O.; Jensen, D.V.; Loft, A.G.; Pedersen, J.K.; Just, S.A.; Danebod, K.; Munk, H.L.; Kristensen, S.; Manilo, N.; et al. Infliximab biosimilar-to-biosimilar switching in patients with inflammatory rheumatic disease: Clinical outcomes in real-world patients from the DANBIO registry. RMD Open. 2022, 8, e002560. [Google Scholar] [CrossRef]
- Jørgensen, K.K.; Olsen, I.C.; Goll, G.L.; Lorentzen, M.; Bolstad, N.; Haavardsholm, E.A.; Lundin, K.E.A.; Mørk, C.; Jahnsen, J.; Kvien, T.K.; et al. Switching from originator infliximab to biosimilar CT-P13 compared with maintained treatment with originator infliximab (NOR-SWITCH): A 52-week, randomised, double-blind, non-inferiority trial. Lancet 2017, 389, 2304–2316. [Google Scholar] [CrossRef]
- Goll, G.L.; Jørgensen, K.K.; Sexton, J.; Olsen, I.C.; Bolstad, N.; Haavardsholm, E.A.; Lundin, K.E.A.; Tveit, K.S.; Lorentzen, M.; Berset, I.P.; et al. Long-term efficacy and safety of biosimilar infliximab (CT-P13) after switching from originator infliximab: Open-label extension of the NOR-SWITCH trial. J. Intern. Med. 2019, 285, 653–669. [Google Scholar] [CrossRef] [PubMed]
- Dapavo, P.; Vujic, I.; Fierro, M.T.; Quaglino, P.; Sanlorenzo, M. The infliximab biosimilar in the treatment of moderate to severe plaque psoriasis. J. Am. Acad. Dermatol. 2016, 75, 736–739. [Google Scholar] [CrossRef] [PubMed]
- Scherlinger, M.; Germain, V.; Labadie, C.; Barnetche, T.; Truchetet, M.E.; Bannwarth, B.; Mehsen-Cetre, N.; Richez, C.; Schaeverbeke, T.; FHU ACRONIM. Switching from originator infliximab to biosimilar CT-P13 in real-life: The weight of patient acceptance. Jt. Bone Spine 2018, 85, 561–567. [Google Scholar] [CrossRef]
- Boone, N.W.; Liu, L.; Romberg-Camps, M.J.; Duijsens, L.; Houwen, C.; van der Kuy, P.H.M.; Janknegt, R.; Peeters, R.; Landewé, R.B.M.; Winkens, B.; et al. The nocebo effect challenges the non-medical infliximab switch in practice. Eur. J. Clin. Pharmacol. 2018, 74, 655–661. [Google Scholar] [CrossRef]
- Glintborg, B.; Sørensen, I.J.; Loft, A.G.; Lindegaard, H.; Linauskas, A.; Hendricks, O.; Hansen, I.M.J.; Jensen, D.V.; Manilo, N.; Espesen, J.; et al. A nationwide non-medical switch from originator infliximab to biosimilar CT-P13 in 802 patients with inflammatory arthritis: 1-year clinical outcomes from the DANBIO registry. Ann. Rheum. Dis. 2017, 76, 1426–1431. [Google Scholar] [CrossRef]
- Tweehuysen, L.; van den Bemt, B.J.F.; van Ingen, I.L.; de Jong, A.J.L.; van der Laan, W.H.; van den Hoogen, F.H.J.; den Broeder, A.A. Subjective Complaints as the Main Reason for Biosimilar Discontinuation after Open-Label Transition from Reference Infliximab to Biosimilar Infliximab. Arthritis Rheumatol. 2018, 70, 60–68. [Google Scholar] [CrossRef]
- Valido, A.; Silva-Dinis, J.; Saavedra, M.J.; Iria, I.; Gonçalves, J.; Lopes, J.P.; Fonseca, J.E. Efficacy, immunogenicity and cost analysis of a systematic switch from originator infliximab to biosimilar CT-P13 of all patients with inflammatory arthritis from a single center. Acta Reum. Port. 2019, 44, 303–311. (In English) [Google Scholar]
- Morita, A.; Nishikawa, K.; Yamada, F.; Yamanaka, K.; Nakajima, H.; Ohtsuki, M. Safety, efficacy, and drug survival of the infliximab biosimilar CT-P13 in post-marketing surveillance of Japanese patients with psoriasis. J. Dermatol. 2022, 49, 957–969. [Google Scholar] [CrossRef]
- Provenzano, G.; Arcuri, C.; Miceli, M.C. Open-label non-mandatory transitioning from originators to biosimilars in routine clinical care. Clin. Rheumatol. 2021, 40, 425–427. [Google Scholar] [CrossRef] [PubMed]
- Gisondi, P.; Virga, C.; Piaserico, S.; Meneguzzo, A.; Odorici, G.; Conti, A.; Girolomoni, G. Switching from one infliximab biosimilar (CT-P13) to another infliximab biosimilar (SB2) in patients with chronic plaque psoriasis. Br. J. Dermatol. 2020, 183, 397–398. [Google Scholar] [CrossRef]
- Lauret, A.; Moltó, A.; Abitbol, V.; Gutermann, L.; Conort, O.; Chast, F.; Goulvestre, C.; Le Jeunne, C.; Chaussade, S.; Roux, C.; et al. Effects of successive switches to different biosimilars infliximab on immunogenicity in chronic inflammatory diseases in daily clinical practice. Semin. Arthritis Rheum. 2020, 50, 1449–1456. [Google Scholar] [CrossRef]
- Di Cesare, A.; Tronconi, G.; Fastame, T.M.; Rosi, E.; Pescitelli, L.; Ricceri, F.; Prignano, F. SB5 adalimumab biosimilar in the treatment of psoriasis and psoriatic arthritis. Derm. Ther. 2020, 33, e13435. [Google Scholar] [CrossRef] [PubMed]
- Gladman, D.D.; Antoni, C.; Mease, P.; Clegg, D.O.; Nash, P. Psoriatic arthritis: Epidemiology, clinical features, course, and outcome. Ann. Rheum. Dis. 2005, 64 (Suppl. 2), ii14–ii17. [Google Scholar] [CrossRef]
- Jfri, A.; Nassim, D.; O’Brien, E.; Gulliver, W.; Nikolakis, G.; Zouboulis, C.C. Prevalence of Hidradenitis Suppurativa: A Systematic Review and Meta-regression Analysis. JAMA Dermatol. 2021, 157, 924–931. [Google Scholar] [CrossRef]
- Available online: https://www.aifa.gov.it/sites/default/files/pp_biosimilari_27.03.2018.pdf (accessed on 15 January 2023).
- Vogler, S.; Schneider, P.; Zuba, M.; Busse, R.; Panteli, D. Policies to Encourage the Use of Biosimilars in European Countries and Their Potential Impact on Pharmaceutical Expenditure. Front. Pharmacol. 2021, 12, 625296. [Google Scholar] [CrossRef]
- Gisbert, J.P.; Chaparro, M. Switching from an originator anti-TNF to a biosimilar in patients with inflammatory bowel disease: Can it be recommended? A systematic review. Gastroenterol. Hepatol. 2018, 41, 389–405. [Google Scholar] [CrossRef]
- Moots, R.; Azevedo, V.; Coindreau, J.L.; Dörner, T.; Mahgoub, E.; Mysler, E.; Scheinberg, M.; Marshall, L. Switching between Reference Biologics and Biosimilars for the Treatment of Rheumatology, Gastroenterology, and Dermatology Inflammatory Conditions: Considerations for the Clinician. Curr. Rheumatol. Rep. 2017, 19, 37. [Google Scholar] [CrossRef] [PubMed]
- Krishna, M.; Nadler, S.G. Immunogenicity to Biotherapeutics—The Role of Anti-drug Immune Complexes. Front. Immunol. 2016, 7, 21. [Google Scholar] [CrossRef]
- Mitrev, N.; Leong, R.W. Therapeutic drug monitoring of anti-tumor necrosis factor-α agents in inflammatory bowel disease. Expert. Opin. Drug. Saf. 2017, 16, 303–317. [Google Scholar] [CrossRef] [PubMed]
- van Schouwenburg, P.A.; van de Stadt, L.A.; de Jong, R.N.; van Buren, E.E.; Kruithof, S.; de Groot, E.; Hart, M.; van Ham, S.M.; Rispens, T.; Aarden, L.; et al. Adalimumab elicits a restricted anti-idiotypic antibody response in autoimmune patients resulting in functional neutralization. Ann. Rheum. Dis. 2013, 72, 104–109. [Google Scholar] [CrossRef]
- Bartelds, G.M.; Krieckaert, C.L.; Nurmohamed, M.T.; van Schouwenburg, P.A.; Lems, W.F.; Twisk, J.W.; Dijkmans, B.A.; Aarden, L.; Wolbink, G.J. Development of antidrug antibodies against adalimumab and association with disease activity and treatment failure during long-term follow-up. JAMA 2011, 305, 1460–1468. [Google Scholar] [CrossRef]
- Colloca, L.; Panaccione, R.; Murphy, T.K. The Clinical Implications of Nocebo Effects for Biosimilar Therapy. Front. Pharmacol. 2019, 10, 1372. [Google Scholar] [CrossRef] [PubMed]
- Colloca, L.; Miller, F.G. The nocebo effect and its relevance for clinical practice. Psychosom. Med. 2011, 73, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Kravvariti, E.; Kitas, G.D.; Sfikakis, P.P. The role of the Nocebo effect in the use of biosimilars in routine rheumatology clinical practice. Mediterr. J. Rheumatol. 2019, 30 (Suppl 1), 63–68. [Google Scholar] [CrossRef]
- Sarnola, K.; Merikoski, M.; Jyrkkä, J.; Hämeen-Anttila, K. Physicians’ perceptions of the uptake of biosimilars: A systematic review. BMJ Open 2020, 10, e034183. [Google Scholar] [CrossRef]
- Petit, J.; Antignac, M.; Poilverd, R.M.; Baratto, R.; Darthout, S.; Desouches, S.; Louati, K.; Deparis, N.; Berenbaum, F.; Beauvais, C. Multidisciplinary team intervention to reduce the nocebo effect when switching from the originator infliximab to a biosimilar. RMD Open 2021, 7, e001396. [Google Scholar] [CrossRef]
- Salaffi, F.; Ciapetti, A.; Carotti, M.; Gasparini, S.; Gutierrez, M. Disease activity in psoriatic arthritis: Comparison of the discriminative capacity and construct validity of six composite indices in a real world. Biomed. Res. Int. 2014, 2014, 528105. [Google Scholar] [CrossRef]
- Rothwell, P.M. External validity of randomised controlled trials: “to whom do the results of this trial apply?”. Lancet 2005, 365, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.iqvia.com/-/media/iqvia/pdfs/library/white-papers/the-impact-of-biosimilar-competition-in-europe-2021.pdf (accessed on 15 January 2023).
- Available online: https://www.verywellhealth.com/top-biologic-drugs-2663233#:~:text=Humira&text=The%20anti%2Dinflammatory%20drug%20Humira,drugs%20worldwide%2C%20regardless%20of%20class (accessed on 15 January 2023).
- Kurki, P.; Barry, S.; Bourges, I.; Tsantili, P.; Wolf-Holz, E. Safety, immunogenicity and interchangeability of biosimilar monoclonal antibodies and fusion proteins: A regulatory perspective. Drugs 2021, 81, 1881–1896. [Google Scholar] [CrossRef]
- Scavone, C.; Sportiello, L.; Rafaniello, C.; Mascolo, A.; Sessa, M.; Rossi, F.; Capuano, A. New era in treatment options of chronic hepatitis C: Focus on safety of new direct-acting antivirals (DAAs). Expert. Opin. Drug. Saf. 2016, 15 (Suppl. 2), 85–100. [Google Scholar] [CrossRef] [PubMed]
- Rafaniello, C.; Pozzi, M.; Pisano, S.; Ferrajolo, C.; Bertella, S.; Sportiello, L.; Carnovale, C.; Sullo, M.G.; Cattaneo, D.; Gentili, M.; et al. Second generation antipsychotics in ‘real-life’ paediatric patients. Adverse drug reactions and clinical outcomes of drug switch. Expert. Opin. Drug. Saf. 2016, 15 (Suppl. 2), 1–8. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).