The Relationship between Vitamin D and TyG Index in Prediabetes and Type 2 Diabetes Mellitus among an Indian Tribal Community: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Anthropometric and Blood Pressure Measurements
2.3. Biochemical Analysis
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DeFronzo, R.A.; Ferrannini, E.; Groop, L.; Henry, R.R.; Herman, W.H. Type 2 diabetes mellitus. Nat. Rev. Dis. Primers 2015, 1, 15019. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M. Pre-diabetes, metabolic syndrome, and cardiovascular risk. J. Am. Coll. Cardiol. 2012, 59, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.B.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Al Kaabi, J. Epidemiology of type 2 diabetes–global burden of disease and forecasted trends. J. Epidemiol. Glob. Health 2020, 10, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Laird, E.; Ward, M.; McSorley, E.; Strain, J.J.; Wallace, J. Vitamin D and bone health: Potential mechanisms. Nutrients 2010, 2, 693–724. [Google Scholar] [CrossRef]
- Rosen, C.J.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; Kovacs, C.S.; et al. IOM committee members respond to Endocrine Society vitamin D guideline. J. Clin. Endocrinol. Metab. 2012, 97, 1146–1152. [Google Scholar] [CrossRef]
- Borissova, A.M.; Tankova, T.; Kirilov, G.; Dakovska, L.; Kovacheva, R. The effect of vitamin D3 on insulin secretion and peripheral insulin sensitivity in type 2 diabetic patients. Int. J. Clin. Pract. 2003, 57, 258–261. [Google Scholar] [CrossRef]
- Pitocco, D.; Crino, A.; Di Stasio, E.; Manfrini, S.; Guglielmi, C.; Spera, S.; Anguissola, G.B.; Visalli, N.; Suraci, C.; Matteoli, M.C.; et al. The effects of calcitriol and nicotinamide on residual pancreatic beta-cell function in patients with recent-onset Type 1 diabetes (IMDIAB XI). Diabet Med. 2006, 23, 920–923. [Google Scholar] [CrossRef]
- Mitri, J.; Pittas, A.G. Vitamin D and diabetes. Endocrinol. Metab. Clin. N. Am. 2014, 43, 205–232. [Google Scholar] [CrossRef]
- Fadda, G.Z.; Akmal, M.; Lipson, L.G.; Massry, S.G. Direct effect of parathyroid hormone on insulin secretion from pancreatic islets. Am. J. Physiol. 1990, 258, E975–E984. [Google Scholar] [CrossRef]
- Gao, Y.; Zheng, T.; Ran, X.; Ren, Y.; Chen, T.; Zhong, L.; Yan, D.; Yan, F.; Wu, Q.; Tian, H. Vitamin D and Incidence of Prediabetes or Type 2 Diabetes: A Four-Year Follow-Up Community-Based Study. Dis. Markers 2018, 2018, 1926308. [Google Scholar] [CrossRef] [PubMed]
- Leung, P.S. The potential protective action of vitamin D in hepatic insulin resistance and pancreatic islet dysfunction in type 2 diabetes mellitus. Nutrients 2016, 8, 147. [Google Scholar] [CrossRef] [PubMed]
- Al Refaie, A.; Baldassini, L.; Mondillo, C.; De Vita, M.; Giglio, E.; Tarquini, R.; Gonnelli, S.; Caffarelli, C. Vitamin D and Dyslipidemia: Is There Really a Link? A Narrative Review. Nutrients 2024, 16, 1144. [Google Scholar] [CrossRef] [PubMed]
- MacGiriley, R.; Phoswa, W.N.; Mokgalaboni, K. Modulatory Properties of Vitamin D in Type 2 Diabetic Patients: A Focus on Inflammation and Dyslipidemia. Nutrients 2023, 15, 4575. [Google Scholar] [CrossRef]
- Relimpio, F. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia 2003, 46, 1707. [Google Scholar] [CrossRef]
- Warram, J.H.; Martin, B.C.; Krolewski, A.S.; Soeldner, J.S.; Kahn, C.R. Slow glucose removal rate and hyperinsulinemia precede the development of type II diabetes in the offspring of diabetic parents. Ann. Intern. Med. 1990, 113, 909–915. [Google Scholar] [CrossRef]
- Cersosimo, E.; Solis-Herrera, C.; Trautmann, M.E.; Malloy, J.; Triplitt, C.L. Assessment of pancreatic β-cell function: Review of methods and clinical applications. Curr. Diabetes Rev. 2014, 10, 2–42. [Google Scholar] [CrossRef]
- Van Minh, H.; Tien, H.A.; Sinh, C.T.; Thang, D.C.; Chen, C.; Tay, J.C.; Siddique, S.; Wang, T.; Sogunuru, G.P.; Chia, Y.; et al. Assessment of preferred methods to measure insulin resistance in Asian patients with hypertension. J. Clin. Hypertens. 2021, 23, 529–537. [Google Scholar] [CrossRef]
- Guerrero-Romero, F.; Simental-Mendía, L.E.; GonzálEz-Ortiz, M.; MartínEz-Abundis, E.; Ramos-Zavala, M.G.; HernánDez-GonzálEz, S.O.; Jacques-Camarena, O.; RodrígUez-Morán, M. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J. Clin. Endocrinol. Metab. 2010, 95, 3347–3351. [Google Scholar] [CrossRef]
- Simental-Mendía, L.E.; Rodríguez-Morán, M.; Guerrero-Romero, F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab. Syndr. Relat. Disord. 2008, 6, 299–304. [Google Scholar] [CrossRef]
- Vasques, A.C.J.; Novaes, F.S.; de Oliveira, M.D.S.; Souza, J.R.M.; Yamanaka, A.; Pareja, J.C.; Tambascia, M.A.; Saad, M.J.A.; Geloneze, B. TyG index performs better than HOMA in a Brazilian population: A hyperglycemic clamp validated study. Diabetes Res. Clin. Pract. 2011, 93, e98–e100. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-L.; Liu, L.; Lo, K.; Huang, J.-Y.; Yu, Y.-L.; Huang, Y.-Q.; Feng, Y.-Q. Association between triglyceride glucose index and risk of new-onset diabetes among Chinese adults: Findings from the China Health and Retirement Longitudinal Study. Front. Cardiovasc. Med. 2020, 7, 610322. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Yang, C.; Shen, R.; Wei, X.; Gong, J.; Pan, Y.; Lv, Y.; Xu, Y. Association between the triglyceride-glucose index and the incidence of diabetes in people with different phenotypes of obesity: A retrospective study. Front. Endocrinol. 2021, 12, 784616. [Google Scholar] [CrossRef]
- Ma, X.; Dong, L.; Shao, Q.; Cheng, Y.; Lv, S.; Sun, Y.; Shen, H.; Wang, Z.; Zhou, Y.; Liu, X. Triglyceride glucose index for predicting cardiovascular outcomes after percutaneous coronary intervention in patients with type 2 diabetes mellitus and acute coronary syndrome. Cardiovasc. Diabetol. 2020, 19, 31. [Google Scholar] [CrossRef]
- Pan, Y.; Zhong, S.; Zhou, K.; Tian, Z.; Chen, F.; Liu, Z.; Geng, Z.; Li, S.; Huang, R.; Wang, H.; et al. Association between diabetes complications and the triglyceride-glucose index in hospitalized patients with type 2 diabetes. J. Diabetes Res. 2021, 2021, 8757996. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Xu, H.; Zhan, J.; Lu, S.; Li, S.; Wang, Y.; Wang, Y.; He, J.; Ni, Y.; Li, L.; et al. Association between the triglyceride-glucose index and vitamin D status in type 2 diabetes mellitus. Nutrients 2023, 15, 639. [Google Scholar] [CrossRef]
- Jia, Y.; Song, T.; Li, Z.; Zhou, L.; Chen, S. The relationship between triglyceride glucose index and vitamin D in type 2 diabetes mellitus. Diabetes Metab. Syndr. Obes. 2022, 15, 511–525. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes-2018. Diabetes Care 2018, 41, S13–S27. [Google Scholar] [CrossRef]
- Zhen, D.; Liu, L.; Guan, C.; Zhao, N.; Tang, X. High prevalence of vitamin D deficiency among middle-aged and elderly individuals in northwestern China: Its relationship to osteoporosis and lifestyle factors. Bone 2015, 71, 1–6. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Simental-Mendía, L.E.; Guerrero-Romero, F. The correct formula for the triglycerides and glucose index. Eur. J. Pediatr. 2020, 179, 1171. [Google Scholar] [CrossRef] [PubMed]
- Aparna, P.; Muthathal, S.; Nongkynrih, B.; Gupta, S.K. Vitamin D deficiency in India. J. Fam. Med. Prim. Care 2018, 7, 324–330. [Google Scholar]
- Jiang, Z.; Pu, R.; Li, N.; Chen, C.; Li, J.; Dai, W.; Wang, Y.; Hu, J.; Zhu, D.; Yu, Q.; et al. High prevalence of vitamin D deficiency in Asia: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2023, 63, 3602–3611. [Google Scholar] [CrossRef]
- Kennel, K.A.; Drake, M.T.; Hurley, D.L. Vitamin D deficiency in adults: When to test and how to treat. Mayo Clin. Proc. 2010, 85, 752–757, quiz 757–758. [Google Scholar] [CrossRef]
- Dhas, Y.; Banerjee, J.; Damle, G.; Mishra, N. Association of vitamin D deficiency with insulin resistance in middle-aged type 2 diabetics. Clin. Chim. Acta 2019, 492, 95–101. [Google Scholar] [CrossRef]
- Salih, Y.A.; Rasool, M.T.; Ahmed, I.H.; Mohammed, A.A. Impact of vitamin D level on glycemic control in diabetes mellitus type 2 in Duhok. Ann. Med. Surg. 2021, 64, 102208. [Google Scholar] [CrossRef]
- Plum, L.A.; DeLuca, H.F. Vitamin D, disease and therapeutic opportunities. Nat. Rev. Drug Discov. 2010, 9, 941–955. [Google Scholar] [CrossRef] [PubMed]
- Pittas, A.G.; Kawahara, T.; Jorde, R.; Dawson-Hughes, B.; Vickery, E.M.; Angellotti, E.; Nelson, J.; Trikalinos, T.A.; Balk, E.M. Vitamin D and risk for type 2 diabetes in people with prediabetes: A systematic review and meta-analysis of individual participant data from 3 randomized clinical trials. Ann. Intern. Med. 2023, 176, 355–363. [Google Scholar] [CrossRef]
- Tai, K.; Need, A.G.; Horowitz, M.; Chapman, I.M. Glucose tolerance and vitamin D: Effects of treating vitamin D deficiency. Nutrition 2008, 24, 950–956. [Google Scholar] [CrossRef]
- Kobza, V.M.; Fleet, J.C.; Zhou, J.; Conley, T.B.; Peacock, M.; IglayReger, H.B.; DePalma, G.; Campbell, W.W. Vitamin D status and resistance exercise training independently affect glucose tolerance in older adults. Nutr. Res. 2013, 33, 349–357. [Google Scholar] [CrossRef]
- Zeitz, U.; Weber, K.; Soegiarto, D.W.; Wolf, E.; Balling, R.; Erben, R.G. Impaired insulin secretory capacity in mice lacking a functional vitamin D receptor. FASEB J. 2003, 17, 509–511. [Google Scholar] [CrossRef] [PubMed]
- Kayaniyil, S.; Vieth, R.; Retnakaran, R.; Knight, J.A.; Qi, Y.; Gerstein, H.C.; Perkins, B.A.; Harris, S.B.; Zinman, B.; Hanley, A.J. Association of vitamin D with insulin resistance and beta-cell dysfunction in subjects at risk for type 2 diabetes. Diabetes Care 2010, 33, 1379–1381. [Google Scholar] [CrossRef]
- Fondjo, L.A.; Owiredu, W.K.B.A.; Sakyi, S.A.; Laing, E.F.; Adotey-Kwofie, M.A.; Antoh, E.O.; Detoh, E. Vitamin D status and its association with insulin resistance among type 2 diabetics: A case-control study in Ghana. PLoS ONE 2017, 12, e0175388. [Google Scholar] [CrossRef] [PubMed]
- Srinath, K.M.; Shashidhara, K.C.; Rajeev, R.; Madhu, B.; Kishaloy, D.; Mahesh, M.G. Pattern of vitamin D status in prediabetic individuals: A case-control study at tertiary hospital in South India. Int. J. Res. Med. Sci. 2016, 4, 1010–1015. [Google Scholar]
- Yu, L.; Zhai, Y.; Shen, S. Association between vitamin D and prediabetes: A PRISMA-compliant meta-analysis. Medicine 2020, 99, e19034. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, H.; Tang, J.; Li, J.; Chong, W.; Hai, Y.; Feng, Y.; Lunsford, L.D.; Xu, P.; Jia, D.; et al. Effects of vitamin D supplementation on prevention of type 2 diabetes in patients with prediabetes: A systematic review and meta-analysis. Diabetes Care 2020, 43, 1650–1658. [Google Scholar] [CrossRef]
- Musazadeh, V.; Kavyani, Z.; Mirhosseini, N.; Dehghan, P.; Vajdi, M. Effect of vitamin D supplementation on type 2 diabetes biomarkers: An umbrella of interventional meta-analyses. Diabetol. Metab. Syndr. 2023, 15, 76. [Google Scholar] [CrossRef]
- Norman, A.W.; Frankel, J.B.; Heldt, A.M.; Grodsky, G.M. Vitamin D deficiency inhibits pancreatic secretion of insulin. Science 1980, 209, 823–825. [Google Scholar] [CrossRef]
- Cade, C.; Norman, A.W. Vitamin D3 improves impaired glucose tolerance and insulin secretion in the vitamin D-deficient rat in vivo. Endocrinology 1986, 119, 84–90. [Google Scholar] [CrossRef]
- Cheng, Q.; Li, Y.C.; Boucher, B.J.; Leung, P.S. A novel role for vitamin D: Modulation of expression and function of the local renin-angiotensin system in mouse pancreatic islets. Diabetologia 2011, 54, 2077–2081. [Google Scholar] [CrossRef]
- Pittas, A.G.; Lau, J.; Hu, F.B.; Dawson-Hughes, B. The role of vitamin D and calcium in type 2 diabetes: A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2007, 92, 2017–2029. [Google Scholar] [CrossRef] [PubMed]
- Wright, D.C.; Hucker, K.A.; Holloszy, J.O.; Han, D.H. Ca2+ and AMPK both mediate stimulation of glucose transport by muscle contractions. Diabetes 2004, 53, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Yuan, Q.; Mao, L.; Chen, F.L.; Ji, F.; Cui, Z.H. Vitamin D deficiency causes insulin resistance by provoking oxidative stress in hepatocytes. Oncotarget 2017, 8, 67605–67613. [Google Scholar] [CrossRef] [PubMed]
- Hurrle, S.; Hsu, W.H. The etiology of oxidative stress in insulin resistance. Biomed J. 2017, 40, 257–262. [Google Scholar] [CrossRef]
- Roberts, C.K.; Hevener, A.L.; Barnard, R.J. Metabolic syndrome and insulin resistance: Underlying causes and modification by exercise training. Compr. Physiol. 2013, 3, 1–58. [Google Scholar]
- Barzegar, N.; Tohidi, M.; Hasheminia, M.; Azizi, F.; Hadaegh, F. The impact of triglyceride-glucose index on incident cardiovascular events during 16 years of follow-up: Tehran Lipid and Glucose Study. Cardiovasc. Diabetol. 2020, 19, 155. [Google Scholar] [CrossRef]
- Lee, S.-H.; Kwon, H.-S.; Park, Y.-M.; Ha, H.-S.; Jeong, S.H.; Yang, H.K.; Lee, J.-H.; Yim, H.-W.; Kang, M.-I.; Lee, W.-C.; et al. Predicting the development of diabetes using the product of triglycerides and glucose: The Chungju Metabolic Disease Cohort (CMC) study. PLoS ONE 2014, 9, e90430. [Google Scholar] [CrossRef]
- Chen, Z.; Wen, J. Elevated Triglyceride-Glucose (TyG) Index Predicts Impaired Islet β-Cell Function: A Hospital-Based Cross-Sectional Study. Front. Endocrinol. 2022, 13, 973655. [Google Scholar] [CrossRef]
- Low, S.; Khoo, K.C.J.; Irwan, B.; Sum, C.F.; Subramaniam, T.; Lim, S.C.; Wong, T.K.M. The role of triglyceride glucose index in development of Type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 2018, 143, 43–49. [Google Scholar] [CrossRef]
- Hameed, E.K. TyG index: A promising biomarker for glycemic control in type 2 diabetes mellitus. Diabetes Metab. Syndr. 2019, 13, 560–563. [Google Scholar] [CrossRef]
- Wen, J.; Wang, A.; Liu, G.; Wang, M.; Zuo, Y.; Li, W.; Zhai, Q.; Mu, Y.; Gaisano, H.Y.; He, Y.; et al. Elevated triglyceride-glucose (TyG) index predicts incidence of prediabetes: A prospective cohort study in China. Lipids Health Dis. 2020, 19, 226. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Wang, X.; Zhong, Y.; Wang, Y.; Wu, J.; Geng, J.; Zhou, Y.; Chen, J.; Guan, P.; Xu, Y.; et al. The Triglyceride-Glucose Index is associated with diabetic retinopathy in Chinese patients with type 2 diabetes: A hospital-based, nested, case-control study. Diabetes Metab. Syndr. Obes. 2021, 14, 1547–1555. [Google Scholar] [CrossRef]
- Liu, L.; Xia, R.; Song, X.; Zhang, B.; He, W.; Zhou, X.; Li, S.; Yuan, G. Association between the triglyceride-glucose index and diabetic nephropathy in patients with type 2 diabetes: A cross-sectional study. J. Diabetes Investig. 2021, 12, 557–565. [Google Scholar] [CrossRef]
- Zhou, J.; Zhu, L.; Li, Y. Association between the triglyceride glucose index and diabetic retinopathy in type 2 diabetes: A meta-analysis. Front. Endocrinol. 2023, 14, 1302127. [Google Scholar] [CrossRef]
- Xiang, Q.; Xu, H.; Liu, Y.; Huang, W. Elevated TyG Index Is Associated with Increased Risk of Vitamin D Deficiency among Elderly Patients with Type 2 Diabetes. Sci. Rep. 2024, 14, 16098. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, W.; Zhao, Z.; Li, W.; Zhang, J. The Triglyceride-Glucose Index is associated with vitamin D status in metabolic-associated fatty liver disease. Diabetes Metab. Syndr. Obes. 2023, 16, 2651–2660. [Google Scholar] [CrossRef] [PubMed]
- Mahat, R.K.; Panda, G.; Nayak, B.P.; Panda, S. Association of Vitamin D with Triglyceride-Glucose Index and Cardiometabolic Risk Factors in Subclinical Hypothyroidism. Hum. Nutr. Metab. 2023, 34, 200226. [Google Scholar] [CrossRef]
- Mahat, R.K.; Rathore, V. Comment on Xiang et al. Association between the Triglyceride-Glucose Index and Vitamin D Status in Type 2 Diabetes Mellitus. Nutrients 2023, 15, 4068. [Google Scholar] [CrossRef]
- Xiang, Q.; Xu, H.; Zhan, J.; Lu, S.; Li, S.; Wang, Y.; Wang, Y.; He, J.; Ni, Y.; Li, L.; et al. Reply to Mahat, R.K.; Rathore, V. Comment on “Xiang et al. Association between the Triglyceride-Glucose Index and Vitamin D Status in Type 2 Diabetes Mellitus”. Nutrients 2023, 15, 4069. [Google Scholar] [CrossRef]
- Mohamad, M.I.; El-Sherbeny, E.E.; Bekhet, M.M. The Effect of Vitamin D Supplementation on Glycemic Control and Lipid Profile in Patients with Type 2 Diabetes Mellitus. J. Am. Coll. Nutr. 2016, 35, 399–404. [Google Scholar] [CrossRef]
- Surdu, A.M.; Pînzariu, O.; Ciobanu, D.-M.; Negru, A.-G.; Căinap, S.-S.; Lazea, C.; Iacob, D.; Săraci, G.; Tirinescu, D.; Borda, I.M.; et al. Vitamin D and Its Role in the Lipid Metabolism and the Development of Atherosclerosis. Biomedicines 2021, 9, 172. [Google Scholar] [CrossRef] [PubMed]
- Asano, L.; Watanabe, M.; Ryoden, Y.; Usuda, K.; Yamaguchi, T.; Khambu, B.; Takashima, M.; Sato, S.-I.; Sakai, J.; Nagasawa, K.; et al. Vitamin D Metabolite, 25-Hydroxyvitamin D, Regulates Lipid Metabolism by Inducing Degradation of SREBP/SCAP. Cell Chem. Biol. 2017, 24, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Rafiq, S.; Jeppesen, P.B. Is Hypovitaminosis D Related to Incidence of Type 2 Diabetes and High Fasting Glucose Level in Healthy Subjects: A Systematic Review and Meta-Analysis of Observational Studies. Nutrients 2018, 10, 59. [Google Scholar] [CrossRef] [PubMed]
- Wamberg, L.; Christiansen, T.; Paulsen, S.K.; Fisker, S.; Rask, P.; Rejnmark, L.; Richelsen, B.; Pedersen, S.B. Expression of vitamin D-metabolizing enzymes in human adipose tissue—The effect of obesity and diet-induced weight loss. Int. J. Obes. 2013, 37, 651–657. [Google Scholar] [CrossRef]
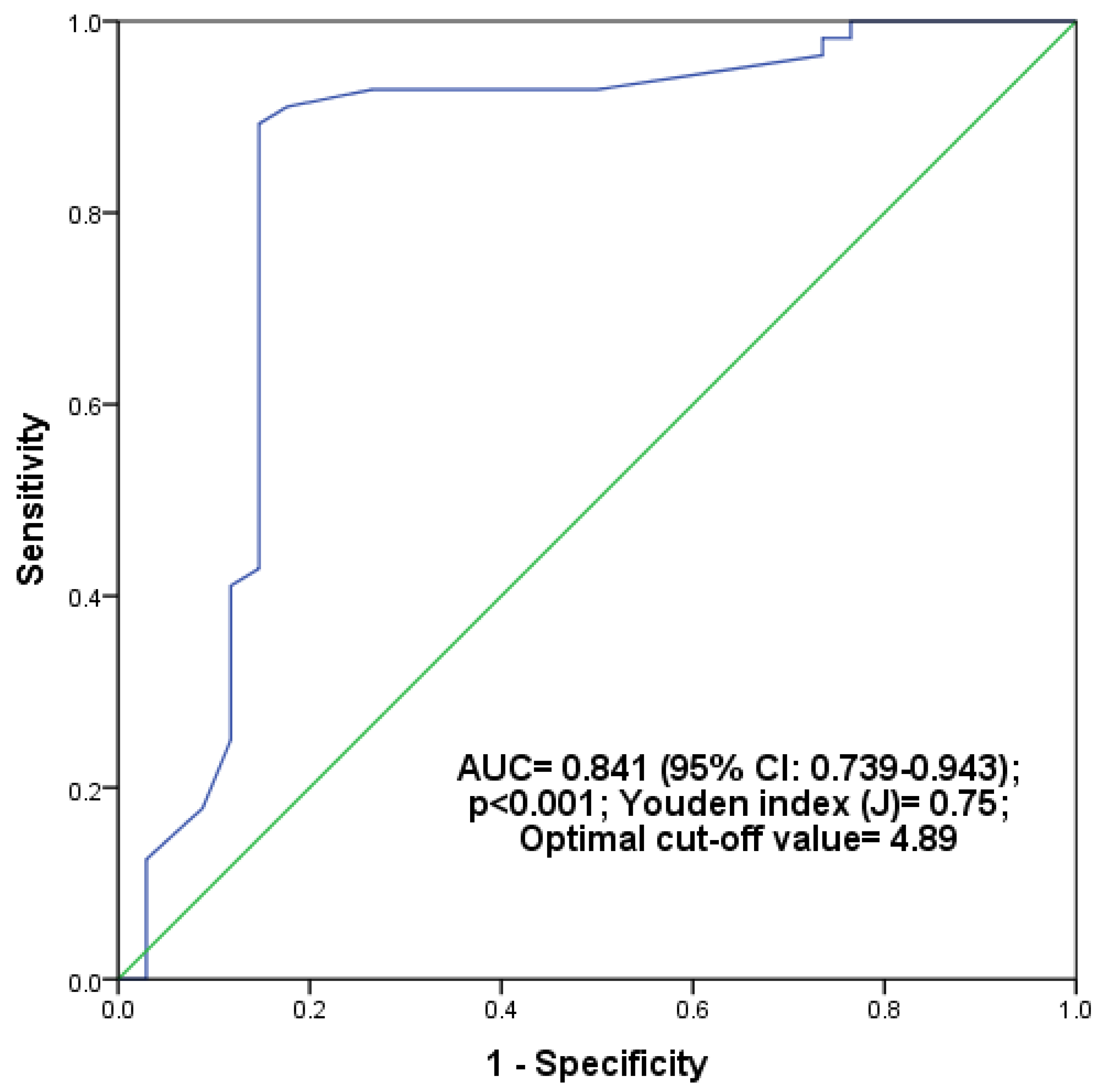
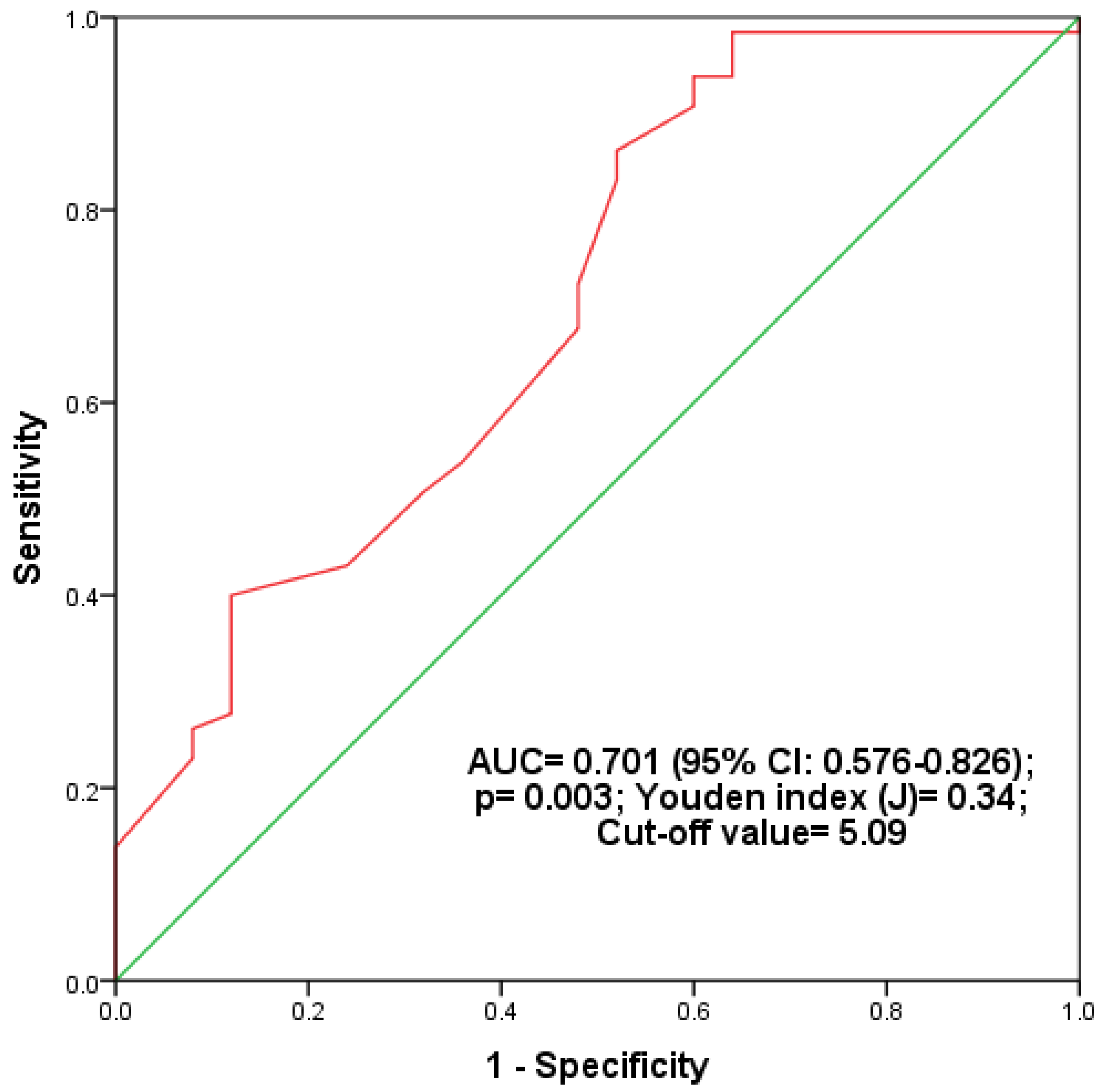
| Variables | T2DM (n = 90) | Prediabetes (n = 90) | Controls (n = 90) | A | B | C | D |
|---|---|---|---|---|---|---|---|
| Age (Years) | 48.64 ± 9.03 | 47.97 ± 8.78 | 47.31 ± 8.87 | 0.604 | 1 | 0.946 | 1 |
| Male/Female (n) | 50/40 | 47/43 | 46/44 | 0.824 | 0.654 | 0.550 | 0.881 |
| BMI (Kg/m2) | 25.1 ± 3.06 | 25.1 ± 2.17 | 22.12 ± 2.48 | <0.001 * | 1 | <0.001 * | <0.001 * |
| WHR | 0.91 ± 0.05 | 0.90 ± 0.03 | 0.85 ± 0.03 | <0.001 * | 0.195 | <0.001 * | <0.001 * |
| SBP (mmHg) | 135.38 ± 9.01 | 124.06 ± 8.1 | 112.5 ± 3.4 | <0.001 * | <0.001 * | <0.001 * | <0.001 * |
| DBP (mmHg) | 88.43 ± 4.91 | 83.39 ± 3.42 | 76.68 ± 3.79 | <0.001 * | <0.001 * | <0.001 * | <0.001 * |
| FPG (mg/dL) | 154.81 ± 13.51 | 112.82 ± 6.5 | 86 ± 6.89 | <0.001 * | <0.001 * | <0.001 * | <0.001 * |
| 2-h PG (mg/dL) | 249.48 ± 21.55 | 161.67 ± 12.07 | 118.62 ± 7.57 | <0.001 * | <0.001 * | <0.001 * | <0.001 * |
| HbA1c (%) | 9.14 ± 1.5 | 6.04 ± 0.19 | 4.69 ± 0.4 | <0.001 * | <0.001 * | <0.001 * | <0.001 * |
| TC (mg/dL) | 229.72 ± 27.51 | 205.22 ± 22.14 | 171.33 ± 7.94 | <0.001 * | <0.001 * | <0.001 * | <0.001 * |
| TG (mg/dL) | 214.89 ± 18.3 | 172.58 ± 26.32 | 125.61 ± 10.12 | <0.001 * | <0.001 * | <0.001 * | <0.001 * |
| HDL-C (mg/dL) | 39.29 ± 4.16 | 44.49 ± 4.62 | 58.19 ± 5.11 | <0.001 * | <0.001 * | <0.001 * | <0.001 * |
| LDL-C (mg/dL) | 147.46 ± 28.1 | 126.22 ± 22.64 | 88.02 ± 10.58 | <0.001 * | <0.001 * | <0.001 * | <0.001 * |
| VLDL-C (mg/dL) | 42.98 ± 3.66 | 34.52 ± 5.26 | 25.12 ± 2.02 | <0.001 * | <0.001 * | <0.001 * | <0.001 * |
| TyG Index | 5.2 ± 0.08 | 4.93 ± 0.09 | 4.64 ± 0.06 | <0.001 * | <0.001 * | <0.001 * | <0.001 * |
| 25(OH)D (ng/mL) | 15 (12–27.75) | 18 (14–39) | 29.5 (17–48.75) | <0.001 * | 0.054 | <0.001 * | 0.032 * |
| Variables | Prediabetes | T2DM | ||||
|---|---|---|---|---|---|---|
| Vitamin D Deficiency (n = 56) | Non-Vitamin D Deficiency (n = 34) | p-Value | Vitamin D Deficiency (n = 65) | Non-Vitamin D Deficiency (n = 25) | p-Value | |
| Age (years) | 48.23 ±9.27 | 47.53 ± 8.04 | 0.715 | 48.42 ± 8.75 | 49.24 ± 9.88 | 0.700 |
| BMI (Kg/m2) | 25.27 ± 2.03 | 24.84 ± 2.39 | 0.363 | 25.48 ± 3.03 | 24.11 ± 2.98 | 0.057 |
| WHR | 0.9 ± 0.03 | 0.9 ± 0.03 | 0.477 | 0.92 ± 0.05 | 0.9 ± 0.05 | 0.130 |
| SBP (mmHg) | 125.34 ± 8.34 | 121.94 ± 7.32 | 0.053 | 135.88 ± 8.5 | 134.08 ± 10.29 | 0.400 |
| DBP (mmHg) | 83.25 ± 3.51 | 83.62 ± 3.32 | 0.624 | 88.88 ± 4.53 | 87.28 ± 5.73 | 0.168 |
| FPG (mg/dL) | 115.43 ± 5.25 | 108.53 ± 6.11 | <0.001 * | 157.38 ± 12.9 | 148.12 ± 13 | 0.003 * |
| 2-h PG (mg/dL) | 165.11 ± 11.35 | 156 ± 11.17 | <0.001 * | 251.42 ± 20.49 | 244.44 ± 23.81 | 0.170 |
| HBA1c (%) | 6.05 ± 0.17 | 6.03 ± 0.21 | 0.656 | 9.39 ± 1.48 | 8.48 ± 1.36 | 0.009 * |
| TC (mg/dL) | 210.52 ± 20.67 | 196.5 ± 21.99 | 0.003 * | 233.52 ± 25.98 | 219.84 ± 29.43 | 0.034 * |
| TG (mg/dL) | 184 (165.75–200) | 151 (136–156) | <0.001 * | 218.71 ± 15.45 | 204.96 ± 21.53 | 0.001 * |
| HDL-C (mg/dL) | 43.43 ± 4.34 | 46.24 ± 4.6 | 0.005 * | 38.63 ± 3.94 | 41 ± 4.31 | 0.015 * |
| LDL-C (mg/dL) | 130.53 ± 21.15 | 119.12 ± 23.51 | 0.019 * | 151.15 ± 26.66 | 137.85 ± 29.98 | 0.044 * |
| VLDL-C (mg/dL) | 36.8 (33.15–40) | 30.2 (27.2–31.2) | <0.001 * | 43.74 ± 3.09 | 40.99 ± 4.31 | 0.001 * |
| TyG Index | 4.97 (4.93–5.03) | 4.85 (4.81–4.87) | <0.001 * | 5.22 ± 0.07 | 5.16 ± 0.08 | <0.001 * |
| 25(OH)D (ng/mL) | 14.66 ± 3.19 | 46.32 ± 8.9 | <0.001 * | 13.49 ± 3.41 | 42.44 ± 8.14 | <0.001 * |
| Variables | Controls | Prediabetes | T2DM | |||
|---|---|---|---|---|---|---|
| ρ | p-Value | ρ | p-Value | ρ | p-Value | |
| Age (years) | −0.10 | 0.362 | −0.04 | 0.741 | −0.06 | 0.555 |
| BMI (Kg/m2) | −0.15 | 0.161 | −0.23 | 0.033 * | −0.22 | 0.038 * |
| WHR | −0.06 | 0.557 | −0.22 | 0.034 * | −0.25 | 0.017 * |
| SBP (mmHg) | −0.06 | 0.580 | −0.24 | 0.021 * | −0.23 | 0.027 * |
| DBP (mmHg) | −0.09 | 0.410 | 0.01 | 0.958 | −0.10 | 0.331 |
| FPG (mg/dL) | −0.05 | 0.669 | −0.26 | 0.012 * | −0.4 | <0.001 * |
| 2-h PG (mg/dL) | −0.13 | 0.213 | −0.25 | 0.019 * | −0.22 | 0.036 * |
| HBA1c (%) | 0.00 | 0.986 | −0.26 | 0.013 * | −0.27 | 0.010 * |
| TC (mg/dL) | −0.13 | 0.229 | −0.26 | 0.012 * | −0.21 | 0.050 |
| TG (mg/dL) | −0.15 | 0.164 | −0.35 | 0.001 * | −0.36 | <0.001 * |
| HDL-C (mg/dL) | 0.08 | 0.446 | 0.23 | 0.031 * | 0.26 | 0.015 * |
| LDL-C (mg/dL) | −0.12 | 0.256 | −0.24 | 0.025 * | −0.17 | 0.105 |
| VLDL-C (mg/dL) | −0.15 | 0.164 | −0.35 | 0.001 * | −0.36 | <0.001 * |
| TyG Index | −0.18 | 0.084 | −0.34 | 0.001 * | −0.42 | <0.001 * |
| B (95% CI) | SE | Beta | t | p | |
|---|---|---|---|---|---|
| Model 1 | −0.78 (−1.13, −0.43) | 0.18 | −0.43 | −4.42 | <0.001 * |
| Model 2 | −0.74 (−1.09, −0.38) | 0.18 | −0.40 | −4.12 | <0.001 * |
| Model 3 | −0.69 (−1.11, −0.27) | 0.21 | −0.38 | −3.27 | 0.002 * |
| B (95% CI) | SE | Beta | t | p | |
|---|---|---|---|---|---|
| Model 1 | −0.57 (−0.81, −0.34) | 0.12 | −0.46 | −4.79 | <0.001 * |
| Model 2 | −0.55 (−0.81, −0.29) | 0.13 | −0.43 | −4.18 | <0.001 * |
| Model 3 | −0.50 (−0.81, −0.19) | 0.16 | −0.40 | −3.18 | 0.002 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahat, R.K.; Rathor, P.K.; Rathore, V.; Arora, M.; Panda, S.; Marndi, G. The Relationship between Vitamin D and TyG Index in Prediabetes and Type 2 Diabetes Mellitus among an Indian Tribal Community: A Cross-Sectional Study. BioMed 2024, 4, 404-418. https://doi.org/10.3390/biomed4040032
Mahat RK, Rathor PK, Rathore V, Arora M, Panda S, Marndi G. The Relationship between Vitamin D and TyG Index in Prediabetes and Type 2 Diabetes Mellitus among an Indian Tribal Community: A Cross-Sectional Study. BioMed. 2024; 4(4):404-418. https://doi.org/10.3390/biomed4040032
Chicago/Turabian StyleMahat, Roshan Kumar, Prasanna Kumar Rathor, Vedika Rathore, Manisha Arora, Suchismita Panda, and Gujaram Marndi. 2024. "The Relationship between Vitamin D and TyG Index in Prediabetes and Type 2 Diabetes Mellitus among an Indian Tribal Community: A Cross-Sectional Study" BioMed 4, no. 4: 404-418. https://doi.org/10.3390/biomed4040032
APA StyleMahat, R. K., Rathor, P. K., Rathore, V., Arora, M., Panda, S., & Marndi, G. (2024). The Relationship between Vitamin D and TyG Index in Prediabetes and Type 2 Diabetes Mellitus among an Indian Tribal Community: A Cross-Sectional Study. BioMed, 4(4), 404-418. https://doi.org/10.3390/biomed4040032






