Emerging Insights into Monkeypox: Clinical Features, Epidemiology, Molecular Insights, and Advancements in Management
Abstract
1. Introduction
2. Clinical Description of Mpox
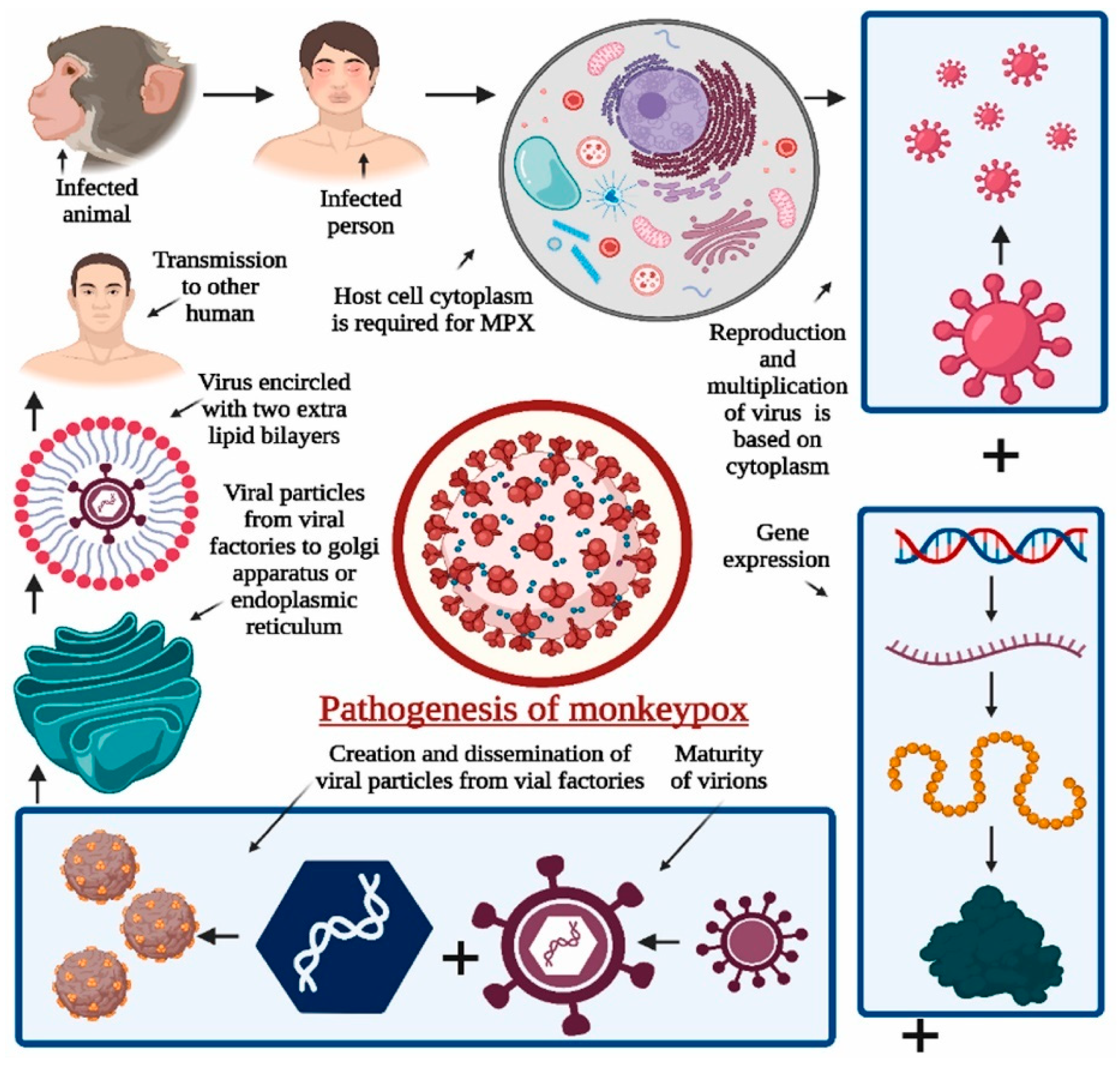
3. Pathogenesis and Transmission of Mpox and Related Orthopoxviruses
4. Epidemiology and Pathology of Mpox
4.1. Mpox Transmission Dynamics and Reservoirs
4.2. Risk Factors for Severe Outcomes and Case Fatality Rates
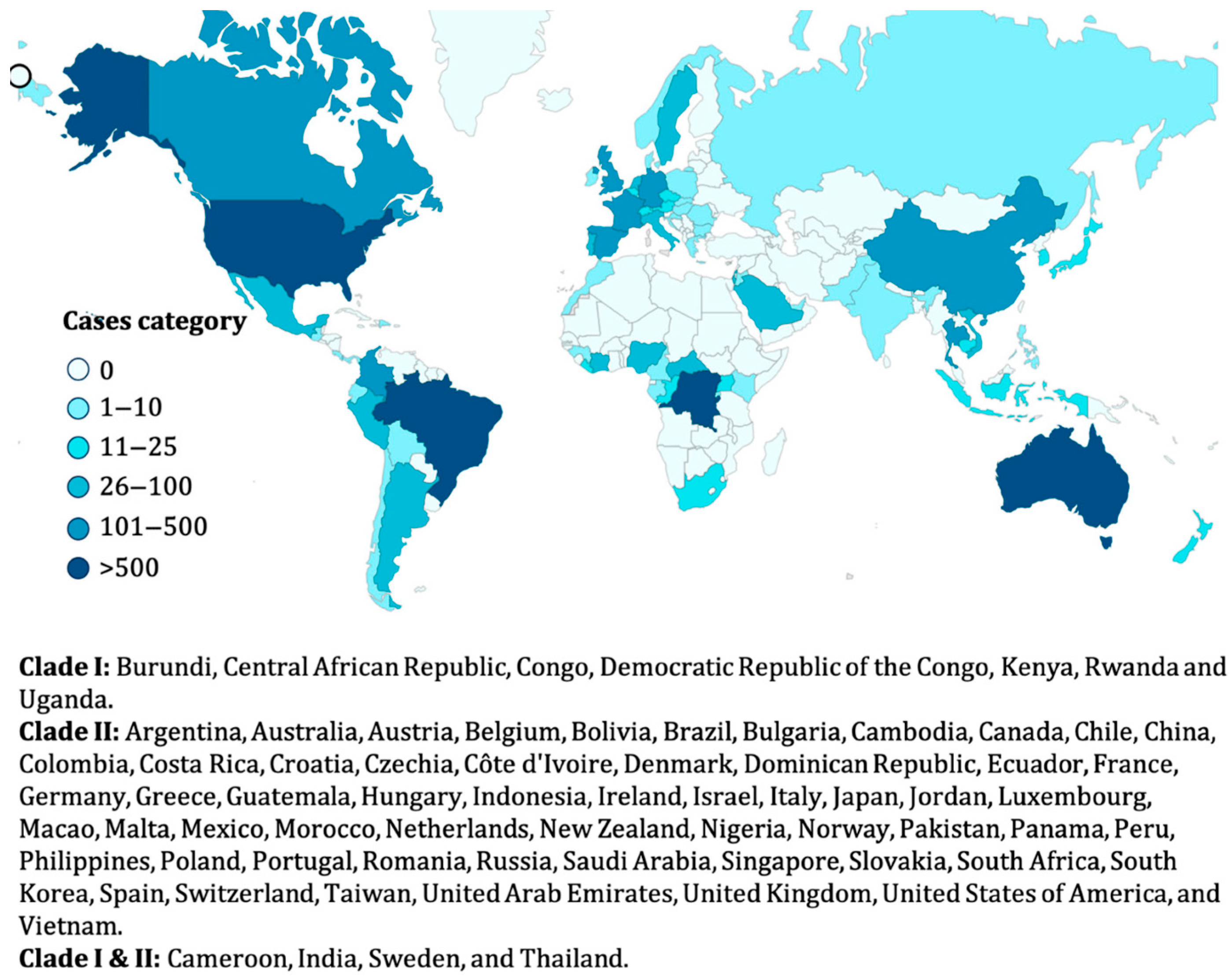
4.3. Recent Outbreaks and Global Prevalence
5. Molecular/Genomic Insights into Mpox
5.1. Key Genomic Characteristics of Mpox Virus
5.2. Evolutionary Trends and Implications for Virulence
5.3. Potential Mutations and Their Clinical Significance
5.4. Genomic Markers as Targets for Diagnostics and Therapies
6. Therapeutic and Preventive Strategies
6.1. Antiviral Therapies and Ongoing Clinical Trials
| Antiviral Therapy | Mechanism of Action | Current Status | Clinical Trial Details | Key Findings/Challenges | Administration Route | Geographic Scope of Trials | Refs |
|---|---|---|---|---|---|---|---|
| Tecovirimat (TPOXX/ST-246) | Inhibits the VP37 protein, blocking viral egress | FDA-approved for smallpox; Phase II/III trials for Mpox | Evaluating efficacy and safety in immunocompromised patients; NCT05550006 | Demonstrates reduced mortality in severe cases; challenges include drug resistance and limited global accessibility. | Oral, intravenous | United States, Europe | [139,140] |
| Cidofovir | Inhibits viral DNA polymerase | Off-label use; ongoing trials | Exploring efficacy in severe Mpox cases; NCT04911880 | Effective in vitro against orthopoxviruses; associated with nephrotoxicity and limited use in resource-constrained settings. | Intravenous | North America, Africa | [43,141] |
| Brincidofovir | Lipid conjugate of cidofovir with lower toxicity | FDA-approved for smallpox; Phase II trials for Mpox | Assessing dosing regimens and safety profiles; NCT05717313 | Improved safety profile over cidofovir; logistical barriers and high cost remain significant obstacles. | Oral | United States, Europe | [141] |
| Vaccinia Immune Globulin Intravenous (VIGIV) | Neutralizes orthopoxvirus through passive immunity | Emergency use authorization | Combined with antiviral therapy in severe or refractory Mpox cases; observational studies ongoing | Limited evidence on Mpox-specific efficacy; primarily used in conjunction with other antivirals for severe immune compromise. | Intravenous | United States, Global availability | [142,143] |
| Monoclonal Antibodies (Investigational) | Target viral proteins to prevent replication | Preclinical and early-phase trials | Investigating multi-epitope targeting strategies for enhanced efficacy | Promising early results in animal models; challenges include scalability and high production costs for widespread use. | Intravenous, subcutaneous | Global | [120] |
| CRISPR-Based Antiviral Therapy | Gene-editing technology to target and deactivate viral DNA | Preclinical research phase | Innovative studies exploring genome-targeting strategies for MPXV; not yet in clinical trials | High specificity and potential for future personalized treatments; barriers include early-stage development and ethical concerns. | TBD (in development) | Global | [37,141] |
| Broad-Spectrum Antivirals (e.g., Tilorone, Valacyclovir, Ribavirin, Favipiravir, and Baloxavir marboxil) | Inhibits viral RNA polymerase | Repurposed for Mpox; exploratory trials | Evaluating efficacy in resource-constrained settings with limited access to specific antivirals | Moderate efficacy in reducing symptoms; inexpensive alternative but limited specific activity against MPXV. | Oral | Asia, Africa | [144] |
6.2. Vaccine Development and Immunization Strategies
6.3. Challenges in Therapeutic and Prophylactic Interventions
7. Public Health Interventions
7.1. Isolation and Quarantine Measures
7.2. Public Health Campaigns and Behavioral Strategies
7.3. The Role of Community Engagement in Controlling Outbreaks
8. Discussion and Postulated Hypotheses on Mpox Evolution
8.1. Factors Driving Re-Emergence and Epidemiological Shifts
8.2. Potential Links with Changing Ecological and Human Behavior
8.3. Predicting Future Trends Based on Zoonotic and Environmental Factors
9. Conclusions and Future Perspectives for Mpox Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sholts, S. The Human Disease: How We Create Pandemics, from Our Bodies to Our Beliefs; MIT Press: Cambridge, MA, USA, 2024. [Google Scholar]
- European Centre for Disease Prevention and Control. Risk Assessment for the EU/EEA of the Mpox Epidemic. Caused by Monkeypox Virus Clade I in Affected African Countries; ECDC: Stockholm, Sweden, 2024.
- Kantele, A.; Chickering, K.; Vapalahti, O.; Rimoin, A.W. Emerging diseases-the monkeypox epidemic in the Democratic Republic of the Congo. Clin. Microbiol. Infect. 2016, 22, 658–659. [Google Scholar] [CrossRef]
- Kelland, K. Disease X: The 100 Days Mission to End Pandemics; Canbury Press: Surrey, UK, 2024. [Google Scholar]
- Vidal, J. Fevered Planet: How Diseases Emerge When We Harm Nature; Bloomsbury Publishing: London, UK, 2023. [Google Scholar]
- Kumar, S.; Guruparan, D.; Karuppanan, K.; Kumar, K.J.S. Comprehensive Insights into Monkeypox (mpox): Recent Advances in Epidemiology, Diagnostic Approaches and Therapeutic Strategies. Pathogens 2025, 14, 1. [Google Scholar] [CrossRef]
- Szymanik, K.H. RNA Phosphatase DUSP11: Target of Viral Piracy and Modulator of RNAP III Transcripts. Ph.D. Thesis, The University of Texas at Austin, Austin, TX, USA, 2024. [Google Scholar]
- Bryant, K.A. A Tale of Two Babies and the ‘Family Tragedy’of Congenital Syphilis. Pediatr. News 2024, 58, 9–10. [Google Scholar]
- Devaux, C.A.; Mediannikov, O.; Medkour, H.; Raoult, D. Infectious disease risk across the growing human-non human primate interface: A review of the evidence. Front. Public Health 2019, 7, 305. [Google Scholar] [CrossRef] [PubMed]
- Leroy, E.M.; Epelboin, A.; Mondonge, V.; Pourrut, X.; Gonzalez, J.-P.; Muyembe-Tamfum, J.-J.; Formenty, P. Human Ebola outbreak resulting from direct exposure to fruit bats in Luebo, Democratic Republic of Congo, 2007. Vector-Borne Zoonotic Dis. 2009, 9, 723–728. [Google Scholar] [CrossRef]
- Zinnah, M.A.; Uddin, M.B.; Hasan, T.; Das, S.; Khatun, F.; Hasan, M.H.; Udonsom, R.; Rahman, M.M.; Ashour, H.M. The re-emergence of mpox: Old illness, modern challenges. Biomedicines 2024, 12, 1457. [Google Scholar] [CrossRef]
- Mohapatra, R.K.; Singh, P.K.; Branda, F.; Mishra, S.; Kutikuppala, L.S.; Suvvari, T.K.; Kandi, V.; Ansari, A.; Desai, D.N.; Alfaresi, M. Transmission dynamics, complications and mitigation strategies of the current mpox outbreak: A comprehensive review with bibliometric study. Rev. Med. Virol. 2024, 34, e2541. [Google Scholar] [CrossRef]
- Papadakis, D.M.; Savvides, A.; Michael, A.; Michopoulos, A. Advancing Sustainable Urban Mobility: Insights from Best Practices and Case Studies. Fuel Commun. 2024, 20, 100125. [Google Scholar] [CrossRef]
- Li, Y.; Wang, L.; Chen, S. An overview of the progress made in research into the Mpox virus. Med. Res. Rev. 2025, 45, 788–812. [Google Scholar] [CrossRef] [PubMed]
- Eslamkhah, S.; Aslan, E.S.; Yavas, C.; Akcalı, N.; Batur, L.K.; Abuaisha, A.; Yildirim, E.E.; Solak, M.; White, K.N. Mpox virus (MPXV): Comprehensive analysis of pandemic risks, pathophysiology, treatments, and mRNA vaccine development. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2025, 398, 6143–6163. [Google Scholar] [CrossRef] [PubMed]
- Murhula Masirika, L.; Hewins, B.; Toloue Ostadgavahi, A.; Dutt, M.; Mutimbwa Mambo, L.; Udahemuka, J.C.; Ndishymie, P.; Bengehya Mbiribindi, J.; Belesi Siangoli, F.; Kelvin, P. The trans-kingdom spectrum of Mpox-like lesion pustules of suspect patients in the Mpox subclade Ib outbreak in Eastern Democratic Republic of the Congo. medRxiv 2025. [Google Scholar] [CrossRef]
- Singh, V.; Dwivedi, S.; Agrawal, R.; Sadashiv; Fatima, G.; Abidi, A. The human Monkeypox virus and host immunity: Emerging diagnostic and therapeutic challenges. Infect. Disord.-Drug Targets 2025, 25, E18715265309361. [Google Scholar] [CrossRef] [PubMed]
- Amin, S.M.; Demerdash, D.E.; El-Sayed, M.M.; Salama, T.R.A.; Elsehrawy, M.G.; Atta, M.H.R. Navigating the fear: Assessing nursing students’ concerns and preventive practices in response to Monkeypox in Egypt. BMC Nurs. 2025, 24, 23. [Google Scholar] [CrossRef] [PubMed]
- Urmi, T.J.; Islam, M.R. The Growing Mpox Infections by Clade I Variant in African Countries Is a Public Health Emergency of International Concern: A Narrative Review. Health Sci. Rep. 2025, 8, e70306. [Google Scholar] [CrossRef]
- Abdoos, P. Emerging Infectious Diseases: Strategies for Prevention and Control. Eurasian J. Chem. Med. Pet. Res. 2025, 4, 136–152. [Google Scholar]
- Du, M.; Deng, J.; Yan, W.; Liu, M.; Liang, W.; Niu, B.; Liu, J. Mpox vaccination hesitancy, previous immunisation coverage, and vaccination readiness in the African region: A multinational survey. eClinicalMedicine 2025, 80, 103047. [Google Scholar] [CrossRef]
- Parás, A.V.; Nieto, L.A.; Gaytán, D.A.C.; Díaz, B.C.; Betancourt, L.J.; Bautista, P.F.H.; Orozco, O.C.; Alvarado, G.V.; Paz, A.M.; Mahey, M.G.R. Risk factors for human infection with mpox among the Mexican population with social security. PLoS ONE 2025, 20, e0313691. [Google Scholar]
- Silva, S.; Kohl, A.; Pena, L.; Pardee, K. Clinical and laboratory diagnosis of monkeypox (mpox): Current status and future directions. iScience 2023, 26, 106759. [Google Scholar] [CrossRef]
- Titanji, B.K.; Hazra, A.; Zucker, J. Mpox Clinical Presentation, Diagnostic Approaches, and Treatment Strategies: A Review. JAMA 2024, 332, 1652–1662. [Google Scholar] [CrossRef]
- Al-Dabbagh, J.; Deeb, E.M.; Younis, R.; Eissa, R. The dermatological manifestations and differential diagnosis of monkeypox: A narrative review. Medicine 2024, 103, e40359. [Google Scholar] [CrossRef] [PubMed]
- Kavey, R.-E.; Kavey, A. Viral Pandemics: From Smallpox to COVID-19 & Mpox; Taylor & Francis: Oxfordshire, UK, 2024. [Google Scholar]
- Cabanillas, B.; Murdaca, G.; Guemari, A.; Torres, M.J.; Azkur, A.K.; Aksoy, E.; Vitte, J.; Fernández-Santamaria, R.; Karavelia, A.; Castagnoli, R. Monkeypox 2024 outbreak: Fifty essential questions and answers. Allergy 2024, 79, 3285–3309. [Google Scholar] [CrossRef]
- Gupta, A.K.; Talukder, M.; Rosen, T.; Piguet, V. Differential Diagnosis, Prevention, and Treatment of mpox (Monkeypox): A Review for Dermatologists. Am. J. Clin. Dermatol. 2023, 24, 541–556. [Google Scholar] [CrossRef] [PubMed]
- Maronese, C.A.; Avallone, G.; Aromolo, I.F.; Spigariolo, C.B.; Quattri, E.; Ramoni, S.; Carrera, C.G.; Marzano, A.V. Mpox: An updated review of dermatological manifestations in the current outbreak. Br. J. Dermatol. 2023, 189, 260–270. [Google Scholar] [CrossRef]
- Isaacs, S.N.; Mitjà, O. Epidemiology, Clinical Manifestations, and Diagnosis of Mpox (Formerly Monkeypox). 2025. Available online: https://www.uptodate.com/contents/epidemiology-clinical-manifestations-and-diagnosis-of-mpox-formerly-monkeypox (accessed on 10 August 2025).
- Cices, A.; Prasad, S.; Akselrad, M.; Sells, N.; Woods, K.; Silverberg, N.B.; Camins, B. Mpox update: Clinical presentation, vaccination guidance, and management. Cutis 2023, 111, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, P.A.; Patro, S.K.; Sandeep, M.; Satapathy, P.; Shamim, M.A.; Kumar, V.; Aggarwal, A.K.; Padhi, B.K.; Sah, R. Oral manifestation of the monkeypox virus: A systematic review and meta-analysis. EClinicalMedicine 2023, 56, 101817. [Google Scholar] [CrossRef]
- De la Herrán-Arita, A.K.; González-Galindo, C.; Inzunza-Leyva, G.K.; Valdez-Flores, M.A.; Norzagaray-Valenzuela, C.D.; Camacho-Zamora, A.; Batiz-Beltrán, J.C.; Urrea-Ramírez, F.J.; Romero-Utrilla, A.; Angulo-Rojo, C.; et al. Clinical Predictors of Monkeypox Diagnosis: A Case-Control Study in a Nonendemic Region during the 2022 Outbreak. Microorganisms 2023, 11, 2287. [Google Scholar] [CrossRef]
- Trestrail, T.; Kodia, K.; Hui, V.W. Gastrointestinal Manifestation of MPox. Curr. Infect. Dis. Rep. 2024, 26, 209–215. [Google Scholar] [CrossRef]
- Hall, R.; Patel, K.; Poullis, A.; Pollok, R.; Honap, S. Separating Infectious Proctitis from Inflammatory Bowel Disease—A Common Clinical Conundrum. Microorganisms 2024, 12, 2395. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Fu, L.; Wang, B.; Sun, Y.; Wu, X.; Peng, X.; Li, Y.; Lin, Y.-F.; Fitzpatrick, T.; Vermund, S.H. Clinical characteristics of human Mpox (monkeypox) in 2022: A systematic review and meta-analysis. Pathogens 2023, 12, 146. [Google Scholar] [CrossRef]
- Okoli, G.N.; Van Caeseele, P.; Askin, N.; Abou-Setta, A.M. Comparative evaluation of the clinical presentation and epidemiology of the 2022 and previous Mpox outbreaks: A rapid review and meta-analysis. Infect. Dis. 2023, 55, 490–508. [Google Scholar] [CrossRef]
- Mohanto, S.; Faiyazuddin, M.; Dilip Gholap, A.; Jc, D.; Bhunia, A.; Subbaram, K.; Gulzar Ahmed, M.; Nag, S.; Shabib Akhtar, M.; Bonilla-Aldana, D.K.; et al. Addressing the resurgence of global monkeypox (Mpox) through advanced drug delivery platforms. Travel Med. Infect. Dis. 2023, 56, 102636. [Google Scholar] [CrossRef] [PubMed]
- Pourriyahi, H.; Aryanian, Z.; Afshar, Z.M.; Goodarzi, A. A systematic review and clinical atlas on mucocutaneous presentations of the current monkeypox outbreak: With a comprehensive approach to all dermatologic and nondermatologic aspects of the new and previous monkeypox outbreaks. J. Med. Virol. 2023, 95, e28230. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, Z. Mpox: A review of laboratory detection techniques. Arch. Virol. 2023, 168, 221. [Google Scholar] [CrossRef]
- Mason, L.M.; Betancur, E.; Riera-Montes, M.; Lienert, F.; Scheele, S. MVA-BN vaccine effectiveness: A systematic review of real-world evidence in outbreak settings. Vaccine 2024, 42, 126409. [Google Scholar] [CrossRef]
- Hazra, A.; Zucker, J.; Bell, E.; Flores, J.; Gordon, L.; Mitjà, O.; Suñer, C.; Lemaignen, A.; Jamard, S.; Nozza, S. Mpox in people with past infection or a complete vaccination course: A global case series. Lancet Infect. Dis. 2024, 24, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Siegrist, E.A.; Sassine, J. Antivirals with Activity Against Mpox: A Clinically Oriented Review. Clin. Infect. Dis. 2023, 76, 155–164. [Google Scholar] [CrossRef]
- Prévost, J.; Sloan, A.; Deschambault, Y.; Tailor, N.; Tierney, K.; Azaransky, K.; Kammanadiminti, S.; Barker, D.; Kodihalli, S.; Safronetz, D. Treatment efficacy of cidofovir and brincidofovir against clade II Monkeypox virus isolates. Antivir. Res. 2024, 231, 105995. [Google Scholar] [CrossRef] [PubMed]
- Damon, I.K. Poxviruses. Man. Clin. Microbiol. 2011, 1647–1658. [Google Scholar]
- Shchelkunova, G.A.; Shchelkunov, S.N. Smallpox, monkeypox and other human orthopoxvirus infections. Viruses 2022, 15, 103. [Google Scholar] [CrossRef]
- Gieryńska, M.; Szulc-Dąbrowska, L.; Struzik, J.; Gregorczyk-Zboroch, K.P.; Mielcarska, M.B.; Toka, F.N.; Schollenberger, A.; Biernacka, Z. Orthopoxvirus Zoonoses—Do We Still Remember and Are Ready to Fight? Pathogens 2023, 12, 363. [Google Scholar] [CrossRef]
- Thèves, C.; Crubézy, E.; Biagini, P. History of smallpox and its spread in human populations. Paleomicrobiol. Hum. 2016, 4, 161–172. [Google Scholar]
- Al-Tammemi, A.a.B.; Albakri, R.; Alabsi, S. The outbreak of human monkeypox in 2022: A changing epidemiology or an impending aftereffect of smallpox eradication? Front. Trop. Dis. 2022, 3, 951380. [Google Scholar] [CrossRef]
- Singh, P.; Sridhar, S.B.; Shareef, J.; Talath, S.; Mohapatra, P.; Khatib, M.N.; Ballal, S.; Kaur, M.; Nathiya, D.; Sharma, S.; et al. The resurgence of monkeypox: Epidemiology, clinical features, and public health implications in the post-smallpox eradication era. New Microbes New Infect. 2024, 62, 101487. [Google Scholar] [CrossRef]
- Petersen, E.; Kantele, A.; Koopmans, M.; Asogun, D.; Yinka-Ogunleye, A.; Ihekweazu, C.; Zumla, A. Human monkeypox: Epidemiologic and clinical characteristics, diagnosis, and prevention. Infect. Dis. Clin. 2019, 33, 1027–1043. [Google Scholar]
- Capobianchi, M.R.; Di Caro, A.; Piubelli, C.; Mori, A.; Bisoffi, Z.; Castilletti, C. Monkeypox 2022 outbreak in non-endemic countries: Open questions relevant for public health, nonpharmacological intervention and literature review. Front. Cell. Infect. Microbiol. 2022, 12, 1005955. [Google Scholar] [CrossRef]
- Ghazy, R.M.; Elrewany, E.; Gebreal, A.; ElMakhzangy, R.; Fadl, N.; Elbanna, E.H.; Tolba, M.M.; Hammad, E.M.; Youssef, N.; Abosheaishaa, H. Systematic review on the efficacy, effectiveness, safety, and immunogenicity of monkeypox vaccine. Vaccines 2023, 11, 1708. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.I.O.; de Oliveira, J.S.; Kroon, E.G.; Trindade, G.d.S.; Drumond, B.P. Here, there, and everywhere: The wide host range and geographic distribution of zoonotic orthopoxviruses. Viruses 2020, 13, 43. [Google Scholar] [CrossRef] [PubMed]
- Babkin, I.V.; Babkina, I.N.; Tikunova, N.V. An update of orthopoxvirus molecular evolution. Viruses 2022, 14, 388. [Google Scholar] [CrossRef] [PubMed]
- Alakunle, E.; Kolawole, D.; Diaz-Canova, D.; Alele, F.; Adegboye, O.; Moens, U.; Okeke, M.I. A comprehensive review of monkeypox virus and mpox characteristics. Front. Cell. Infect. Microbiol. 2024, 14, 1360586. [Google Scholar] [CrossRef]
- Bishnoi, A.; Sharma, A.; Mehta, H.; Vinay, K. Emerging and re-emerging viral exanthems among children: What a physician should know. Trans. R. Soc. Trop. Med. Hyg. 2025, 119, 13–26. [Google Scholar] [CrossRef]
- Gerlicki, C.M. Viral diseases affecting the skin. Dermatol. Rev. 2024, 5, e225. [Google Scholar] [CrossRef]
- Atceken, N.; Bayaki, I.; Can, B.; Yigci, D.; Tasoglu, S. Mpox disease, diagnosis, and point of care platforms. Bioeng. Transl. Med. 2024, 10, e10733. [Google Scholar] [CrossRef] [PubMed]
- Protopapas, K.; Dimopoulou, D.; Kalesis, N.; Akinosoglou, K.; Moschopoulos, C.D. Mpox and Lessons Learned in the Light of the Recent Outbreak: A Narrative Review. Viruses 2024, 16, 1620. [Google Scholar] [CrossRef]
- Bhat, S.; Saha, S.; Garg, T.; Sehrawat, H.; Chopade, B.A.; Gupta, V. Insights into the challenging multi-country outbreak of Mpox: A comprehensive review. J. Med. Microbiol. 2023, 72, 001725. [Google Scholar] [CrossRef]
- Kanoujia, J.; Tarannum, S.; Kaurav, M.; Raina, N.; Jain, K.; Gupta, M. Insight into Recent Updates on Vaccines Development and Immunology of Monkeypox Infection. Curr. Treat. Options Infect. Dis. 2024, 16, 118–128. [Google Scholar] [CrossRef]
- Curaudeau, M.; Besombes, C.; Nakouné, E.; Fontanet, A.; Gessain, A.; Hassanin, A. Identifying the most probable mammal reservoir hosts for monkeypox virus based on ecological niche comparisons. Viruses 2023, 15, 727. [Google Scholar] [CrossRef]
- Acharya, A.; Kumar, N.; Singh, K.; Byrareddy, S.N. Mpox in MSM: Tackling Stigma, Minimizing Risk Factors, Exploring Pathogenesis, and Treatment Approaches. Biomed. J. 2024, 48, 100746. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, C.; Jaspe, R.; Sulbarán, Y.; Rodríguez, L.; Alarcón, V.; Monsalve, I.; García, J.M.; Zambrano, J.L.; Rangel, H.; Pujol, F.H. Epidemiological and virological characterization of mpox cases in Venezuela during the multinational 2022–2023 outbreak. Investig. Clín. 2024, 65, 445–453. [Google Scholar]
- Verma, A.; Khatib, M.N.; Sharma, G.D.; Singh, M.P.; Bushi, G.; Ballal, S.; Kumar, S.; Bhat, M.; Sharma, S.; Ndabashinze, R. Mpox 2024: New variant, new challenges, and the looming pandemic. Clin. Infect. Pract. 2024, 24, 100394. [Google Scholar] [CrossRef]
- Aggarwal, S.; Agarwal, P.; Nigam, K.; Vijay, N.; Yadav, P.; Gupta, N. Mapping the Landscape of Health Research Priorities for Effective Pandemic Preparedness in Human Mpox Virus Disease. Pathogens 2023, 12, 1352. [Google Scholar] [CrossRef]
- Ou, G.; Tang, Y.; Liu, J.; Hao, Y.; Chen, Z.; Huang, T.; Li, S.; Niu, S.; Peng, Y.; Feng, J. Automated robot and artificial intelligence-powered wastewater surveillance for proactive mpox outbreak prediction. Biosaf. Health 2024, 6, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Das, H.K. Exploring the dynamics of monkeypox transmission with data-driven methods and a deterministic model. Front. Epidemiol. 2024, 4, 1334964. [Google Scholar] [CrossRef]
- Chatzispyros, C. Literature Review on Mathematical and Phylodynamic Modelling of Mpox Outbreaks in Men Who Have Sex with Men Populations. Master Thesis, Utrecht University, Utrecht, The Netherlands, 2024. [Google Scholar]
- Asif, S.; Zhao, M.; Li, Y.; Tang, F.; Ur Rehman Khan, S.; Zhu, Y. AI-Based Approaches for the Diagnosis of Mpox: Challenges and Future Prospects. Arch. Comput. Methods Eng. 2024, 31, 3585–3617. [Google Scholar] [CrossRef]
- Chauhan, R.P.; Fogel, R.; Limson, J. Overview of diagnostic methods, disease prevalence and transmission of MPOX (formerly monkeypox) in humans and animal reservoirs. Microorganisms 2023, 11, 1186. [Google Scholar] [CrossRef]
- Duda, R.; Betoulet, J.M.; Besombes, C.; Mbrenga, F.; Borzykh, Y.; Nakouné, E.; Giles-Vernick, T. A time of decline: An eco-anthropological and ethnohistorical investigation of mpox in the Central African Republic. PLoS Glob. Public Health 2024, 4, e0002937. [Google Scholar] [CrossRef] [PubMed]
- Banuet-Martinez, M.; Yang, Y.; Jafari, B.; Kaur, A.; Butt, Z.A.; Chen, H.H.; Yanushkevich, S.; Moyles, I.R.; Heffernan, J.M.; Korosec, C.S. Monkeypox: A review of epidemiological modelling studies and how modelling has led to mechanistic insight. Epidemiol. Infect. 2023, 151, e121. [Google Scholar] [CrossRef] [PubMed]
- Olawade, D.B.; Wada, O.Z.; Fidelis, S.C.; Oluwole, O.S.; Alisi, C.S.; Orimabuyaku, N.F.; Clement David-Olawade, A. Strengthening Africa’s response to Mpox (monkeypox): Insights from historical outbreaks and the present global spread. Sci. One Health 2024, 3, 100085. [Google Scholar] [CrossRef]
- Bragazzi, N.L.; Iyaniwura, S.A.; Han, Q.; Woldegerima, W.A.; Kong, J.D. Quantifying the basic reproduction number and underestimated fraction of Mpox cases worldwide at the onset of the outbreak. J. R. Soc. Interface 2024, 21, 20230637. [Google Scholar] [CrossRef]
- Schwartz, D.A. High rates of miscarriage and stillbirth among pregnant women with clade I mpox (monkeypox) are confirmed during 2023–2024 DR Congo outbreak in South Kivu Province. Viruses 2024, 16, 1123. [Google Scholar] [CrossRef]
- Schwartz, D.A.; Ha, S.; Dashraath, P.; Baud, D.; Pittman, P.R.; Adams Waldorf, K.M. Mpox Virus in Pregnancy, the Placenta, and Newborn: An Emerging Poxvirus with Similarities to Smallpox and Other Orthopoxvirus Agents Causing Maternal and Fetal Disease. Arch. Pathol. Lab. Med. 2023, 147, 746–757. [Google Scholar] [CrossRef]
- Bragazzi, N.L.; Woldegerima, W.A.; Wu, J.; Converti, M.; Szarpak, L.; Crapanzano, A.; Odeh, M.; Farah, R.; Khamisy-Farah, R. Epidemiological and Clinical Characteristics of Mpox in Cisgender and Transgender Women and Non-Binary Individuals Assigned to the Female Sex at Birth: A Comprehensive, Critical Global Perspective. Viruses 2024, 16, 325. [Google Scholar] [CrossRef]
- Corma-Gómez, A.; Cabello, A.; Orviz, E.; Morante-Ruiz, M.; Ayerdi, O.; Al-Hayani, A.; Muñoz-Gómez, A.; Santos, I.D.L.; Gómez-Ayerbe, C.; Rodrigo, D. Long or complicated mpox in patients with uncontrolled HIV infection. J. Med. Virol. 2024, 96, e29511. [Google Scholar] [CrossRef]
- Laurenson-Schafer, H.; Sklenovská, N.; Hoxha, A.; Kerr, S.M.; Ndumbi, P.; Fitzner, J.; Almiron, M.; de Sousa, L.A.; Briand, S.; Cenciarelli, O. Description of the first global outbreak of mpox: An analysis of global surveillance data. Lancet Glob. Health 2023, 11, e1012–e1023. [Google Scholar] [CrossRef]
- Whittles, L.K.; Mbala-Kingebeni, P.; Ferguson, N.M. Age-patterns of severity of clade I mpox in historically endemic countries. medRxiv 2024. [Google Scholar] [CrossRef]
- Fu, L.; Wang, B.; Wu, K.; Yang, L.; Hong, Z.; Wang, Z.; Meng, X.; Ma, P.; Qi, X.; Xu, G. Epidemiological characteristics, clinical manifestations, and mental health status of human mpox cases: A multicenter cross-sectional study in China. J. Med. Virol. 2023, 95, e29198. [Google Scholar] [CrossRef] [PubMed]
- Mohamed Abdoul-Latif, F.; Ainane, A.; Mohamed, H.; Merito Ali, A.; Houmed Aboubaker, I.; Jutur, P.P.; Ainane, T. Mpox Resurgence: A Multifaceted Analysis for Global Preparedness. Viruses 2024, 16, 1737. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.R. Mpox surveillance: The need for enhanced testing and genomic epidemiology. Lancet 2024, 404, 1784–1785. [Google Scholar] [CrossRef]
- Karagoz, A.; Tombuloglu, H.; Alsaeed, M.; Tombuloglu, G.; AlRubaish, A.A.; Mahmoud, A.; Smajlović, S.; Ćordić, S.; Rabaan, A.A.; Alsuhaimi, E. Monkeypox (mpox) virus: Classification, origin, transmission, genome organization, antiviral drugs, and molecular diagnosis. J. Infect. Public Health 2023, 16, 531–541. [Google Scholar] [CrossRef]
- Monzón, S.; Varona, S.; Negredo, A.; Vidal-Freire, S.; Patiño-Galindo, J.A.; Ferressini-Gerpe, N.; Zaballos, A.; Orviz, E.; Ayerdi, O.; Muñoz-Gómez, A. Monkeypox virus genomic accordion strategies. Nat. Commun. 2024, 15, 3059. [Google Scholar] [CrossRef]
- Cordsmeier, A.; Herrmann, A.; Gege, C.; Kohlhof, H.; Korn, K.; Ensser, A. Molecular analysis of the 2022 mpox outbreak and antiviral activity of dihydroorotate dehydrogenase inhibitors against orthopoxviruses. Antivir. Res. 2025, 233, 106043. [Google Scholar] [CrossRef]
- Fang, D.; Liu, Y.; Dou, D.; Su, B. The unique immune evasion mechanisms of the mpox virus and their implication for developing new vaccines and immunotherapies. Virol. Sin. 2024, 39, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Weaver, J.R.; Isaacs, S.N. Monkeypox virus and insights into its immunomodulatory proteins. Immunol. Rev. 2008, 225, 96–113. [Google Scholar] [CrossRef]
- Lucena-Neto, F.D.; Falcão, L.F.M.; Vieira-Junior, A.S.; Moraes, E.C.S.; David, J.P.F.; Silva, C.C.; Sousa, J.R.; Duarte, M.I.S.; Vasconcelos, P.F.C.; Quaresma, J.A.S. Monkeypox Virus Immune Evasion and Eye Manifestation: Beyond Eyelid Implications. Viruses 2023, 15, 2301. [Google Scholar] [CrossRef] [PubMed]
- Araf, Y.; Nipa, J.F.; Naher, S.; Maliha, S.T.; Rahman, H.; Arafat, K.I.; Munif, M.R.; Uddin, M.J.; Jeba, N.; Saha, S. Insights into the Transmission, Host Range, Genomics, Vaccination, and Current Epidemiology of the Monkeypox Virus. Vet. Med. Int. 2024, 2024, 8839830. [Google Scholar] [CrossRef]
- Okwor, T.; Mbala, P.K.; Evans, D.H.; Kindrachuk, J. A contemporary review of clade-specific virological differences in monkeypox viruses. Clin. Microbiol. Infect. 2023, 29, 1502–1507. [Google Scholar] [CrossRef]
- Young, B.; Seifert, S.N.; Lawson, C.; Koehler, H. Exploring the genomic basis of Mpox virus-host transmission and pathogenesis. mSphere 2024, 9, e00576-24. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Shukla, N.; Bhatia, A.; Mahfooz, S.; Narayan, J. Comparative genome analysis reveals driving forces behind Monkeypox virus evolution and sheds light on the role of ATC trinucleotide motif. Virus Evol. 2024, 10, veae043. [Google Scholar] [CrossRef]
- Ndodo, N.; Ashcroft, J.; Lewandowski, K.; Yinka-Ogunleye, A.; Chukwu, C.; Ahmad, A.; King, D.; Akinpelu, A.; Maluquer de Motes, C.; Ribeca, P. Distinct monkeypox virus lineages co-circulating in humans before 2022. Nat. Med. 2023, 29, 2317–2324. [Google Scholar] [CrossRef]
- Huang, Y.; Bergant, V.; Grass, V.; Emslander, Q.; Hamad, M.S.; Hubel, P.; Mergner, J.; Piras, A.; Krey, K.; Henrici, A.; et al. Multi-omics characterization of the monkeypox virus infection. Nat. Commun. 2024, 15, 6778. [Google Scholar] [CrossRef]
- Cai, X.; Zhou, T.; Shi, W.; Cai, Y.; Zhou, J. Monkeypox Virus Crosstalk with HIV: An Integrated Skin Transcriptome and Machine Learning Study. ACS Omega 2023, 8, 47283–47294. [Google Scholar] [CrossRef]
- Alakunle, E.; Moens, U.; Nchinda, G.; Okeke, M.I. Monkeypox virus in Nigeria: Infection biology, epidemiology, and evolution. Viruses 2020, 12, 1257. [Google Scholar] [CrossRef]
- Anil, S.; Joseph, B.; Thomas, M.; Sweety, V.K.; Suresh, N.; Waltimo, T. Monkeypox: A viral zoonotic disease of rising global concern. Infect. Dis. Immun. 2024, 4, 121–131. [Google Scholar] [CrossRef]
- Scarpa, F.; Azzena, I.; Ciccozzi, A.; Branda, F.; Locci, C.; Perra, M.; Pascale, N.; Romano, C.; Ceccarelli, G.; Terrazzano, G. Update of the genetic variability of monkeypox virus clade iib lineage b. 1. Microorganisms 2024, 12, 1874. [Google Scholar] [CrossRef]
- Zheng, J.; Zeng, J.; Long, H.; Chen, J.; Liu, K.; Chen, Y.; Du, X. Recombination and selection trajectory of the monkeypox virus during its adaptation in the human population. J. Med. Virol. 2024, 96, e29825. [Google Scholar] [CrossRef]
- Kumar, A.; Jhanwar, P.; Gulati, A.; Tatu, U. Genomic analyses of recently emerging clades of mpox virus reveal gene deletion and single nucleotide polymorphisms that correlate with altered virulence and transmission. bioRxiv 2024. [Google Scholar] [CrossRef]
- Musuka, G.; Moyo, E.; Tungwarara, N.; Mhango, M.; Pierre, G.; Saramba, E.; Iradukunda, P.G.; Dzinamarira, T. A critical review of mpox outbreaks, risk factors, and prevention efforts in Africa: Lessons learned and evolving practices. IJID Reg. 2024, 12, 100402. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.M.; Lei, Y.L.; Li, M.; Zhong, L.; Li, S. The monkeypox virus-host interplays. Cell Insight 2024, 3, 100185. [Google Scholar] [CrossRef] [PubMed]
- Tigistu-Sahle, F.; Mekuria, Z.H.; Satoskar, A.R.; Sales, G.F.; Gebreyes, W.A.; Oliveira, C.J. Challenges and opportunities of molecular epidemiology: Using omics to address complex One Health issues in tropical settings. Front. Trop. Dis. 2023, 4, 1151336. [Google Scholar] [CrossRef]
- Paredes, M.I.; Ahmed, N.; Figgins, M.; Colizza, V.; Lemey, P.; McCrone, J.T.; Müller, N.; Tran-Kiem, C.; Bedford, T. Underdetected dispersal and extensive local transmission drove the 2022 mpox epidemic. Cell 2024, 187, 1374–1386.e13. [Google Scholar] [CrossRef]
- Luna, N.; Muñoz, M.; Bonilla-Aldana, D.K.; Patiño, L.H.; Kasminskaya, Y.; Paniz-Mondolfi, A.; Ramírez, J.D. Monkeypox virus (MPXV) genomics: A mutational and phylogenomic analyses of B. 1 lineages. Travel Med. Infect. Dis. 2023, 52, 102551. [Google Scholar] [CrossRef]
- Forni, D.; Cagliani, R.; Pozzoli, U.; Sironi, M. An APOBEC3 mutational signature in the genomes of human-infecting orthopoxviruses. mSphere 2023, 8, e00062-23. [Google Scholar] [CrossRef]
- Dobrovolná, M.; Brázda, V.; Warner, E.F.; Bidula, S. Inverted repeats in the monkeypox virus genome are hot spots for mutation. J. Med. Virol. 2023, 95, e28322. [Google Scholar] [CrossRef]
- Li, H.; Zhang, H.; Ding, K.; Wang, X.-H.; Sun, G.-Y.; Liu, Z.-X.; Luo, Y. The evolving epidemiology of monkeypox virus. Cytokine Growth Factor Rev. 2022, 68, 1–12. [Google Scholar] [CrossRef]
- Andrei, G.; Snoeck, R. Differences in pathogenicity among the mpox virus clades: Impact on drug discovery and vaccine development. Trends Pharmacol. Sci. 2023, 44, 719–739. [Google Scholar] [CrossRef] [PubMed]
- Masirika, L.M.; Udahemuka, J.C.; Schuele, L.; Ndishimye, P.; Otani, S.; Mbiribindi, J.B.; Marekani, J.M.; Mambo, L.M.; Bubala, N.M.; Boter, M.; et al. Ongoing mpox outbreak in Kamituga, South Kivu province, associated with monkeypox virus of a novel Clade I sub-lineage, Democratic Republic of the Congo, 2024. Euro Surveill 2024, 29, 2400106. [Google Scholar] [CrossRef] [PubMed]
- Ali, Y.; Imtiaz, H.; Tahir, M.M.; Gul, F.; Saddozai, U.A.K.; ur Rehman, A.; Ren, Z.-G.; Khattak, S.; Ji, X.-Y. Fragment-based approaches identified tecovirimat-competitive novel drug candidate for targeting the F13 protein of the monkeypox virus. Viruses 2023, 15, 570. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.G.; Gigante, C.M.; Wynn, N.T.; Matheny, A.; Davidson, W.; Yang, Y.; Condori, R.E.; O’Connell, K.; Kovar, L.; Williams, T.L.; et al. Tecovirimat Resistance in Mpox Patients, United States, 2022–2023. Emerg. Infect. Dis. 2023, 29, 2426–2432. [Google Scholar] [CrossRef]
- Lim, C.K.; Roberts, J.; Moso, M.; Liew, K.C.; Taouk, M.L.; Williams, E.; Tran, T.; Steinig, E.; Caly, L.; Williamson, D.A. Mpox diagnostics: Review of current and emerging technologies. J. Med. Virol. 2023, 95, e28429. [Google Scholar] [CrossRef] [PubMed]
- Mani, S.; Ganesan, A.; Arunagiri, T.; Kumaran, V.R.; Kannaiah, K.P.; Vellapandian, C.; Chanduluru, H.K. Epidemiology, Virology, and Mutation Landscape of Monkeypox Virus from Past to Present. Cureus 2024, 16, e67872. [Google Scholar] [CrossRef]
- Ozaktas, T.; Dizkirici, A.; Carbone, A.; Tekpinar, M. Comprehensive Mutational Landscape Analysis of Monkeypox Virus Proteome. bioRxiv 2024. [Google Scholar] [CrossRef]
- Malik, S.; Ahmed, A.; Ahsan, O.; Muhammad, K.; Waheed, Y. Monkeypox Virus: A Comprehensive Overview of Viral Pathology, Immune Response, and Antiviral Strategies. Vaccines 2023, 11, 1345. [Google Scholar] [CrossRef]
- Lu, J.; Xing, H.; Wang, C.; Tang, M.; Wu, C.; Ye, F.; Yin, L.; Yang, Y.; Tan, W.; Shen, L. Mpox (formerly monkeypox): Pathogenesis, prevention, and treatment. Signal Transduct. Target. Ther. 2023, 8, 458. [Google Scholar] [CrossRef]
- Mujuru, S. K4DD Mpox Health Evidence Summary No 3; Institute of Development Studies: Brighton, UK, 2024. [Google Scholar]
- Zhu, J.; Yu, J.; Qin, H.; Chen, X.; Wu, C.; Hong, X.; Zhang, Y.; Zhang, Z. Exploring the key genomic variation in monkeypox virus during the 2022 outbreak. BMC Genom. Data 2023, 24, 67. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.H.; Lee, M.; Lee, S.E.; Kim, J.W.; Shin, H.; Choi, M.M.; Yi, H.; Kim, M.K.; Jeon, J.; Park, J.S.; et al. Genomic Analysis of Monkeypox Virus During the 2023 Epidemic in Korea. J. Korean Med. Sci. 2024, 39, e165. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Laxmi; Sharma, K.; Sridhar, S.B.; Talath, S.; Shareef, J.; Mehta, R.; Satapathy, P.; Sah, R. Clade Ib: A new emerging threat in the Mpox outbreak. Front. Pharmacol. 2024, 15, 1504154. [Google Scholar] [CrossRef] [PubMed]
- Otieno, J.R.; Ruis, C.; Onoja, A.B.; Kuppalli, K.; Hoxha, A.; Nitsche, A.; Brinkmann, A.; Michel, J.; Mbala-Kingebeni, P.; Mukadi-Bamuleka, D. Global genomic surveillance of monkeypox virus. Nat. Med. 2025, 31, 342–350. [Google Scholar] [CrossRef]
- Li, X.; Habibipour, S.; Chou, T.; Yang, O.O. The role of APOBEC3-induced mutations in the differential evolution of monkeypox virus. Virus Evol. 2023, 9, vead058. [Google Scholar] [CrossRef]
- de Oliveira Thomasi, R.M.; da Silva Correa, T.; Silva do Carmo, D.; Rodrigues, D.F.; da Silva Correa, L.V.; Xavier, S.R.; Silva, L.S.; da Silva, J.O.; Dos Santos, M.; da Silva Dantas, A. Molecular methods for diagnosis of monkeypox: A mini-review. Curr. Mol. Med. 2024, 24, 1208–1218. [Google Scholar] [CrossRef]
- Branda, F.; Romano, C.; Ciccozzi, M.; Giovanetti, M.; Scarpa, F.; Ciccozzi, A.; Maruotti, A. Mpox: An Overview of Pathogenesis, Diagnosis, and Public Health Implications. J. Clin. Med. 2024, 13, 2234. [Google Scholar] [CrossRef]
- Louten, J.; Beach, M.; Palermino, K.; Weeks, M.; Holenstein, G. MicroRNAs Expressed during Viral Infection: Biomarker Potential and Therapeutic Considerations. Biomark. Insights 2015, 10, 25–52. [Google Scholar] [CrossRef]
- Loganathan, T.; Fletcher, J.; Abraham, P.; Kannangai, R.; Chakraborty, C.; El Allali, A.; Alsamman, A.M.; Zayed, H.; Doss C, G.P. Expression analysis and mapping of Viral—Host Protein interactions of Poxviridae suggests a lead candidate molecule targeting Mpox. BMC Infect. Dis. 2024, 24, 483. [Google Scholar] [CrossRef]
- Maurya, V.K.; Kumar, S.; Maurya, S.; Ansari, S.; Paweska, J.T.; Abdel-Moneim, A.S.; Saxena, S.K. Structure-based drug designing for potential antiviral activity of selected natural product against Monkeypox (Mpox) virus and its host targets. Virus Dis. 2024, 35, 589–608. [Google Scholar] [CrossRef]
- Shabil, M.; Khatib, M.N.; Ballal, S.; Bansal, P.; Tomar, B.S.; Ashraf, A.; Kumar, M.R.; Sinha, A.; Rawat, P.; Gaidhane, A.M.; et al. Effectiveness of Tecovirimat in Mpox Cases: A Systematic Review of Current Evidence. J. Med. Virol. 2024, 96, e70122. [Google Scholar] [CrossRef]
- Demir, K.K.; Desjardins, M.; Fortin, C.; Grandjean-Lapierre, S.; Chakravarti, A.; Coutlée, F.; Zaharatos, G.; Morin, J.; Tremblay, C.; Longtin, J. Treatment of severe human mpox virus infection with tecovirimat: A case series. Can. Commun. Dis. Rep. 2023, 49, 76–80. [Google Scholar] [CrossRef]
- He, S.; Zhao, J.; Chen, J.; Liang, J.; Hu, X.; Zhang, X.; Zeng, H.; Sun, G. Urogenital Manifestations in Mpox (Monkeypox) Infection: A Comprehensive Review of Epidemiology, Pathogenesis, and Therapeutic Approaches. Infect. Drug Resist. 2025, 18, 209–226. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Atutxa, I.; Mondragon-Teran, P.; Huerta-Saquero, A.; Villanueva-Flores, F. Advancements in monkeypox vaccines development: A critical review of emerging technologies. Front. Immunol. 2024, 15, 1456060. [Google Scholar] [CrossRef] [PubMed]
- Christodoulidou, M.M.; Mabbott, N.A. Efficacy of smallpox vaccines against Mpox infections in humans. Immunother. Adv. 2023, 3, ltad020. [Google Scholar] [CrossRef]
- Fung, S.; Chiu, W.-K. Environmental and Ecological Determinants and Population Health. In Sustainable Health Promotion Practices and the Global Economy; Routledge: Oxfordshire, UK, 2025; pp. 67–88. [Google Scholar]
- Wang, Y.; Chen, H.; Lin, K.; Han, Y.; Gu, Z.; Wei, H.; Mu, K.; Wang, D.; Liu, L.; Jin, R.; et al. Ultrasensitive single-step CRISPR detection of monkeypox virus in minutes with a vest-pocket diagnostic device. Nat. Commun. 2024, 15, 3279. [Google Scholar] [CrossRef]
- DeLaurentis, C.E.; Kiser, J.; Zucker, J. New Perspectives on Antimicrobial Agents: Tecovirimat for Treatment of Human Monkeypox Virus. Antimicrob. Agents Chemother. 2022, 66, e0122622. [Google Scholar] [CrossRef] [PubMed]
- O’Laughlin, K. Clinical use of tecovirimat (Tpoxx) for treatment of monkeypox under an investigational new drug protocol—United States, May–August 2022. MMWR. Morb. Mortal. Wkly. Rep. 2022, 71, 1190–1195. [Google Scholar] [CrossRef]
- Bruno, G.; Buccoliero, G.B. Antivirals against monkeypox (Mpox) in humans: An updated narrative review. Life 2023, 13, 1969. [Google Scholar] [CrossRef]
- Lozano, J.M.; Muller, S. Monkeypox: Potential vaccine development strategies. Trends Pharmacol. Sci. 2023, 44, 15–19. [Google Scholar] [CrossRef]
- Thet, A.K.; Kelly, P.J.; Kasule, S.N.; Shah, A.K.; Chawala, A.; Latif, A.; Chilimuri, S.S.; Zeana, C.B. The use of Vaccinia Immune Globulin in the Treatment of Severe Mpox. Virus Infection in Human Immunodeficiency Virus/AIDS. Clin. Infect. Dis. 2023, 76, 1671–1673. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, M.; Zabihian, A.; Hajsaeedi, M.; Hooshmand, M. Antivirals for monkeypox virus: Proposing an effective machine/deep learning framework. PLoS ONE 2024, 19, e0299342. [Google Scholar] [CrossRef]
- Sanz-Muñoz, I.; Sánchez-dePrada, L.; Sánchez-Martínez, J.; Rojo-Rello, S.; Domínguez-Gil, M.; Hernán-García, C.; Fernández-Espinilla, V.; de Lejarazu-Leonardo, R.O.; Castrodeza-Sanz, J.; Eiros, J.M. Possible Mpox Protection from Smallpox Vaccine-Generated Antibodies among Older Adults. Emerg. Infect. Dis. 2023, 29, 656–658. [Google Scholar] [CrossRef] [PubMed]
- WHO Organization. WHO Advisory Committee on Variola Virus Research: Report of the Twenty-Fifth Meeting, Geneva, Switzerland, 25–26 October 2023; World Health Organization: Geneva, Switzerland, 2024.
- Ganesan, A.; Arunagiri, T.; Mani, S.; Kumaran, V.R.; Kannaiah, K.P.; Chanduluru, H.K. From pox to protection: Understanding Monkeypox pathophysiology and immune resilience. Trop. Med. Health 2025, 53, 33. [Google Scholar] [CrossRef]
- Adamo, S.; Gao, Y.; Sekine, T.; Mily, A.; Wu, J.; Storgärd, E.; Westergren, V.; Filen, F.; Treutiger, C.-J.; Sandberg, J.K. Memory profiles distinguish cross-reactive and virus-specific T cell immunity to mpox. Cell Host Microbe 2023, 31, 928–936.e924. [Google Scholar] [CrossRef] [PubMed]
- Agrati, C.; Cossarizza, A.; Mazzotta, V.; Grassi, G.; Casetti, R.; De Biasi, S.; Pinnetti, C.; Gili, S.; Mondi, A.; Cristofanelli, F.; et al. Immunological signature in human cases of monkeypox infection in 2022 outbreak: An observational study. Lancet Infect. Dis. 2023, 23, 320–330. [Google Scholar] [CrossRef]
- Abebaw, D.; Akelew, Y.; Adugna, A.; Teffera, Z.H.; Tegegne, B.A.; Fenta, A.; Amare, G.A.; Jemal, M.; Baylie, T.; Atnaf, A. Antigen recognition and immune response to monkeypox virus infection: Implications for Mpox vaccine design—A narrative review. Infez. Med. 2025, 33, 151–162. [Google Scholar] [CrossRef]
- Shafaati, M.; Forghani, S.; Shahsavand Davoudi, A.; Samiee, R.; Mohammadi, K.; Akbarpour, S.; Seifi, A.; Salehi, M.; Zare, M. Current advances and challenges in mpox vaccine development: A global landscape. Ther. Adv. Vaccines Immunother. 2025, 13, 25151355251314339. [Google Scholar] [CrossRef]
- Sah, R.; Paul, D.; Mohanty, A.; Shah, A.; Mohanasundaram, A.S.; Padhi, B.K. Monkeypox (Mpox) vaccines and their side effects: The other side of the coin. Int. J. Surg. 2023, 109, 215–217. [Google Scholar] [CrossRef]
- Prompetchara, E.; Ketloy, C.; Khawsang, C.; Palaga, T.; Ruxrungtham, K. Mpox global health emergency: Insights into the virus, immune responses, and advancements in vaccines PART II: Insights into the advancements in vaccines. Asian Pac. J. Allergy Immunol. 2024, 42, 191–206. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Su, S.-B.; Chen, K.-T. A Review of epidemiology, diagnosis, and management of Mpox: The role of One Health. Glob. Health Med. 2025, 7, 1–12. [Google Scholar] [CrossRef]
- Rizk, Y.; Lippi, G.; Henry, B.M.; Notarte, K.I.; Rizk, J.G. Update on Mpox Management: Epidemiology, Vaccines and Therapeutics, and Regulatory Changes. Drugs 2024, 85, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Grabenstein, J.D.; Hacker, A. Vaccines against mpox: MVA-BN and LC16m8. Expert Rev. Vaccines 2024, 23, 796–811. [Google Scholar] [CrossRef] [PubMed]
- Saalbach, K.P. Treatment and Vaccination for Smallpox and Monkeypox. Poxviruses 2024, 1451, 301–316. [Google Scholar]
- Huang, Q.; Sun, Y.; Jia, M.; Jiang, M.; Xu, Y.; Feng, L.; Yang, W. An effectiveness study of vaccination and quarantine combination strategies for containing mpox transmission on simulated college campuses. Infect. Dis. Model. 2024, 9, 805–815. [Google Scholar] [CrossRef]
- Amer, F.; Khalil, H.E.; Elahmady, M.; ElBadawy, N.E.; Zahran, W.A.; Abdelnasser, M.; Rodríguez-Morales, A.J.; Wegdan, A.A.; Tash, R.M.E. Mpox: Risks and approaches to prevention. J. Infect. Public Health 2023, 16, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Philemon, M.; Olopade, I.; Ogbaji, E. Mathematical Analysis of the Effect of Quarantine on the Dynamical Transmission of Monkey Pox. Asian J. Pure Appl. Math. 2023, 5, 473–492. [Google Scholar]
- Ahmed, S.K.; Abdulqadir, S.O.; Hussein, S.H.; Omar, R.M.; Ahmed, N.A.; Essa, R.A.; Dhama, K.; Lorenzo, J.M.; Abdulla, A.Q. The impact of monkeypox outbreak on mental health and counteracting strategies: A call to action. Int. J. Surg. 2022, 106, 106943. [Google Scholar] [CrossRef]
- León-Figueroa, D.A.; Barboza, J.J.; Siddiq, A.; Sah, R.; Valladares-Garrido, M.J.; Rodriguez-Morales, A.J. Knowledge and attitude towards mpox: Systematic review and meta-analysis. PLoS ONE 2024, 19, e0308478. [Google Scholar] [CrossRef]
- Nimbi, F.M.; Rosati, F.; Baiocco, R.; Giovanardi, G.; Lingiardi, V. Monkeypox spread among men who have sex with men: How do people explain this relationship? A quali-quantitative study of beliefs among heterosexual and non-heterosexual Italian individuals. Psychol. Sex. 2024, 15, 415–431. [Google Scholar] [CrossRef]
- Chan, Z.Y.S.; Chong, S.Y.; Niaupari, S.; Harrison-Quintana, J.; Lim, J.T.; Dickens, B.; Kularathne, Y.; Wong, C.S.; Tan, R.K.J. Receptiveness to monkeypox vaccines and public health communication strategies among gay, bisexual and other men who have sex with men in Singapore: Cross-sectional quantitative and qualitative insights. Sex. Transm. Infect. 2024, 100, 362–367. [Google Scholar] [CrossRef]
- Norberg, A.N.; Norberg, P.R.B.M.; Manhães, F.C.; Souza, D.G.d.; Queiroz, M.M.d.C.; Neto, C.H.G.; Ribeiro, P.C.; Boechat, J.C.d.S.; Viana, K.S.; Silva, M.T.R.d. Public Health Strategies Against Social Stigma in the Mpox Outbreak: A Systematic Review. J. Adv. Med. Med. Res. 2024, 36, 33–47. [Google Scholar] [CrossRef]
- Lawrence, A. Socio-Demographic Factors Influencing Monkeypox Vaccination Intentions Among Healthcare Workers and the General Population of Makurdi, Nigeria: A Cross-Sectional Study. Cureus 2024, 16, e71828. [Google Scholar] [CrossRef]
- Bose, D.L.; Hundal, A.; Singh, S.; Singh, S.; Seth, K.; Hadi, S.U.; Saran, A.; Joseph, J.; Goyal, K.; Salve, S. Evidence and gap map report: Social and Behavior Change Communication (SBCC) interventions for strengthening HIV prevention and research among adolescent girls and young women (AGYW) in low- and middle-income countries (LMICs). Campbell Syst. Rev. 2023, 19, e1297. [Google Scholar] [CrossRef]
- Abdulrahim, A.; Gulumbe, B.H.; Idris, I.; Abubakar, T.M.; Sobur, K.A.; Lawal, A.I.; Shehu, A. Strengthening global health preparedness amid Mpox spread in Africa. Discov. Public Health 2025, 22, 9. [Google Scholar] [CrossRef]
- Tambo, E.; Noungoue Ngounou, P.J.; Njobet, M.P.; Tappa, N.T.; Ngogang, J.; Hunter, M.; Shaw, S.Y.; Rimoin, A.W.; Placide, M.; Kindrachuk, J. Assessment of risk perception and determinants of mpox for strengthening community engagement in local populations in Cameroon. medRxiv 2024. [Google Scholar] [CrossRef]
- Branda, F.; Ceccarelli, G.; Ciccozzi, M.; Scarpa, F. Strengthening community resilience: Lessons from COVID-19 for mpox prevention. Lancet 2024, 404, 929. [Google Scholar] [CrossRef] [PubMed]
- El Dine, F.B.; Gebreal, A.; Samhouri, D.; Estifanos, H.; Kourampi, I.; Abdelrhem, H.; Mostafa, H.A.a.; Elshaar, A.G.; Suvvari, T.K.; Ghazy, R.M. Ethical considerations during Mpox Outbreak: A scoping review. BMC Med. Ethics 2024, 25, 79. [Google Scholar] [CrossRef]
- Gilmore, J.; Comer, D.; Field, D.J.; Parlour, R.; Shanley, A.; Noone, C. Recognising and responding to the community needs of gay and bisexual men around mpox. PLoS ONE 2024, 19, e0313325. [Google Scholar] [CrossRef] [PubMed]
- Taube, J.C.; Rest, E.C.; Lloyd-Smith, J.O.; Bansal, S. The global landscape of smallpox vaccination history and implications for current and future orthopoxvirus susceptibility: A modelling study. Lancet Infect. Dis. 2023, 23, 454–462. [Google Scholar] [CrossRef]
- Vaughan, A.M.; Cenciarelli, O.; Colombe, S.; Alves de Sousa, L.; Fischer, N.; Gossner, C.M.; Pires, J.; Scardina, G.; Aspelund, G.; Avercenko, M.; et al. A large multi-country outbreak of monkeypox across 41 countries in the WHO European Region, 7 March to 23 August 2022. Euro Surveill 2022, 27, 2200620. [Google Scholar] [CrossRef] [PubMed]
- Marziano, V.; Guzzetta, G.; Longini, I.; Merler, S. Epidemiologic quantities for Monkeypox Virus Clade I from historical data with implications for current outbreaks, Democratic Republic of the Congo. Emerg. Infect. Dis. 2024, 30, 2042. [Google Scholar] [CrossRef]
- Xiu, V. Characteristics of the Sexual Networks of Gay, Bisexual, and Other Men Who Have Sex with Men and Impact of Past Interventions on Mpox Transmission During the 2022 Outbreak in Montréal, Toronto, and Vancouver (Canada). Master’s Thesis, McGill University, Montreal, QC, Canada, 2024. [Google Scholar]
- Molla, J.; Sekkak, I.; Mundo Ortiz, A.; Moyles, I.; Nasri, B. Mathematical modeling of mpox: A scoping review. One Health 2023, 16, 100540. [Google Scholar] [CrossRef] [PubMed]
- Iftikhar, H.; Daniyal, M.; Qureshi, M.; Tawiah, K.; Ansah, R.K.; Afriyie, J.K. A hybrid forecasting technique for infection and death from the mpox virus. Digit. Health 2023, 9, 20552076231204748. [Google Scholar] [CrossRef]
- Kaur, A.; Kumar, A.; Kumari, G.; Muduli, R.; Das, M.; Kundu, R.; Mukherjee, S.; Majumdar, T. Rational design and computational evaluation of a multi-epitope vaccine for monkeypox virus: Insights into binding stability and immunological memory. Heliyon 2024, 10, e36154. [Google Scholar] [CrossRef]
- Cuba, W.M.; Huaman Alfaro, J.C.; Iftikhar, H.; López-Gonzales, J.L. Modeling and analysis of monkeypox outbreak using a new time series ensemble technique. Axioms 2024, 13, 554. [Google Scholar] [CrossRef]
- Singh, V.; Khan, S.A.; Yadav, S.K.; Akhter, Y. Modeling Global Monkeypox Infection Spread Data: A Comparative Study of Time Series Regression and Machine Learning Models. Curr. Microbiol. 2023, 81, 15. [Google Scholar] [CrossRef]
- Jena, D.; Sridhar, S.B.; Shareef, J.; Talath, S.; Ballal, S.; Kumar, S.; Bhat, M.; Sharma, S.; Kumar, M.R.; Chauhan, A.S.; et al. Time series modelling and forecasting of Monkeypox outbreak trends Africa’s in most affected countries. New Microbes New Infect. 2024, 62, 101526. [Google Scholar] [CrossRef]
- Moss, B. Understanding the biology of monkeypox virus to prevent future outbreaks. Nat. Microbiol. 2024, 9, 1408–1416. [Google Scholar] [CrossRef] [PubMed]

| Clinical Feature/Sign | Estimated Frequency in Mpox Cases (%) | Key Differential Diagnoses | Refs |
|---|---|---|---|
| Skin lesions (papules → vesicles → pseudo-pustules): Lesion count, non-endemic: 1–20 lesions- Endemic: >10 lesions (often >100): Lesions at different stages often present simultaneously | 95% | Chickenpox, smallpox, herpes zoster, molluscum contagiosum, HSV-1/2, Varicella-zoster, eczema vaccinatum, syphilis, scabies | [28,29] |
| Anogenital lesions (clustered, painful, localized vesicles, often in genital areas) | 36–73% | HSV, syphilis (chancre, condyloma lata), LGV, chancroid | [23,30] |
| Perioral/oral lesions (clustered, painful, localized vesicles, often in oral areas) | 14–25% | Herpes simplex, aphthous ulcers | [31] |
| Fever | ~58% (Common but less frequent than in endemic cases) | Influenza, measles, mononucleosis, Varicella-zoster, measles, bacterial lymphadenitis, EBV, CMV, HIV, bacterial infections, Influenza | [23] |
| Lymphadenopathy (inguinal/submandibular) | ~53% inguinal (Common but less frequent than in endemic cases) | HIV acute, streptococcal pharyngitis, bacterial infections, Varicella-zoster, measles, bacterial lymphadenitis, EBV, CMV, HIV, bacterial infections, Influenza | [23,32] |
| Proctitis/rectal pain (anal pain/inflammation) | 15–30% in MSM | STI-related proctitis (LGV, gonorrhea, chlamydia, HSV) | [33,34] |
| Headache, myalgia, fatigue | Common but unquantified | Influenza, COVID-19, EBV | [23] |
| Polymorphic lesion stages (uneven) | Common | Unlike synchronous vaccination reactions | [23,35] |
| Cough | ~26–38% | Influenza, adenovirus, pertussis | [23,36] |
| Ocular symptoms (conjunctivitis, etc.) | 5–16% | Conjunctivitis, herpes simplex ocular infection | [23] |
| Vaccine | Type | Approval Status | Target Population | Administration Strategy | Refs |
|---|---|---|---|---|---|
| JYNNEOS (Modified Vaccinia Ankara-Bavarian Nordic (MVA-BN) (Imvamune® or Imvanex®)) | Live, non-replicating vaccine | Approved in the U.S., Europe and Canada for smallpox; authorized for Mpox during outbreaks WHO prequalified in September 2024 | Adults, including those with HIV; under investigation for pediatric use General population; focus on outbreak control in endemic regions | Two-dose series, subcutaneous injection; studies ongoing for single-dose efficacy | [17,153,154] |
| ACAM2000 | Live, replicating vaccine | Approved in the U.S. for smallpox; available for Mpox under Expanded Access IND | Adults at high risk for Mpox exposure; not recommended for immunocompromised individuals | Single-dose, percutaneous administration using a bifurcated needle | [17,155] |
| KM Biologics Vaccine (LC16m8) | Inactivated vaccine | WHO Emergency Use Listing granted in November 2024 | Children over 1 year old and adults; particularly in outbreak regions like Congo | Single-dose, intramuscular injection; Japan donating 3 million doses to Congo | [153,156] |
| Tonix Pharmaceuticals TNX-801 | Live, attenuated vaccine | Experimental; preclinical development | Intended for broader population; aims for single-dose immunity | Single-dose, intradermal injection; designed for enhanced stability and ease of distribution | [62,157] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mushebenge, A.G.-A.; Mphuthi, D.D. Emerging Insights into Monkeypox: Clinical Features, Epidemiology, Molecular Insights, and Advancements in Management. BioMed 2025, 5, 21. https://doi.org/10.3390/biomed5030021
Mushebenge AG-A, Mphuthi DD. Emerging Insights into Monkeypox: Clinical Features, Epidemiology, Molecular Insights, and Advancements in Management. BioMed. 2025; 5(3):21. https://doi.org/10.3390/biomed5030021
Chicago/Turabian StyleMushebenge, Aganze Gloire-Aimé, and David Ditaba Mphuthi. 2025. "Emerging Insights into Monkeypox: Clinical Features, Epidemiology, Molecular Insights, and Advancements in Management" BioMed 5, no. 3: 21. https://doi.org/10.3390/biomed5030021
APA StyleMushebenge, A. G.-A., & Mphuthi, D. D. (2025). Emerging Insights into Monkeypox: Clinical Features, Epidemiology, Molecular Insights, and Advancements in Management. BioMed, 5(3), 21. https://doi.org/10.3390/biomed5030021






