Abstract
The emergence of COVID-19 in March 2020 challenged Zimbabwe to respond with limited medical facilities and therapeutic options. Based on early clinical indications of efficacy for the macrocyclic lactone, Ivermectin (IVM), against COVID-19, IVM-based combination treatments were deployed to treat it. Oxygen saturation (SpO2) data were retrospectively analyzed for 34 severe, hypoxic COVID-19 patients all on room air (without supplemental oxygen). The patients, median age 56.5, were treated at clinics or at home between August 2020 and May 2021. All but three of these 34 patients had significantly increased SpO2 values within 24 h after the first IVM dose. The mean increase in SpO2 as a percentage of full normalization to SpO2 = 97 was 55.1% at +12 h and 62.3% at +24 h after the first IVM dose (paired t-test, p < 0.0000001). These results parallel similar sharp, rapid increases in SpO2, all on room air, for 24 mostly severe COVID-19 patients in the USA (California) who were given an IVM-based combination treatment. All patients in both of these critical series recovered. These rapid increases in SpO2 values after IVM treatment stand in sharp contrast to declines in SpO2 and associated pulmonary function through the second week following the onset of moderate or severe COVID-19 symptoms under standard care.
1. Introduction
Evaluating the efficacy of COVID-19 therapeutics is challenging, since most patients typically recover; the worldwide cumulative case fatality rate was 2% as of September 2021 [1]. Thus, randomized clinical trial (RCT) results are important, yet interpretations, for example, of the mixed results from more than 25 RCTs for COVID-19 treatment with the drug Ivermectin (IVM) [2,3,4,5], including three recent studies with negative results [6,7,8], have been controversial. Yet, such conflicting results have emerged even among RCTs having large patient cohorts, for example those for remdesivir, for which opposite conclusions were drawn by the U.S. FDA (positive) [9] and the World Health Organization (negative) [10] as to efficacy against COVID-19.
Against the backdrop of these mixed RCT results for IVM, a quantifiable determination of whether rapid, major improvements in pulmonary function are achieved shortly after IVM administration to COVID-19 patients can provide an additional means to study efficacy. Such a route of investigation offers additional potential insights when conducted in conjunction with important new studies that indicate potential biological mechanisms by which IVM could rapidly neutralize the morbidity of a SARS-CoV-2 infection [11,12]. The simplest indicator of lung function is blood oxygen saturation level, SpO2, measured with a pulse oximeter. However, the administration of supplemental oxygen to any COVID-19 patient would preclude meaningful pre- and post-treatment comparisons of SpO2 values. For this retrospective study conducted in Zimbabwe, ironically, challenges that constrained treatment capabilities also provided the opportunity to track changes in SpO2 values, all recorded on room air (without supplemental oxygen), for 34 hypoxic, severe COVID-19 patients within 12 to 48 h after beginning IVM-based combination treatment (see Figure 1). Details of the inclusion and exclusion criteria applied that resulted in the selection of these 34 patients are provided in the Materials and Methods Section below. It should be noted that while inferences can be drawn as to these changes in SpO2 values over a 48 h period following administration of ivermectin, as detailed below, other conclusions are limited by a lack of control groups, randomization, or follow-up.
Zimbabwe, a landlocked country in Southern Africa that shares a border with South Africa, had its first reported case of COVID-19 in March 2020 [13]. Eight cases and one death from COVID-19 followed in the same month [14]. COVID-19 wards were created at a general practice clinic by converting two staff rooms into a four-bed ward and a storeroom into a two-bed ward. Available equipment included several oxygen cylinders, an oxygen concentrator, six beds, and three monitors for SpO2 and blood flow parameters. The staff consisted of the lead author, another primary care physician, and either one or two nurses at different times, each on 12 h shifts. During the initial months of the pandemic, in the absence of proven therapies and protocols, the standard of care evolved through early August 2020 to include corticosteroids, anticoagulants, and antibiotics, as used elsewhere in the world during this period.
However, the efficacy of these treatments was found to be limited, and by the end of July 2020, several COVID-19 deaths were recorded in the country. Based on reports of initial success using IVM for COVID-19 treatment from colleagues in Johannesburg, South Africa, the College of Primary Care Physicians of Zimbabwe (CPCPZ) adopted and included IVM in their COVID-19 treatment protocol from 8 August 2020, starting initially with a 10–12 mg stat dose. Treatment of COVID-19 with IVM continued after the first patients showed improved outcomes, with more rapid recoveries achieved at doses higher than the standard of 200 ug/kg, as initially used. In August 2020, after it became apparent that IVM added to standard of care was significantly reducing the death rate, together with the hospital system being overwhelmed, CPCPZ physicians decided to treat COVID-19 patients where an IVM-based protocol could be administered, including at local general practice clinics that had nursing care and oxygen and at some patients’ homes with nursing support and oxygen supplementation as available.
IVM for COVID-19 Treatment
The decision to include IVM in COVID-19 treatment protocols in Zimbabwe was made as the pandemic swept through that nation, overwhelming limited clinical care facilities, with no drug developed to treat COVID-19 being generally accepted as effective. A published case–control study of IVM treatment for COVID-19 conducted at four U.S. hospitals [15] that had been initially released in a preprint in June 2020 found a 40% reduction in mortality among 173 patients treated with low-dose IVM vs. 107 case-matched controls (15% vs. 25.2% deaths). Interest in IVM was supported by its Nobel prize-honored pedigree and its extensive use to treat a variety of human diseases in over 3.7 billion doses worldwide since 1987 [2,16,17]. Another favorable characteristic of this drug is its extraordinary record of safety, well tolerated at high doses [18,19], including in studies for COVID-19 treatment [6,20]. It is generally non-toxic even at doses far exceeding the therapeutic range [21,22]. Since August 2020, inpatient and outpatient treatments of COVID-19 with IVM have been applied across 25 countries [2], with more than 20 RCTs conducted for IVM treatment regimens [2,3,4,5].
A comprehensive review of clinical studies for IVM treatment of COVID-19 by the Nobel co-laureate for IVM, Dr Satoshi Omura, and colleagues, concluded that IVM yielded major reductions in mortality [2]. As noted above, however, three recent RCTs for IVM treatment of COVID-19 had negative results [6,7,8]. However, one of these had multiple protocol violations, with 38 doses of IVM mistakenly substituted for placebo and blinding violated with a glucose placebo [6,23], while related concerns were raised for another [7,24]. Two animal studies of IVM treatment at low human-equivalent doses, one for the SARS-CoV-2 virus in golden hamsters [25] and another for a related betacoronavirus (MHV-A59) in mice [26], found statistically significant treatment benefits. An indicated key biological mechanism of IVM, competitive binding with the SARS-CoV-2 spike protein [11,12], is likely non-epitope-specific, possibly yielding full efficacy against emerging viral mutant strains.
The demonstrated safety of IVM at much higher than standard doses [6,18,19,20] allowed the latitude for dose escalation for IVM treatment of COVID-19 over time, and combination therapy centered around IVM plus doxycycline and zinc became the standard COVID-19 treatment protocol used by the CPCPZ. This application of multiple drugs against COVID-19 was based on Zimbabwe’s experience with prior infectious diseases, for which early, aggressive use of multiple drugs has been a core treatment principle. The potential efficacy of these adjuncts was later supported by successful clinical trial results with treatments using IVM in combination with doxycycline [27] or with doxycycline and zinc [28]. This combination therapy for COVID-19 has been researched and advanced by Thomas Borody [5], who, in 1990, published the first clinical trial of using a triple therapy of three inexpensive repurposed drugs for H. pylori [29], the underlying bacterial cause of peptic ulcers. This triple therapy of repurposed drugs became the worldwide standard of care for peptic ulcers a decade later, after the patents for the palliative drugs Tagamet and Zantac expired, and the discovery of H. pylori as the cause of peptic ulcers was honored with the Nobel Prize for Medicine in 2005 [5].
2. Results
Table 1 shows the age group, sex, and range of SpO2 values prior to treatment for the 34 patients in this study. Figure 1 and Figure 2 and Table S1 show the progression of pre-treatment and post-treatment SpO2 values within 48 h after the start of treatment (including first dose of IVM) for the 34 COVID-19 patients of this study, all of whom were treated with IVM, doxycycline, and zinc. All of these patients recovered. SpO2 values are shown at pre-treatment, all recorded within one hour before the start of treatment (first dose of IVM) and within 12, 24, and 48 h after start of treatment. The SpO2 value shown for a given patient at time x is that for the latest post-treatment time ≤ x (thus, for example, for a patient having an SpO2 measurement at Hour 17 as the only one in the first 48 h after initial treatment, which would be used as their +24 and +48 h values).

Table 1.
Patient age group, sex, and range of pre-treatment SpO2 values.
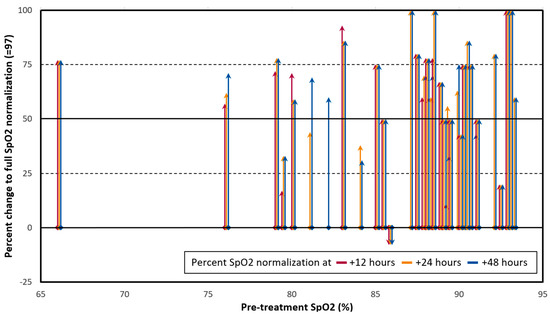
Figure 1.
Changes in pre- to post-treatment SpO2 values, all on room air, for 34 severe COVID-19 patients treated with Ivermectin (IVM), doxycycline, and zinc, as percentages of optimal normalization to SpO2 = 97. Red, orange, and blue lines represent, respectively, changes in SpO2 values at +12, +24, and +48 h after the first dose of IVM. Arrows were shifted to the right slightly (<1.5 × axis units), as needed for clarity. For pre- and post-treatment SpO2 values S0 and S1, the y axis is the percent of optimal normalization to SpO2 = 97, that is: 100 × (S1 − S0)/(97 − S0), capped at 100%. Mean (±SD) SpO2 changes (y values) were 55.1% ± 28.0% at +12 h, 62.3% ± 26.3% at +24 h, and 64.3% ± 24.5% at +48 h.
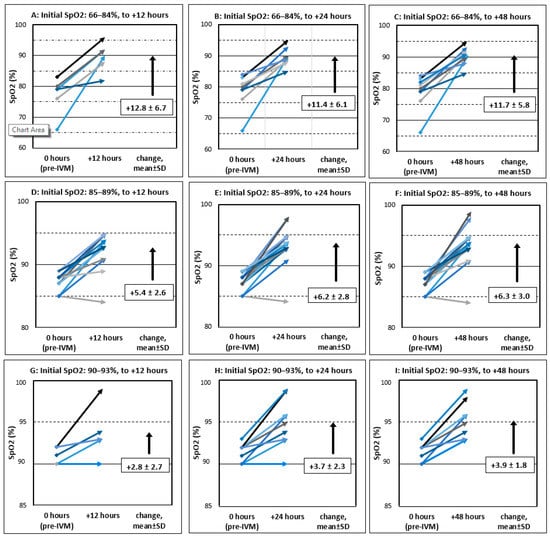
Figure 2.
Pre- and post-treatment SpO2 values, all on room air, for 34 severe COVID-19 patients treated with IVM, doxycycline, and zinc. Graphs A–I are in horizontal groupings by the range of pre-treatment SpO2 values and in vertical groupings by elapsed time after first dose of IVM. (The lowest SpO2 value in the stipulated range of 51–93% was 66%.) For these elapsed times of x = 12, 24, and 48 h, the SpO2 value shown for a given patient is at the latest post-treatment time ≤ x. All pre-treatment SpO2 values are from within one hour before the start of treatment.
Figure 1 shows, for all 34 patients, SpO2 value changes from before IVM administration to post-treatment as percentages of full normalization to an optimal SpO2 value of 97 (95 is considered the minimum normal SpO2 value for a healthy child or adult by the U.S. CDC [30]). Red, orange, and blue lines show, respectively, SpO2 values at +12, +24, and +48 h post-treatment. The mean (±SD) SpO2 changes as this specified percent of optimal normalization were 55.1% ± 28.0% at +12 h, 62.3% ± 26.3% at +24 h, and 64.3% ± 24.5% at +48 h. As shown in Figure 1, the bulk of the patients had 50% or more SpO2 normalization within 48 h, while the sickest 3 (pre-treatment SpO2 ranging from 66 to 78%) improved by over 70%.
Figure 2 shows all pre- and post-treatment SpO2 values grouped into nine graphs (A–I) by the range of pre-treatment SpO2 values and post-treatment times (after first IVM dose) of +12, +24, and +48 h. As shown in Figure 2 and identically in Table 2, those patients with the lowest, mid-range, and highest pre-treatment SpO2 values had mean SpO2 increases at +12 h of 12.8, 5.4, and 2.8, respectively (all SpO2 values in percentage units). Figure 3 shows all pre- and post-treatment SpO2 values throughout the entire observation period for each of the 34 patients. Note that these values were recorded less frequently after a patient’s SpO2 value had increased above severe COVID-19 levels (≤93) and general condition had improved.

Table 2.
Mean (± SD) changes in SpO2 values from pre- to post-treatment with IVM-based combination therapy.
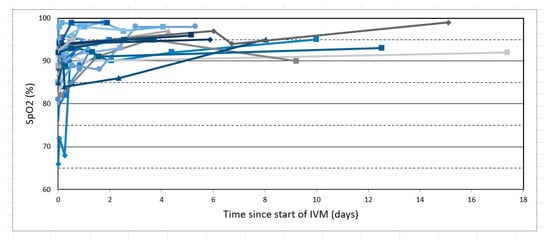
Figure 3.
All SpO2 values (on room air) for the 34 severe COVID-19 patients of this study, all treated with IVM-based combination therapy including IVM, doxycycline, and zinc. Each patient is represented by joined line segments of a different color, with markers designating SpO2 values recorded.
Figure 4 shows successive SpO2 values for one patient who had a particularly rapid increase in these values after his first dose of IVM. This patient was a 25-year-old male, treated by a CPCPZ physician at a GP clinic without a supplemental oxygen capability. He received his first 12 mg IVM dose (repeated over the next four days) immediately after entering the clinic with respiratory distress and bilateral pneumonia indicated by stethoscopic examination. His COVID-19 diagnosis was confirmed by a positive result from a rapid antigen test. As shown, his SpO2 values increased from that recorded immediately before treatment (79%) to values at 45 min (87%), 90 min (92%), and 3 h (95%) post-treatment. He was discharged later that same day, and his home SpO2 readings then fluctuated between 92% and 95% over the next three days. By the fourth day after discharge, his SpO2 stabilized at 97%, his pulse dropped to 77 from prior values over 100, and he resumed working from home. Several other patients in this series likewise experienced some degree of tachycardia; however, these were generally manifested prior to the administration of IVM and did not become more pronounced after IVM dosing. While the increases in SpO2 for most of these 34 patients occurred less rapidly, over 48 h, than for this case, as shown in Figure 1, Figure 2 and Figure 3 and Table S1, several other patients had SpO2 increases of 12% or more within 12 h after the first IVM dose (Table S1): SpO2 76% to 88% for a 38-year-old female; 83% to 96% for a 45-year-old male; 66% to 90% for a 49-year-old female; and 80% to 92% for a 75-year-old male.
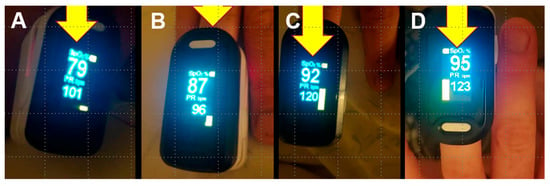
Figure 4.
Changes in SpO2 over 3 h for a COVID-19 patient after administration of IVM. SpO2 values are shown (all on room air), with pulse rates (bpm) below those, at pre-treatment (A) and for 45 min (B), 90 min (C), and 3 h (D) after first dose of IVM. This patient, a 25-year-old male, was diagnosed with COVID-19 by a positive result from a rapid antigen test and treated with combination therapy including IVM, doxycycline, and zinc plus other oral and nebulized adjunct agents from among those itemized above.
As shown in Figure 2, all but two of the 34 patients in this study had increases in SpO2 within the first 48 h after the first dose of IVM. This overall increase in SpO2 continued throughout the entire observation period (Figure 3). Because SpO2 values that are at best stable and typically decreasing for several days after onset of disease symptoms are a well-established norm for moderate and severe COVID-19 patients under standard care, as discussed and shown in Figure 5 and Figure 6 in the Discussion Section below, these sharp, rapid SpO2 increases for all but two of the 34 patients in this study are noteworthy. Furthermore, given this expected change in SpO2 of ≤0 within 12, 24, or 48 h after the first IVM dose in the absence of an efficacious intervention against COVID-19, one-tailed paired t-test calculations can be performed to assess whether the change in SpO2 values was significantly greater than zero at each time period tracked. Taking into account some missing post-treatment values (see Table S1), there are 25, 33, and 34 pairs of pre- and post-treatment SpO2 values at +12 h, +24, and +48 h, respectively. Applying these paired t-test calculations, the SpO2 increases were highly significant for each time period: t = 6.28, p = 8.5 × 10−7 at +12 h; t = 8.42, p = 6.36 × 10−10 at +24 h, and t = 8.81, p = 3.47 × 10−10 at +48 h.
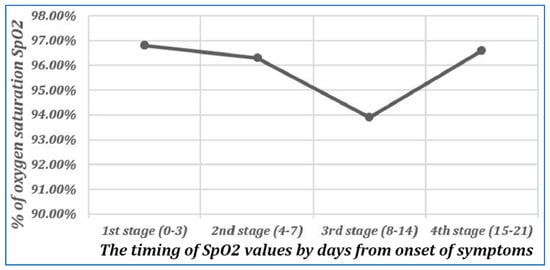
Figure 5.
Changes in oxygen saturation during different stages of follow-up of cases with COVID-19 infection. Reproduced from Osman et al., 2020 [31] (CC-BY 4.0). These values were obtained from 125 PCR-confirmed COVID-19 inpatients, March-August 2020, with all values obtained on room air except for 3 patients in Stages 1 and 2, 13 in Stage 3, and 1 in Stage 4. As shown, the mean value of SPO2 decreases from Days 0–3 to Days 8–14; it is inversely correlated with the severity of pulmonary CT abnormalities in these patients throughout the full period of observation.
No serious adverse effects (AEs) from IVM treatment were observed in any patient, although transient AEs such as blurred vision characteristic of higher-dose IVM administration were observed in some patients given doses as high as 100 mg. While comparative results using higher vs. lower doses were not systematically tracked, the practice of increasing IVM doses for patients not initially responding to treatment had generally successful outcomes and became the standard protocol after December 2020.
3. Discussion
This study is a retrospective review of clinical data collected amid the challenges of providing treatment with limited facilities and resources to COVID-19 patients with severe disease. Under such conditions, it was not possible to obtain blood test values for all patients, including values for lymphocyte count, LDH, D-Dimer, and CRP, which were thus not analyzed. On the other hand, the lack of availability of oxygen supplementation for many of the patients treated in Zimbabwe provided the rare opportunity to track changes in SpO2 values all recorded on room air before and after administration of IVM for this group of 34 severe COVID-19 patients, 74% of them presenting with SpO2 values below 90% and 29% presenting with SpO2 85% and below.
It should be noted that this retrospective review is limited in its conclusions by the lack of control groups, randomization, or follow-up. Although, as noted, all of these 34 severe COVID-19 patients in this series survived, no conclusions as to mortality benefits can be drawn due to the absence of randomized controls. The one clinical parameter for which conclusions can be reasonably drawn is for the change in SpO2 values during the course of a 48 h period following IVM administration, since as detailed below, there is an extensive set of consistent baseline data on changes in SpO2 during the course of a COVID-19 infection under standard care.
The mean (±SD) SpO2 normalization, as reported above, was 55.1% ± 28.0% at +12 h, which rose to 62.3% ± 26.3% at +24 h and then to 64.3% ± 24.5% at +48 h after the first IVM dose. Paired t-test calculations yield p < 0.0000001 for each of the SpO2 increases, at +12, +24, or +48 h, having occurred by chance if the IVM-based combination therapy applied had no clinical activity against COVID-19. These highly significant p-values are based on the well-grounded assumption of expected change in SpO2 values of ≤0 during this time period. Moderate and severe COVID-19 patients under standard care typically manifest decreasing SpO2 values in tandem with increasing pulmonary CT abnormalities from the day of onset of disease symptoms through the second week following, as established in several studies that tracked SpO2 values, pulmonary abnormalities, or both [31,32,33,34,35,36,37]. For example, Figure 5 shows SpO2 values recorded over three weeks for COVID-19 patients under standard care [31], with decreasing SpO2 values through Stage 3, 8–14 days after the onset of symptoms.
The most useful baseline of SpO2 values for comparison would be those on room air for COVID-19 patients having pre-treatment SpO2 values ≤ 93% (severe COVID-19), as obtained in this study. Yet such SpO2 data are rarely obtained, since severe COVID-19 patients would typically receive supplemental oxygen. However, results without supplemental oxygen were provided in one study of COVID-19 patients in Nigeria (Thairu et al., 2022), which tracked SpO2 values prior to standard treatment and for ten days following [38] as compared with results of a prior study in which IVM treatment was provided in a similarly scheduled regimen [39]. The patients in the standard treatment series were given varying combinations of lopinavir/ritonavir (Alluvia), remdesivir, azithromycin, and enoxaparin (low-molecular-weight heparin, administered to all patients), along with zinc sulfate and vitamin C. The standard treatment regimen used by Thairu et al., 2022, was aggressive; some studies using just heparin as a mainstay of COVID-19 treatment reported successful results [40,41,42]. In a molecular modeling study that screened 100 agents [43], heparin was found to have binding affinity to the SARS-CoV-2 spike protein second highest to that of IVM, indicating the potential for heparin, like IVM, to inhibit virally caused morbidities affecting blood oxygenation efficiency [12].
The results for Thairu et al., 2022, its standard treatment group, as shown in Figure 6, are for those 26 COVID-19 patients who had pre-treatment SpO2 values ≤ 93% (severe COVID-19), and who were tracked only on room air per limitations on availability of supplemental oxygen [38,44]. Mean SpO2 remained at or below its pre-treatment value through Day 4 and then began to increase at Day 5. Despite the potentially greater than typical improvements in SpO2 for patients under standard care in Thairu et al., 2022, given use of enoxaparin, mean SpO2 did not increase through Day 4, as shown in Figure 6, in contrast to the sharp increases of SpO2 values through Day 2 for our study’s 34 patients.
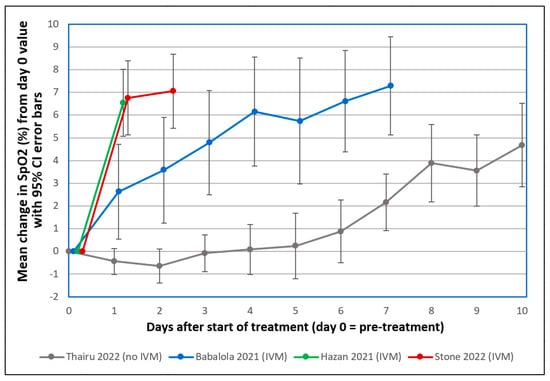
Figure 6.
Mean changes in oxygen saturation (SpO2) for severe COVID-19 patients following treatments including or excluding IVM. Patients tracked over various time periods from each regimen were those with SpO2 values all recorded on room air, having pre-treatment (day 0) values ≤ 93%. The y axis value at day n is the mean of changes in SpO2 values from day 0 to day n, with error bars designating 95% confidence intervals. ● Thairu et al., 2022 [38,44]: 26 patients, median age 45, treated with varying combinations of lopinavir/ritonavir (Alluvia), remdesivir, azithromycin, and enoxaparin plus zinc sulfate and vitamin C. ● Babalola et al., 2021 [39,44]: 19 patients, median age 33, treated with IVM, zinc and vitamin C, with some also given azithromycin and hydroxychloroquine. ● Hazan et al., 2021 [45]: 19 patients, median age 63, treated with IVM, doxycycline and zinc. ● Stone et al., 2022 (this study): 34 patients, median age 56.5, treated with IVM, doxycycline and zinc, as described.
Our study’s results, including the recoveries of all 34 patients, parallel those recently reported by Hazan et al., 2022, for which SpO2 values all on room air for 24 RT-qPCR confirmed COVID-19 patients in California (USA) who were tracked before and +24 h after treatment with IVM, doxycycline, and zinc [45]. For the 19 severe COVID-19 patients in that series who had pre-treatment SpO2 values of 90% or below (minimum = 77%) and had post-treatment SpO2 values recorded at +24 h (see Table S2), the mean (±SD) SpO2 values rose from 86.7% ± 4.5% pre-treatment to 93.3% ± 2.6% at +24 h after the first IVM dose. Per our calculation of the percentage of normalization to SpO2 = 97, the mean (±SD) relative SpO2 increase at 24 h post-treatment for these 19 patients was 65.2% ± 17.5%, which is close to the mean (±SD) of 62.3% ± 26.3% for the 34 patients in our study. The one-tailed paired t-test for these increased SpO2 values in these 19 patients yielded t = 9.34, p < 0.0000001, which, as for our Zimbabwe study, is highly significant.
In a third study, Babalola et al., 2021, for 19 severe COVID-19 patients in Nigeria, SpO2 values increased significantly one day after the first dose of IVM (p < 0.008, one-tailed paired t-test) [39,44]. SpO2 values increased even more markedly at two days after the first dose of IVM (p < 0.0023, one-tailed paired t-test). All of the patients in the Hazan and Babalola studies recovered (38 of these 43 total patients having severe COVID-19 status, as noted), as did all our Zimbabwe patients.
The similar results for the 34 severe COVID-19 patients in our Zimbabwe SpO2 series and the 19 severe COVID-19 patients of Hazan et al., 2022 [45], are both sharper than the significant SpO2 increases obtained by Babalola et al., 2021 [39] and suggest that the triple therapy of IVM, doxycycline, and zinc provides enhanced efficacy with respect to IVM used with other adjuncts. One limitation of our study is that except for nebulized nano-silver, used for all patients at the start of the treatment regimen, other adjuncts were deployed on a case-by-case basis, per the patient’s condition. Furthermore, IVM dosages were increased during the study period based on the observations that no serious AEs were observed at higher doses, and higher doses appeared to be more effective for the patients with the most severe symptoms. A follow-up clinical study of IVM-based combination treatment of COVID-19 would benefit from a more structured specification and tracking of dosages and adjuncts used.
The distinct improvements in respiratory function for our study parallel those reported by Hazan et al., 2022, and Babalola et al., 2021, and provide a quantifiable demonstration of rapid clinical improvement in severe COVID-19 patients after IVM treatment. Such rapid improvements had been observed since the first major clinical trial in Florida, USA, of IVM treatment of COVID-19 [15]. The lead investigator of that clinical trial had observed that stabilization and then improvement in breathing function along with blood oxygenation as tracked by SpO2 frequently occurred within 12–48 h after IVM treatment, even for patients who had been deteriorating rapidly and had required supplemental oxygen at up to a 50% mixture [46]. That Florida study also reported a 40% reduction in mortality in the IVM-treated group compared with propensity-matched case controls. Likewise, for a larger series of 92 severe COVID-19 patients treated in Zimbabwe with IVM, doxycycline, and zinc and other agents, the number of case fatalities (two) and deteriorations followed by recoveries (two) were much lower than predicted for a comparable set of patients using a well-regarded risk assessment model [47]. Yet, this risk model assessment is suggestive only, and as stated earlier, no conclusions regarding mortality or deteriorations can be drawn given the lack of controls in this study.
The SpO2 increases within a day after IVM treatment observed in this study and by Hazan et al., 2022, and Babalola et al., 2021, offer a distinct indication that IVM yields rapid, directly observable resolution of pulmonary dysfunction as tracked by SpO2 values for COVID-19 patients. This finding offers clues as to the potential biological mechanism of IVM activity against SARS-CoV-2 since, for example, even an effective freeze on viral replication or rapid repair of damaged pulmonary alveoli would be unlikely to yield such rapid clinical improvements. One indicated biological mechanism of IVM activity, competitive binding with the SARS-CoV-2 spike protein [11,12,48], may act quickly, through a reversal of virally induced blood cell clumping, to increase pulmonary capillary flow and, in turn, account for normalization of blood oxygenation. Another plausible mechanism of IVM activity, based on strong binding affinity of IVM to the alpha 7 cholinergic receptor a7nAChR, as shown in silico [11], is the activation of the cholinergic anti-inflammatory pathway under the control of the vagus nerve [49], which is regulated by acetylcholine and potentiated by the high-affinity binding of IVM (a positive allosteric modulator) to a7nAChR [50] expressed on bronchial, vascular, as well as to cytokine-producing cells (i.e., TNF, IL1- and IL6-secreting macrophages, lymphocytes, and mast cells) [11,51]. Through the stimulation of a related a7nAChR pathway, IVM may also be able to induce vasodilation [11].
It should be noted that similar rapid normalizations of SpO2 deficits were observed in this study and Hazan et al., 2022 [45], which both used IVM, doxycycline, and zinc; and in Rajter et al., 2020, conducted in Florida [15], which used IVM added to the standard of care, compared with controls using the standard of care without IVM. In Babalola et al., 2021 [39], similar rapid increases in SpO2 were observed using IVM, zinc, and vitamin C, as well as hydroxychloroquine and azithromycin for some patients (no significant differences in outcomes were found with or without those two additional agents; the results with and without those two added agents are combined in Figure 6). Although it is reasonable to deduce that since IVM was the common agent in all these studies and the biological mechanisms noted provide a reasonable explanation for their reported rapid increases in SpO2, without appropriate controls, it is impossible to positively conclude that it was IVM and not various combinations of other adjuncts that caused the SpO2 increases observed.
For our study, rapid increases in SpO2 values after the start of IVM-based combination treatment occurred for all but two of these 34 patients and resulted in recoveries for all of them. As noted above, no serious adverse effects (AEs) from IVM treatment were observed in any patient, and while comparative results using higher vs. lower doses were not systematically tracked, the practice of increasing IVM doses for patients not initially responding to treatment had generally successful outcomes.
4. Materials and Methods
This study is a retrospective review of clinical data collected for 34 severe COVID-19 patients for whom SpO2 values were monitored, all on room air, during the course of their treatment, with therapeutic agents selected by their physicians to offer the greatest chances for clinical benefits and recovery. Clinical data were collected for additional patients in Zimbabwe for whom SpO2 values on room air were not recorded, thus not fitting the inclusion criteria of this analysis, with mortality and deterioration outcomes for that larger patient series having been previously reported [47].
4.1. Outcomes
Outcomes tracked were changes in SpO2 values from within one hour before treatment to 12, 24, and 48 h after start of treatment (first dose of IVM), for 34 COVID-19 patients for whom a pre-treatment SpO2 value and at least one SpO2 value up to +48 h after start of treatment were available, all obtained on room air.
4.2. Participants
The 34 COVID-19 patients of this study were from Harare, treated by CPCPZ physicians either at local clinics or at patients’ homes.
4.3. Inclusion and Exclusion Criteria
Patients selected for analysis were of age 18 or older and had treatment start dates between 8 August 2020 and 31 May 2021. Patients selected had an SpO2 value on intake of 51–93%, with all SpO2 values recorded and documented to have been obtained on room air (in almost every case because oxygen was not available), and at least one SpO2 value obtained within 48 h after IVM administration. These patients thus fit the U.S. National Institutes of Health’s definition of severe COVID-19, a sufficient condition of which is an SpO2 value of 93% or below [52]. Patients were required to have been found COVID-19 positive either by a positive PCR test (real-time PCR, nasal-pharyngeal swabs) or a clinical diagnosis made by criteria including exposure to a COVID-19 patient, hypoxia, lymphopenia, monocytosis, elevated LDH, elevated dimer, and/or radiology consistent with pulmonary abnormalities caused by the virus. PCR testing was not performed for all patients because, in addition to having significantly depressed SpO2 values ≤ 93%, several patients had other symptoms of serious concern and required immediate treatment before the results of a PCR test could be obtained.
4.4. Treatment
All patients were administered an IVM-based combination therapy also including doxycycline and zinc plus selected other agents from the standard of care used prior to August 2020. These agents used in combination with IVM included corticosteroids, clopidogrel, aspirin, enoxaparin, a nebulized nano-silver preparation (applied for all patients), rivaroxaban, zinc sulfate, azithromycin, doxycycline, and, in some cases, an IV antibiotic. Patients treated in a clinic were assessed by a nurse upon admittance, with blood drawn and PCR tests conducted as feasible given the patient’s condition and with severe symptoms necessitating immediate treatment. For those patients who contacted a CPCPZ physician from home requesting treatment, an online questionnaire was first completed by the patient, after which, if COVID-19 was still a suspected diagnosis, a nurse visit to the home was conducted, with associated follow-up per the procedures described for in-clinic patients.
As evidence of IVM safety and tolerability accrued following its use beginning in August 2020, its stat dose of 10 mg as used for the earliest patients was increased on 11 September 2020 to 10–12 mg every four days for three doses. Subsequently, the dosage was further increased to 12 mg IVM on the day of admission and then on Days 4 and 8 plus doxycycline (100 mg b.i.d.) and zinc sulfate (60 mg/day). The latter regimen was used up through December 2020, when the second pandemic wave emerged in Zimbabwe. At that time, additional evidence of the safety and tolerability of this regimen supported further dose escalation to a standard IVM dose regimen of 12 mg daily for five consecutive days, with adjunct use of doxycycline and zinc sulfate continued at the doses noted. In some cases, for which this standard treatment regimen did not yield significant clinical gains within a few days, even higher doses of IVM were used, in some cases as high as 100 mg for a single dose. Transient adverse effects (AEs) such as blurred vision characteristic of high-dose IVM often occurred at those dose levels, but no serious AEs associated with IVM were manifested in any patient.
4.5. Data Collection
SpO2 values were tracked using monitors that continually displayed readings for pulse rate and blood pressure and waveform images of blood pulses. For those patients treated at home, the intake nurse provided a pulse oximeter to the patient, unless the patient had one that the nurse deemed of reliable quality. The patient or a family member took SpO2 readings regularly, using the same oximeter as used for the pre-treatment reading. In most cases, these readings were taken daily, with much lower frequency after SpO2 values had risen significantly and the patient’s clinical condition had correspondingly improved. Patients were instructed to message the nurse immediately if SpO2 ever decreased from a higher value to 93% or below or if any new clinical symptoms of concern developed.
4.6. Analytical Methods
For the 34-patient severe COVID-19 patients of this study, a measure of percent normalization toward a fully optimal SpO2 value of 97 was applied, which for pre- and post-treatment SpO2 values S0 and S1, was: 100 × (S1−S0)/(97−S0), capped at 100%. Pre- and post-treatment SpO2 values are plotted for all 34 patients using this percent normalization measure in Figure 1 and are also presented directly in Figure 2 and Figure 3 and Table S1.
5. Conclusions
Marked, rapid normalizations of blood oxygenation, p < 0.0000001 (paired t-test), were obtained in each time period analyzed for the 34 severe COVID-19 patients in this study, all treated with IVM-based combination therapy. These results parallel similar rapid SpO2 increases reported by Hazan et al., 2022, for 19 severe COVID-19 patients having pre-treatment SpO2 ≤ 90, also at p < 0.0000001, with all SpO2 values likewise on room air. Similar results for IVM treatment were also obtained by Babalola et al., 2021, for 19 severe COVID-19 patients, with paired t-test values of p < 0.008 at Day 1 and p < 0.0023 at Day 2. These sharp, rapid increases in SpO2, accompanied by the survival of all patients in this Zimbabwe study and the California (Hazan) and Nigeria (Babalola) clinical series stand in sharp contrast to the, at best, stable and typically decreasing SpO2 values for several days after the onset of moderate or severe COVID-19 symptoms, even under aggressive non-IVM-based treatment regimens. These SpO2 increases rapidly following the first IVM dose establish a cause-and-effect clinical benefit for IVM-based combination treatment of this disease.
For the 34 patients of this study, treatment at home or in clinics with basic facilities freed up hospital resources for other patients, and the treatment approach modeled in these studies could significantly relieve the pressure on overwhelmed health facilities. IVM is widely available worldwide, inexpensive, and one of the safest drugs in modern medicine, with its safety in “improving the health and wellbeing of millions” noted explicitly by the Nobel Committee in awarding its 2015 prize for the discovery of IVM [53]. These study results, therefore, support the extended deployment of IVM-based combination treatment for COVID-19, complementary to immunizations for prevention.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biologics2030015/s1, Table S1: Changes in SpO2 for 34 COVID-19 patients treated with IVM, doxycycline, and zinc.; Table S2: Changes in SpO2 for 19 COVID-19 patients treated with IVM, doxycycline, and zinc as reported by Hazan et al., 2022.
Author Contributions
Conceptualization, J.C.S., P.N. and M.G.G.; methodology, J.C.S., M.G.G. and C.A.; investigation: J.C.S. and P.N.; software, B.M.D. and D.E.S.; validation, B.M.D., J.D. and D.E.S.; formal analysis, B.M.D., J.D. and D.E.S.; writing—original draft preparation, J.C.S., D.E.S. and C.A.; writing—review and editing, B.M.D., J.D., P.N. and D.E.S.; project administration, J.C.S. and P.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of The Medical Research Council of Zimbabwe (Protocol Code E293, 23 June 2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data underlying the study cannot be made publicly available on an unrestricted basis due to ethical concerns about patient confidentiality. Data will be made available to qualified researchers upon request to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AE | Adverse effect |
| CPCPZ | Primary Care Physicians of Zimbabwe |
| COVID-19 | coronavirus disease 2019 |
| IVM | ivermectin |
| RCT | randomized clinical trial |
References
- Worldometer Coronovirus Statistics. Available online: https://www.worldometers.info/coronavirus/#countries (accessed on 22 September 2021).
- Yagisawa, M.; Foster, P.J.; Hanaki, H.; Omura, S. Global trends in clinical studies of ivermectin in COVID-19. Jpn. J. Antibiot. 2021, 74, 44–95. [Google Scholar]
- Kory, P.; Meduri, G.U.; Varon, J.; Iglesias, J.; Marik, P.E. Review of the emerging evidence demonstrating the efficacy of ivermectin in the prophylaxis and treatment of COVID-19. Am. J. Ther. 2021, 28, e299–e318. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.; Garratt, A.; Levi, J.; Falconer, J.; Ellis, L.; McCann, K.; Pilkington, V.; Qavi, A.; Wang, J.; Wentzel, H. Meta-analysis of randomized trials of ivermectin to treat SARS-CoV-2 infection. Open Forum Infect. Dis. 2021, 8, ofab358. [Google Scholar] [CrossRef] [PubMed]
- Santin, A.D.; Scheim, D.E.; McCullough, P.A.; Yagisawa, M.; Borody, T.J. Ivermectin: A multifaceted drug of Nobel prize-honored distinction with indicated efficacy against a new global scourge, COVID-19. N. Microbes N. Infect. 2021, 43, 100924. [Google Scholar] [CrossRef]
- López-Medina, E.; López, P.; Hurtado, I.C.; Dávalos, D.M.; Ramirez, O.; Martínez, E.; Díazgranados, J.A.; Oñate, J.M.; Chavarriaga, H.; Herrera, S.; et al. Effect of ivermectin on time to resolution of symptoms among adults with mild COVID-19: A randomized clinical trial. JAMA 2021, 325, 1426–1435. [Google Scholar] [CrossRef]
- Lim, S.C.L.; Hor, C.P.; Tay, K.H.; Mat Jelani, A.; Tan, W.H.; Ker, H.B.; Chow, T.S.; Zaid, M.; Cheah, W.K.; Lim, H.H.; et al. Efficacy of ivermectin treatment on disease progression among adults with mild to moderate COVID-19 and comorbidities: The I-TECH randomized clinical trial. JAMA Intern. Med. 2022, 182, 426–435. [Google Scholar] [CrossRef]
- Reis, G.; Silva, E.A.S.M.; Silva, D.C.M.; Thabane, L.; Milagres, A.C.; Ferreira, T.S.; dos Santos, C.V.Q.; Campos, V.H.S.; Nogueira, A.M.R.; de Almeida, A.P.F.G.; et al. Effect of early treatment with ivermectin among patients with COVID-19. N. Engl. J. Med. 2022, 386, 1721–1731. [Google Scholar] [CrossRef]
- U.S. National Institutes of Health. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines: Therapeutic Management of Patients with COVID-19; U.S. National Institutes of Health: Bethesda, MD, USA, 2021. Available online: https://www.covid19treatmentguidelines.nih.gov/ (accessed on 12 May 2022).
- World Health Organization. WHO Recommends against the Use of Remdesivir in COVID-19 Patients; World Health Organization: Geneva, Switzerland, 2020; Available online: https://www.who.int/news-room/feature-stories/detail/who-recommends-against-the-use-of-remdesivir-in-covid-19-patients (accessed on 12 May 2022).
- Aminpour, M.; Cannariato, M.; Safaeeardebili, M.E.; Preto, J.; Moracchiato, A.; Doria, D.; Donato, F.; Zizzi, E.A.; Deriu, M.A.; Scheim, D.E.; et al. In silico analysis of the multi-targeted mode of action of ivermectin and related compounds. Computation 2022, 10, 51. [Google Scholar] [CrossRef]
- Scheim, D.E. A deadly embrace: Hemagglutination mediated by SARS-CoV-2 spike protein at its 22 N-glycosylation sites, red blood cell surface sialoglycoproteins, and antibody. Int. J. Mol. Sci. 2022, 23, 2558. [Google Scholar] [CrossRef]
- Chirisa, S. Zimbabwe Confirms Its First Case of Coronavirus, 20 March 2020. Available online: https://iharare.com/zimbabwe-confirms-first-case-of-coronavirus-2/ (accessed on 12 May 2022).
- WHO. Coronavirus Disease 2019 (COVID-19), Situation Report—72; World Health Organization: Geneva, Switzerland, 2020; Available online: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200401-sitrep-72-covid-19.pdf?sfvrsn=3dd8971b_2 (accessed on 12 May 2022).
- Rajter, J.C.; Sherman, M.S.; Fatteh, N.; Vogel, F.; Sacks, J.; Rajter, J.-J. Use of ivermectin is associated with lower mortality in hospitalized patients with COVID-19 (ICON study). Chest 2020, 159, 85–92. [Google Scholar] [CrossRef]
- Campbell, W.C. History of avermectin and ivermectin, with notes on the history of other macrocyclic lactone antiparasitic agents. Curr. Pharm. Biotechnol. 2012, 13, 853–865. [Google Scholar] [CrossRef] [PubMed]
- Crump, A.; Ōmura, S. Ivermectin, ‘wonder drug’ from Japan: The human use perspective. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2011, 87, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Guzzo, C.A.; Furtek, C.I.; Porras, A.G.; Chen, C.; Tipping, R.; Clineschmidt, C.M.; Sciberras, D.G.; Hsieh, J.Y.; Lasseter, K.C. Safety, tolerability, and pharmacokinetics of escalating high doses of ivermectin in healthy adult subjects. J. Clin. Pharmacol. 2002, 42, 1122–1133. [Google Scholar] [CrossRef] [PubMed]
- Navarro, M.; Camprubí, D.; Requena-Méndez, A.; Buonfrate, D.; Giorli, G.; Kamgno, J.; Gardon, J.; Boussinesq, M.; Muñoz, J.; Krolewiecki, A. Safety of high-dose ivermectin: A systematic review and meta-analysis. J. Antimicrob. Chemother. 2020, 75, 827–834. [Google Scholar] [CrossRef]
- Krolewiecki, A.; Lifschitz, A.; Moragas, M.; Travacio, M.; Valentini, R.; Alonso, D.F.; Solari, R.; Tinelli, M.A.; Cimino, R.O.; Álvarez, L.; et al. Antiviral effect of high-dose ivermectin in adults with COVID-19: A proof-of-concept randomized trial. EClinicalMedicine 2021, 37, 100959. [Google Scholar] [CrossRef]
- de Castro, C.G., Jr.; Gregianin, L.J.; Burger, J.A. Continuous high-dose ivermectin appears to be safe in patients with acute myelogenous leukemia and could inform clinical repurposing for COVID-19 infection. Leuk Lymphoma 2020, 61, 2536–2537. [Google Scholar] [CrossRef]
- Chung, K.; Yang, C.C.; Wu, M.L.; Deng, J.F.; Tsai, W.J. Agricultural avermectins: An uncommon but potentially fatal cause of pesticide poisoning. Ann. Emerg. Med. 1999, 34, 51–57. [Google Scholar] [CrossRef]
- Scheim, D.E.; Hibberd, J.A.; Chamie-Quintero, J.J. Protocol Violations in López-Medina et al.: 38 Switched Ivermectin (IVM) and Placebo Doses, Failure of Blinding, Ubiquitous IVM Use OTC in Cali, and Nearly Identical AEs for the IVM and Control Groups. Available online: https://doi.org/10.31219/osf.io/u7ewz (accessed on 13 February 2022). [CrossRef]
- Scheim, D.E. The Drug Used in Lim et al. 2022, Source Not Specified, Had <1% Incidence of AEs Distinctive and Common for Ivermectin at This Study’s Very High Dose, 2 mg/kg. Available online: https://doi.org/10.31219/osf.io/5cwmr (accessed on 12 May 2022). [CrossRef]
- de Melo, G.D.; Lazarini, F.; Levallois, S.; Hautefort, C.; Michel, V.; Larrous, F.; Verillaud, B.; Aparicio, C.; Wagner, S.; Gheusi, G.; et al. COVID-19-related anosmia is associated with viral persistence and inflammation in human olfactory epithelium and brain infection in hamsters. Sci. Transl. Med. 2021, 13, eabf8396. [Google Scholar]
- Arévalo, A.P.; Pagotto, R.; Pórfido, J.L.; Daghero, H.; Segovia, M.; Yamasaki, K.; Varela, B.; Hill, M.; Verdes, J.M.; Duhalde Vega, M.; et al. Ivermectin reduces in vivo coronavirus infection in a mouse experimental model. Sci. Rep. 2021, 11, 7132. [Google Scholar] [CrossRef]
- Mahmud, R.; Rahman, M.M.; Alam, I.; Ahmed, K.G.U.; Kabir, A.K.M.H.; Sayeed, S.K.J.B.; Rassel, M.A.; Monayem, F.B.; Islam, M.S.; Islam, M.M.; et al. Ivermectin in combination with doxycycline for treating COVID-19 symptoms: A randomized trial. J. Int. Med. Res. 2021, 49, 03000605211013550. [Google Scholar] [CrossRef]
- Hashim, H.A.; Maulood, M.F.; Rasheed, A.M.; Fatak, D.F.; Kabah, K.K.; Abdulamir, A.S. Controlled randomized clinical trial on using Ivermectin with Doxycycline for treating COVID-19 patients in Baghdad, Iraq. medRxiv 2020. [Google Scholar] [CrossRef]
- George, L.L.; Borody, T.J.; Andrews, P.; Devine, M.; Moore-Jones, D.; Walton, M.; Brandl, S. Cure of duodenal ulcer after eradication of Helicobacter pylori. Med. J. Aust. 1990, 153, 145–149. [Google Scholar] [CrossRef] [PubMed]
- U.S. Centers for Disease Control and Prevention (CDC). Coronavirus Disease 2019 (COVID-19). The Basics of Oxygen Monitoring and Oxygen Therapy during the COVID-19 Pandemic. Available online: https://www.cdc.gov/coronavirus/2019-ncov/videos/oxygen-therapy/Basics_of_Oxygen_Monitoring_and_Oxygen_Therapy_Transcript.pdf (accessed on 23 September 2021).
- Osman, A.M.; Farouk, S.; Osman, N.M.; Abdrabou, A.M. Longitudinal assessment of chest computerized tomography and oxygen saturation for patients with COVID-19. Egypt. J. Radiol. Nucl. Med. 2020, 51, 255. [Google Scholar] [CrossRef]
- Metwally, M.I.; Basha, M.A.A.; Zaitoun, M.M.A.; Abdalla, H.M.; Nofal, H.A.E.; Hendawy, H.; Manajrah, E.; Hijazy, R.f.; Akbazli, L.; Negida, A.; et al. Clinical and radiological imaging as prognostic predictors in COVID-19 patients. Egypt. J. Radiol. Nucl. Med. 2021, 52, 100. [Google Scholar] [CrossRef]
- Aoki, R.; Iwasawa, T.; Hagiwara, E.; Komatsu, S.; Utsunomiya, D.; Ogura, T. Pulmonary vascular enlargement and lesion extent on computed tomography are correlated with COVID-19 disease severity. Jpn. J. Radiol. 2021, 39, 451–458. [Google Scholar] [CrossRef]
- Ding, X.; Xu, J.; Zhou, J.; Long, Q. Chest CT findings of COVID-19 pneumonia by duration of symptoms. Eur. J. Radiol. 2020, 127, 109009. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, C.; Hu, Y.; Li, C.; Ren, Q.; Zhang, X.; Shi, H.; Zhou, M. Temporal changes of CT findings in 90 patients with COVID-19 pneumonia: A longitudinal study. Radiology 2020, 296, E55–E64. [Google Scholar] [CrossRef]
- Quispe-Cholan, A.; Anticona-De-La-Cruz, Y.; Cornejo-Cruz, M.; Quispe-Chirinos, O.; Moreno-Lazaro, V.; Chavez-Cruzado, E. Tomographic findings in patients with COVID-19 according to evolution of the disease. Egypt. J. Radiol. Nucl. Med. 2020, 51, 215. [Google Scholar] [CrossRef]
- Annunziata, A.; Coppola, A.; Carannante, N.; Simioli, F.; Lanza, M.; Di Micco, P.; Fiorentino, G. Home management of patients with moderate or severe respiratory failure secondary to COVID-19, using remote monitoring and oxygen with or without HFNC. Pathogens 2021, 10, 413. [Google Scholar] [CrossRef]
- Thairu, Y.; Babalola, O.E.; Ajayi, A.A.; Ndanusa, Y.; Ogedengbe, J.O.; Omede, O. A comparison of Ivermectin and non Ivermectin based regimen for COVID-19 in Abuja: Effects on virus clearance, days-to-discharge and mortality. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Babalola, O.E.; Ndanusa, Y.; Adesuyi, A.; Ogedengbe, O.J.; Thairu, Y.; Ogu, O. A randomized controlled trial of ivermectin monotherapy versus HCQ, IVM, and AZ combination therapy in COVID-19 patients in Nigeria. J. Infect. Dis. Epidemiol. 2021, 7, 233. [Google Scholar] [CrossRef]
- Ayerbe, L.; Risco, C.; Ayis, S. The association between treatment with heparin and survival in patients with Covid-19. J. Thromb. Thrombolysis 2020, 50, 298–301. [Google Scholar] [CrossRef] [PubMed]
- Negri, E.M.; Piloto, B.M.; Morinaga, L.K.; Jardim, C.V.P.; Lamy, S.A.E.-D.; Ferreira, M.A.; D’Amico, E.A.; Deheinzelin, D. Heparin therapy improving hypoxia in COVID-19 patients—A case series. Front. Physiol. 2020, 11, 1341. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Huang, M.; Li, D.; Tang, N. Difference of coagulation features between severe pneumonia induced by SARS-CoV2 and non-SARS-CoV2. J. Thromb. Thrombolysis 2020, 51, 1107–1110. [Google Scholar] [CrossRef] [PubMed]
- Dayer, M. Coronavirus (2019-nCoV) deactivation via spike glycoprotein shielding by old drugs, bioinformatic study. Preprints.org 2020. [Google Scholar] [CrossRef]
- Babalola, O.E.; (Bingham University, New Karu, Nigeria). Personal communication, 2022. This communication provided deidentified patient level data for SpO2 changes post-treatment from two studies which he coauthored (per references [38] and [39] as cited above), one using standard treatment excluding IVM (#38) and the other including IVM in the treatment regimen (#39), for those values obtained exclusively on room air and with pre-treatment SpO2 values ≤ 93%.
- Hazan, S.; Dave, S.; Gunaratne, A.W.; Dolai, S.; Clancy, R.L.; McCullough, P.A.; Borody, T.J. Effectiveness of ivermectin-based multidrug therapy in severely hypoxic, ambulatory COVID-19 patients. Future Microbiol. 2022, 17, 339–350. [Google Scholar] [CrossRef]
- Rajter, J.J.; (Broward Health Medical Center, Fort Lauderdale, FL, USA). Personal communication, 2020.
- Stone, J.C.; Ndarukwa, P.; Scheim, D.E.; Dancis, B.M.; Dancis, J.; Gill, M.G.; Aldous, C. Rapid increase of SpO2 on room air for 34 severe COVID-19 patients after ivermectin-based combination treatment: 55–62% normalization within 12–24 hours. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Zaidi, A.K.; Dehgani-Mobaraki, P. The mechanisms of action of ivermectin against SARS-CoV-2—An extensive review. J. Antibiot. 2022, 75, 60–71. [Google Scholar] [CrossRef]
- Wang, H.; Yu, M.; Ochani, M.; Amella, C.A.; Tanovic, M.; Susarla, S.; Li, J.H.; Wang, H.; Yang, H.; Ulloa, L.; et al. Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation. Nature 2003, 421, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Krause, R.M.; Buisson, B.; Bertrand, S.; Corringer, P.J.; Galzi, J.L.; Changeux, J.P.; Bertrand, D. Ivermectin: A positive allosteric effector of the alpha7 neuronal nicotinic acetylcholine receptor. Mol. Pharmacol. 1998, 53, 283–294. [Google Scholar] [CrossRef] [Green Version]
- Ren, C.; Tong, Y.L.; Li, J.C.; Lu, Z.Q.; Yao, Y.M. The protective effect of alpha 7 nicotinic acetylcholine receptor activation on critical illness and its mechanism. Int. J. Biol. Sci. 2017, 13, 46–56. [Google Scholar] [CrossRef] [Green Version]
- U.S. National Institutes of Health (NIH). Clinical Spectrum of SARS-CoV-2 Infection. Available online: https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/ (accessed on 2 March 2022).
- The Nobel Assembly at Karolinska Institutet. The 2015 Nobel Prize in Physiology or Medicine—Press Release; The Nobel Assembly at Karolinska Institutet: Solna, Sweden, 2015; Available online: https://www.nobelprize.org/prizes/medicine/2015/press-release/ (accessed on 12 May 2022).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).