Abstract
Recently, in Italy, a flowchart to be used by General Practitioners for the at-home treatment of patients with COVID-19, has been released. It states that early at-home treatment for SARS-CoV-2 infection is possible due to the availability of specific antiviral drugs to be used in at-risk patients, and that non-steroidal anti-inflammatory drugs (NSAIDs) have an important function in combating the virus. Therefore, the use of NSAIDs is not only rational but also effective in cases that cannot be treated using antivirals. These seemingly simple concepts have been applied in Italy since the beginning of the pandemic by doctors that belong to Italian groups created in order to help COVID-19 patients early at home, at a time of organizational difficulties within Italian health institutions and government. However, this approach was largely boycotted by both the Italian Ministry of Health and medical institutions, which mainly suggested the use of paracetamol as symptomatic, and a wait-and-watch approach for the first three days from the onset of symptoms. In this article, we analyze the rationale for the use of NSAIDs and, in particular, the multi-targeted approach including indomethacin in synergism with flavonoids and low-dose aspirin, as early at-home treatment of patients with COVID-19. Applying these simple concepts from the beginning could have reduced the high lethality of the disease during the first two years of the pandemic and prevented hospital overload. In perspective, it is still necessary to systematically address the comparison between different therapeutic approaches to this viral disease on an experimental basis.
1. Introduction
Between 1991 and 1992 the epidemiologists at McMaster University in Hamilton (Ontario, Canada) published a series of studies in which the statute of evidence-based medicine (EBM) was defined for the first time [1]. EBM refers to the application of the best available research to clinical care, which requires the integration of evidence with clinical expertise and patient values [2]. EBM brought about an epochal turning point in the way medicine is practiced. This approach has simplified and made the way medicine is applied more precise and safer.
Unfortunately, however, between the end of 2019 and the beginning of 2020, we found ourselves facing a pandemic for the first time in the modern era, determined by a virus (SARS-CoV-2), little-known, which started in China and spread rapidly around the world. This virus immediately showed that it had rapid diffusibility and high lethality, particularly in older subjects and those with comorbidities [3].
At the time, we had no EBM-supported guidelines to help us deal with COVID-19, the disease generated by the SARS-CoV-2 infection. Therefore, it was necessary to return to doctoring like before the advent of EBM and the guidelines, because to obtain indications based on evidence of effectiveness took time, money, and organization. In the meantime, something had to be done; patients could not be left to their own devices without medical support [4]. Despite this, two fronts of doctors were formed who thought in completely opposite ways, namely, that of doctors closely linked to EBM, who claimed, therefore, not to use untested drugs for this disease, and that of doctors, mostly older, who asserted that it was not ethical not deal with the disease at least by using drugs already on the market, which had a rationale based on their known pharmacological mechanisms [5]. That is, drugs that could logically interfere with the multiplication of the virus and counteract the inflammation and thrombosis that is triggered by the infection, in the hope of preventing worsening of the disease, thus reducing hospitalizations and deaths.
A little-known viral disease had to be treated with all the weapons at our disposal. The weapons to combat viral diseases are first and foremost vaccines (provided they are effective and safe) and antiviral drugs, and, in the case of this virus, drugs that could attenuate, if not completely reset, the pathophysiological mechanisms that it uses to determine aggravation of the disease. Unfortunately, however, from the beginning of the pandemic, the governments in most countries almost exclusively espoused the route of vaccines, even boycotting, openly in some countries, the rational use of potentially effective drugs already on the market, albeit with other indications. Yet, it is now well known that mRNA vaccines against SARS-CoV-2 are difficult to develop, particularly due to the rapid variability of this type of virus, which ends up making vaccines partially ineffective, particularly in preventing virus transmission [6]. Among the various drugs already on the market, we had many drugs available, which, based on their mechanisms of action could be efficacious to counteract SARS-CoV-2 infection, preventing it from worsening. Among them, firstly anti-inflammatory non-steroidal drugs.
This is a descriptive review in which we analyze the rationale for the use of NSAIDs and, in particular, a multi-targeted approach including indomethacin in synergism with flavonoids and low-dose aspirin, as early at-home treatment of patients with mild-to-moderate COVID-19. The articles were selected in Internet Archive and in PubMed by using the words Non-steroidal anti-inflammatory drugs, Indomethacin, Flavonoids, Quercetin, Hesperidin, SARS-CoV-2, COVID-19, and COVID-19 guidelines in Italy.
2. Non-Steroidal Anti-Inflammatory Drugs in the Fight against COVID-19
It would seem obvious, in light of the fact that the virus can, in some cases, aggravate disease-causing uncontrolled inflammation, up to cytokine storm and thrombosis [7,8], to hypothesize the use of anti-inflammatory drugs aimed at reducing the aggressiveness of the disease, as has already been done for some other diseases such as the flu. However, unfortunately, this thesis was espoused by few, also because, at the very beginning of the pandemic, a warning was issued not to use ibuprofen during COVID-19 because this could lead to a worsening of the disease [9]. Furthermore, the first guidelines issued by the Italian Ministry of Health and the Italian Medicines Agency (AIFA), indicated only the use of paracetamol as symptomatic and to carefully observe the progress of the disease in the first 72 h from the onset of symptoms [10].
However, despite ministerial guidelines, some Italian doctors began using non-steroidal anti-inflammatory drugs in the hope of preventing the development of uncontrolled inflammation in the lungs and vessels. Many of these doctors met in groups, to be able to discuss with each other and to try to determine a common line of conduct. Of these, certainly, the largest group was that of Early Home Therapy for COVID-19 (www.terapiadomiciciliarecovid19.org (accessed on 19 April 2020)) founded by a Neapolitan lawyer, Erich Grimaldi, to help treat COVID-19 at home in those patients who could not find help from their general practitioners. The results obtained by this and other groups, as later documented by scientific publications, have been very good, showing a significant reduction in both hospitalizations and lethality, particularly when prompt action was taken at the first onset of symptoms, using non-steroidal anti-inflammatory drugs [11,12,13,14,15]. These results were also obtained in older patients and in subjects with numerous risk factors.
One study published in July 2021 by an important Italian group, reported that effective treatment algorithms implemented based on a pharmacological and pathophysiological rationale can greatly reduce hospitalizations of patients with COVID-19 and that this result has important implications both for patients and the health system [11]. In this case, the treatment consisted principally of anti-inflammatory agents, especially relatively selective cyclooxygenase-2 (COX-2) inhibitors, administered early at the very beginning of the onset of symptoms.
Our group also published the results of a retrospective observational study of outcomes and hospitalization rates of patients in Italy with a confirmed diagnosis of early COVID-19 [12]. The study was performed on 158 patients divided into two groups. Group 1 of 85 patients was treated as early as possible (<72 h from the onset of symptoms), while group 2 of 73 patients was treated >72 h after the onset of symptoms, because they consulted the doctor late. The NSAID used in this case was indomethacin at a dose of 75 or 100 mg a day, according to weight <70 or >100 kg, integrated with flavonoids, cardioaspirin, and omeprazole. The results of this study showed a significant reduction in symptom duration and hospitalizations in group 1, indicating the efficacy of the drugs used when they were administered promptly at the early onset of symptoms.
These results were also confirmed by a further retrospective multicenter larger study of 966 patients with COVID-19 treated with different NSAIDs and of a subgroup of 339 older patients with a mean age of 60 years and with multiple risk factors. Prompt intervention with NSAIDs produced better results compared to later intervention [15].
A further publication by Consolaro et al. evaluated the outcomes, by a matched-cohort study, in 108 consecutive patients with mild COVID-19 managed promptly at home, according to the proposed treatment algorithm and in another 108 patients treated with another therapeutic schedule [13]. This study showed a significant reduction in both symptom duration and hospitalizations in the group treated according to the recommended algorithm. Moreover, in this case, NSAIDs with an action relatively selective on COX-2 were preferred. Another recent paper by Cosentino et al. reports the results of an observational retrospective study performed on data provided by volunteer doctors who belong to the IppocrateOrg Association in Italy, on 392 COVID-19 patients [14]. In this case, the treatment, mostly with early NSAID application, produced a great reduction in hospitalizations with a very low number of deaths.
The results of a randomized double-blind placebo-controlled trial were reported recently, showing that mefenamic acid, a non-steroidal anti-inflammatory drug, markedly reduced the extent of symptoms and the time to reach an acceptable health status of the patients [16].
An attractive opinion article clearly asserted that the early administration of NSAIDs, among others, ibuprofen, in COVID-19 patients is not only safe but may also prevent the occurrence of complications that could worsen the course of the disease, and explained some of the suggested protective mechanisms of NSAIDs [17]. Recently, another important review article on early at-home treatment using NSAIDs in patients with mild-to-moderate SARS-CoV-2 infection was published, reporting that early disease symptoms variably reflect an underlying inflammatory response to the viral infection, and that, for this reason, the use of NSAIDs in the initial stage of the disease could be a valid therapeutic strategy [18]. In this publication, the authors recognize the validity of the approach pioneered by our group, which includes the use of indomethacin [11].
Most of these studies, although observational and retrospective, demonstrate that the early treatment at home with a NSAID improves the outcomes of the disease reducing the duration of symptoms and the number of patients evolving towards interstitial pneumonia, and both the number of hospitalizations and deaths [11,12,13,14,15]. In addition, one of the studies also reported that in the group of patients for whom the therapy was started within 72 h from the beginning of disease symptoms, a significantly lower number of patients had increased D-dimer levels, as compared with the number of patients for whom the treatment had been started later [12].
3. Potential of Indomethacin in the Treatment of COVID-19
Of the various non-steroidal anti-inflammatory drugs, indomethacin seems to have the best characteristics for counteracting the pathophysiological mechanisms used by the virus to worsen the disease.
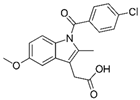
Indomethacin is a molecule that belongs to the class of NSAIDs. Indomethacin was put on the market in the mid-60s, and so has been used for about 60 years. It has a powerful analgesic, antipyretic, and anti-inflammatory action, greater than acetylsalicylic acid and most of the other NSAIDs that subsequently came into use, and is extremely low-cost (between EUR 2 and 3 per package). Over 45 years ago, it was absolutely the most used anti-inflammatory drug with multiple indications such as osteo-articular diseases and, in particular, acute pericarditis (post-viral and uremic) and myocarditis (post-viral) [19]. In these latter types of pathology, indomethacin has a very rapid action on chest pain and fever, with evident improvement of the disease already in the first days of therapy; even though, on average, the therapy cycle lasts at least a month. Since the dosage of indomethacin in these diseases is rather high (50 mg orally 3 or 4 times a day after meals), to avoid the most frequent side effect, i.e., gastritis with stomach pain, it is always associated with gastric protection. Recurrences of pericarditis, in progress or after discontinuation of indomethacin therapy, are very rare and, in this case, colchicine is associated with the drug. Indomethacin is also used intravenously to close the ductus arteriosus of Botallo in premature births and, being a prostaglandin antagonist, as a tocolytic, to slow down uterine contractions in premature births [20].
It has been reported with computational and experimental methods that indomethacin is a potent inhibitor of coronavirus replication in vitro against human SARS-CoV-1 and 2, and in vivo against canine coronavirus [21,22], without cytotoxic effects, and that, having both anti-inflammatory and antiviral activity, it could be repurposed as a treatment in COVID-19 therapy [23]. A retrospective study reported a beneficial effect of indomethacin in refractory headaches in COVID-19 and post-COVID-19 patients [24]. A recent manuscript reported the results of a randomized controlled study of indomethacin versus paracetamol, added to standard background therapy, in patients with mild-to-moderate COVID-19. The study’s main objective was the prevention of desaturation at a value <93% of O2 [25]. Importantly, the results showed that no patients in the indomethacin group desaturated, while as many as 20 patients desaturated in the paracetamol group.
Indomethacin can be administered orally, rectally, or intravenously, and by topical use, in particular eye drops. The side and unwanted effects of indomethacin are prevalent at the level of the gastrointestinal tract, although, more rarely, dizziness, vertigo, headache, drowsiness, etc., may occur. Side effects, as with most drugs, are more frequent at high doses so that, often, by reducing the dosage, they tend to reduce their intensity until they disappear. This is one of the reasons why we looked for substances that could have a synergistic mechanism with indomethacin in the therapy of COVID-19 and which, added to the anti-inflammatory therapy, would allow us to reduce the dosage to between 75 and 100 mg per day, which, in clinical experience, are dosages largely better tolerated. These substances have been identified in hesperidin and quercetin, two nutraceutical substances of the flavonoid class.
The anti-inflammatory actions of indomethacin are exerted in various ways, some of which are specific to the molecule and, as such, make it more indicated in COVID-19.
3.1. Cyclooxygenases Inhibition
The traditional action is that of inhibition of cyclooxygenases 1 and 2; therefore, a broad-spectrum inhibitory action on prostaglandin synthesis, common to other inhibitors such as diclofenac, ibuprofen, etc. Since the predominant pharmacological activity is the inhibition of COX-2 overexpression and overproduction of pro-inflammatory cytokines and chemokines, these drugs represent a robust treatment option for SARS-CoV-2 infection [26].
3.2. BCL2-Associated Agonist of Cell Death
Indomethacin blocks the BCL2-associated agonist of cell death (BAD) pro-apoptotic protein, which, in turn, is linked to the synthesis of cytokines IL-6, IL-8, and IL-23 [27] and to the activation of MAPK8 and MAPK10 (mitogen-activated protein kinases), which are key mediators of inflammation, vasoconstriction, and thrombosis. This means that the effect of indomethacin on human cells is much broader than the inhibition of cyclo-oxygenases and directly regulates delicate and complex biological mechanisms such as pro-inflammatory cytokines.
3.3. Renin–Angiotensin System
In close correlation with the previous point, one of the most interesting aspects emerges, which is the regulation of the renin–angiotensin system (RAS). Indeed, a “network pharmacology” study identified that three target proteins of the 6MNA anti-inflammatories, rofecoxib and indomethacin (MAPK8, MAPK10, and BAD), are mainly associated with the RAS signaling pathway [28]. Therefore, inactivation of these proteins may also be a viable strategy to alleviate the organ injury induced by COVID-19, which, as we have seen, depends heavily on dysregulation of that system. Precisely, the cited work [28] compared many NSAIDs in terms of their ability to interact with the key mechanisms of COVID-19: Celecoxib, Diclofenac, Diflunisal, Etodolac, Fenoprofen, Flubiprofen, Ibuprofen, Indomethacin, Ketoprofen, Ketorolac, Mefenamic acid, Meloxicam, Naproxen, Oxaprozin, Piroxicam, Rofecoxib, Sulindac, Tolmetin, Valdecoxib, and 6MNA (prodrug of Nabumetone). Of the three NSAIDs 6MNA, rofecoxib and indomethacin were the most potent candidates to fight COVID-19 due to their ability to regulate the RAS. It should be noted that rofecoxib was withdrawn due to adverse cardiovascular events.
3.4. Bradykinin
Finally, the RAS system, unbalanced in COVID-19 due to an excess of angiotensin II and a lack of ACE2, leads to an increase in bradykinin, an important mediator of acute inflammation (especially exudation and pain) [29] involved in vasodilation, plasma extravasation, bronchoconstriction, and nociception. Since ACE2 enzyme activity is involved in the degradation of bradykinin [30,31], its absence can cause serious consequences by being responsible for a “bradykinin storm” in the acute phases of the inflammatory process of COVID-19 [29,31,32] and, due to its characteristics, indomethacin is among the most suitable drugs to counteract the effects of this imbalance [33,34].
3.5. Antiviral Action of Indomethacin
As mentioned above, the particular interest in indomethacin, among the many NSAIDs available, is related also to the fact that this drug, in addition to its powerful anti-inflammatory and anti-platelets actions, has direct antiviral properties against several viruses, including cytomegalovirus, herpes virus 6, and hepatitis B virus [35,36,37]. According to Amici [38], in a model of vesicular stomatitis infection, indomethacin-activated PKR (double-stranded RNA-dependent protein kinase), resulting in the phosphorylation of elF2α and, in turn, disrupting translation of the viral protein and inhibiting viral replication. More recently, indomethacin has been shown to have antiviral properties in vitro on human SARS-CoV, canine coronavirus, and recently on SARS-CoV-2, without cytotoxic effects [22,38]. Direct evidence of the antiviral efficacy of indomethacin against SARS-CoV-2 has been provided in cellular models and in vivo in another canine model of infection [39]. Compared with a control anti-inflammatory drug (aspirin), indomethacin significantly reduced mortality in experimentally infected dogs, suggesting that the effect is independent of anti-inflammatory action. Molecular docking studies have suggested that indomethacin is able to down-regulate genes involved in virus entry (ACE2 and TMPRSS2), as well as other genes involved in the same pathways [40], and is a potential antagonist of the main SARS-CoV-2 protease [41] and of non-structural protein 10 [42]. Models based on systems biology suggest that the action of indomethacin against SARS-CoV-2 depends on its preferred interaction with viral proteins [43]. Moreover, indomethacin inhibits the function of membrane-associated prostaglandin E synthase (PTGES2), which catalyzes the conversion of prostaglandin H2 to prostaglandin E2 [44]. Since PTGES2 interacts with the viral protein NSP7, the complex function of NSP7/NSP8 is slowed down or blocked, and this could constitute a mechanism of inhibition of viral growth.
A group of Indian researchers performed in silico screening of existing drugs against the crucial proteins of SARS-CoV-2, and a few existing drugs, among which was indomethacin, were shortlisted. In addition, they analyzed the gene expression data of SARS-CoV-2 in human lung epithelial cells and investigated which molecules can reverse the cellular mRNA expression profiles in the diseased state. Indomethacin was found effective to treat SARS-CoV-2 infection. The in silico findings on indomethacin were also successfully validated by in vitro testing in Vero CCL-81 cells with ah IC₅₀ of 12 µM [45]. Relevant scientific literature demonstrates that indomethacin has shown both in vitro and in vivo to decrease viral replication in SARS-CoV and in SARS-CoV-2.
4. The Logic of a Target Synergistic Therapy
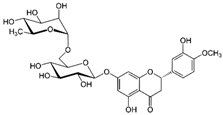
NSAIDs may be combined with other substances to mitigate side effects and increase efficacy by exploiting the synergistic mechanisms of the associated substances. Our cited observational study reported results obtained using indomethacin for the treatment of COVID-19, combined with two flavonoids (hesperidin and quercetin), an anti-platelet dose of aspirin, vitamin C, and omeprazole [12] (Table 1, Figure 1).

Table 1.
Basic components of the synergistic multi-therapy used by Fazio et al. [12] and described in this review.
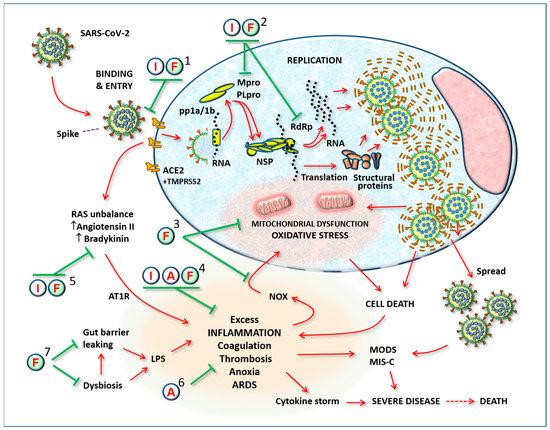
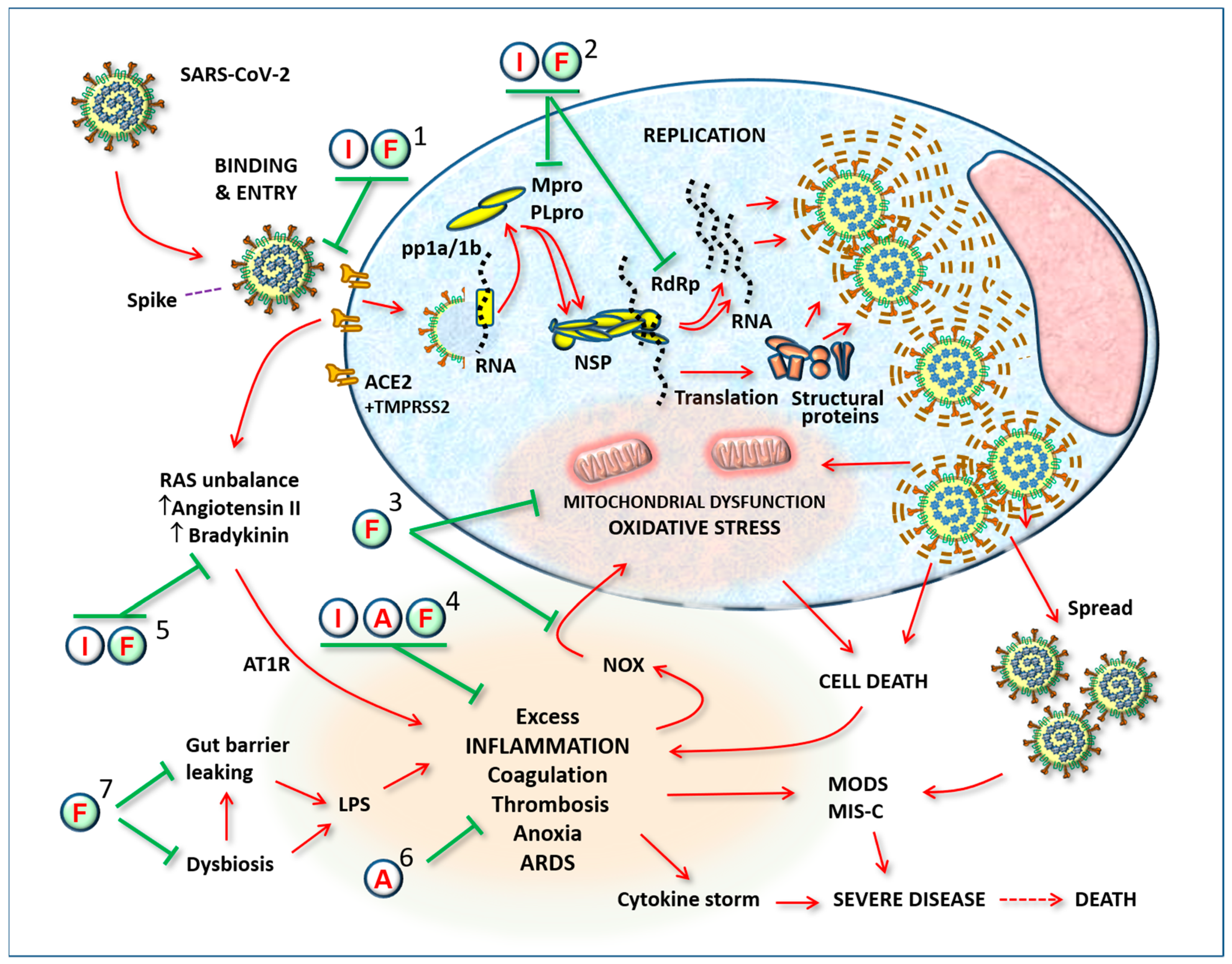
Figure 1.
General outline of viral infection and the complications caused by its spread in the body. The substances proposed here for a possible therapeutic effect in coronavirus infection may act in the various stages: (1) preventing binding of the virus to receptors or inhibiting the function of the receptor itself, when it initiates the process of internalization; (2) inhibiting replication by blocking proteases, Non-structural proteins, RNA polymerases, or the assembly of new virus particles; (3) by helping the cell resist viral attack, i.e., by stopping the process of cytotoxicity mediated by oxygen free radicals; (4) by modulating excess inflammatory reactions; (5) by modulating RAS imbalance; (6) by preventing thrombosis; (7) by modulating gut microbiota and preventing systemic release of pro-inflammatory LPS. Abbreviations: SARS-CoV-2: severe acute respiratory syndrome-Coronavirus-2; I: Indomethacin; F: Flavonoids; A: Low-dose aspirin; ACE2: angiotensin-converting enzyme-2; pp1a/1b: polypeptides 1a/1b; Mpro: Major protease (3CL-pro); PLpro: papain-like protease; TMPRSS2: transmembrane serine protease 2; NSP: non-structural proteins; RdRp: RNA-dependent RNA polymerase; LPS: Lipopolysaccharide; NOX: NADPH oxidase; pp1a/1b: polypeptides 1a/1b; Mpro: Major protease (3CL-pro); PLpro: papain-like protease; TMPRSS2: transmembrane serine protease 2; NSP: non-structural proteins; RdRp: RNA-dependent RNA polymerase; LPS: Lipopolysaccharide; NOX: NADPH oxidase; RAS: Renin–Angiotensin System; AT1R: Angiotensin II receptor-1; ARDS: acute respiratory distress syndrome; MODS: multiple organ dysfunction syndrome; MIS-C: multisystemic inflammatory syndrome of children.
This combination also made a slight reduction in dosages possible, particularly of indomethacin. The two flavonoids were chosen for their potent antioxidant action, but also for their anti-inflammatory and antiviral actions, which were strongly synergic with those of indomethacin [46,47]. An anti-platelet dose of aspirin was associated in order to boost the effects already present in indomethacin, aiming to completely block the platelet hyper-aggregation that the virus can cause once it has entered the bloodstream [47,48,49,50,51]. This treatment regimen, when started within 72 h of the onset of symptoms, showed relevant positive effects, practically eliminating hospitalizations, significantly reducing the duration of symptoms, and nullifying the onset of side effects [11,47].
Figure 1 illustrates the targets of the substances used, focusing on a typical SARS-CoV-2-infected cell and the systemic consequences of its spread. The numbers indicate where the substances used can act synergistically to prevent disease progression.
It should be considered that this anti-inflammatory activity of indomethacin can indirectly have antiviral effects. Indeed, in the case of another common virus (cytomegalovirus), PGE2 stimulates the activity of the main immediate-early promoter, which controls the synthesis of viral regulatory proteins that are essential for its replication [36,52]. Other potential advantages of indomethacin are its lysosomotropic features, enabling the drug to counteract the endosomal pathway of virus entry [53].
4.1. Antioxidant and Antiviral Potentials of Flavonoids
In theory, the ideal therapy should include drugs that block the virus on the mucous membranes (the surface linings of cavities such as the respiratory and digestive systems), preventing it from entering the bloodstream and spreading to vital organs, for sufficient time to allow the affected person to form antibodies, particularly mucosal secretory IgA, capable of controlling and rendering the virus harmless. This function can also be exploited by natural antiviral substances present in food or provided as supplements [54,55,56,57,58,59,60,61,62]. Promising results have been reported for clinical trials carried out to test the efficacy of quercetin as a complementary treatment in COVID-19 patients [63,64,65,66].
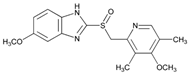
During SARS-CoV-2 virus infection, it is possible that large quantities of reactive oxygen species are generated, causing damage to nearby cells and tissues [67,68,69]. In serious cases, endothelial injury, coagulopathy, and pulmonary thrombosis cause hypoxia, mitochondrial chain anomalies, mitochondrial dysfunction, oxidative stress, and DNA damage [70,71]. Given the importance of oxidative stress in COVID-19, a therapeutic approach including antioxidants has been proposed by authoritative researchers for this disease too, and various publications have been reviewed [61,72]. It has been proposed that early treatment with flavonoids could be a suitable way to restore the redox balance and prevent cell damage and the consequent inflammatory storm due to lung damage and respiratory dysfunction [73,74,75,76,77,78]. Polyphenols such as hesperidin and quercetin exert a defensive action against oxidative stress, both as “scavenger” molecules, which eliminate free radicals by deactivating them, and as inducers of endogenous antioxidant enzymes.
The Chinese group of Wu et al. [79] was the first to identify hesperidin as a potential anti-SARS-CoV-2 remedy, using molecular docking techniques. So-called “molecular docking” can be used to virtually make two molecules react and predict how a protein (receptor or target) interacts with bioactive compounds (“ligands”). Large databases of manmade compounds and an entire database of natural products were screened. The most extraordinary result is that, of all the compounds examined, hesperidin was found to be the most suitable for binding to the SARS-CoV-2 Spike, wedging itself in the middle surface groove of the RBD, where some amino acids form a “hydrophobic pocket” suitable for containing the substance. Various authors then confirmed the affinity of hesperidin with the RBD fragment of the Spike protein and its ability to hinder the link with ACE2 or to make the interaction unstable [80,81,82,83,84,85]. Today, literature on the subject has been greatly enriched, also thanks to the writers’ contributions [35,74,86], other Italian authors [72,87,88,89], and international researchers [90,91,92,93,94].
Energy landscape studies revealed that fisetin, quercetin, and campherol also bind with the ACE2-Spike complex with low-binding free energy [95]. Quercetin formed hydrogen bonds with eight amino acid residues and hydrophobic interaction with five others. Another group reported studies showing that quercetin has a high affinity with the viral Spike, blocking sites of interaction with cell receptors [96]. Based on its ability to interact with non-structural proteins 8 and 10, quercetin is considered to be a candidate antiviral drug even for mutated SARS-CoV-2 strains [49].
Surprisingly, the affinity of hesperidin with coronavirus proteins is not limited to the Spike but also to the Mpro and PLpro enzymes (Figure 1, point 2). After the screening of thousands of potential Mpro binding molecules, using molecular docking techniques, various authors discovered a strong affinity between hesperidin and these proteases [79,81,97,98]. This flavanone is therefore fully and rightly included among the candidates for exercising clear direct antiviral action. This specific bond of hesperidin has also been confirmed by other authors [81,84]. Quercetin has also been shown to inhibit the Mpro of various coronaviruses, in particular of SARS-CoV [98], MERS-CoV [99], and SARS-CoV-2 [100,101,102].
Quercetin has also been investigated for its properties that inhibit various stages of the inflammatory process [103]. Quercetin also inhibits the NLR family pyrin domain containing 3 (NLRP3) inflammasome, affecting TXNIP (thioredoxin interacting protein) [104] and the secretory response of activated mast cells in human and animal models [105,106,107,108,109]. The biological action of this flavonol has also been explored in the laboratory directed by Bellavite, evaluating the release of histamine from human basophils [110,111]. These inhibitory properties of quercetin on histamine release are also interesting for COVID-19, given that lung mast cells are involved in the phenomenon of worsening of the pulmonary picture in the event of a “cytokine storm” [112,113] and histamine released from mast cells can amplify the inflammatory process in SARS-CoV-2-infected lungs [114].
Finally, flavonoids also have significant benefits on the intestinal barrier function by contributing to the regulation of the microbiota [115]. Available data indicate that the oral cavity and intestine may be an active sites of SARS-CoV-2 infection [116,117,118,119], and that the modulation of the microbiota can have beneficial effects in the context of the disease [120,121].
4.2. Vitamin C
According to various authors, in a normal diet Vitamin C contributes from 15% to 30% of the total antioxidant power of blood plasma [122]. Vitamin C is believed to prevent LDL oxidation, protect human vascular smooth muscle cells from apoptosis [123], and strengthen immune function [124]. Studies on animals infected with the influenza virus have shown that vitamin C stimulates antiviral immune responses and reduces inflammation in the lungs [125,126]. Taking these results, the low cost, and the high safety of natural foods rich in vitamin C into account, it has been suggested that it could be useful to increase the daily intake of these foods during the spread of COVID-19 [88,127,128]. However, one must also consider that high doses of ascorbate can also be harmful [129]. The antioxidant activity of vitamin C works in synergy with quercetin, thanks to its ability to recycle the flavonol molecule, protecting it from oxidation and recycling its oxidized quinone form after the scavenger action on free radicals [130].
4.3. Low-Dose Aspirin
The synergistic therapeutic scheme includes aspirin at an antiplatelet dosage, given that thrombosis is one of the most feared complications, especially in vascular and heart disease patients.
Low-dose aspirin has been widely used due to its clearly demonstrated antithrombotic action. Its use in SARS-CoV-2 infection could be useful for reducing or dampening platelet hyper-aggregation, which can be caused by the binding of the Spike of the virus with platelet ACE2 receptors, and used for the purpose of preventing micro- or macro-thrombosis during the initial phase of the disease [131,132]. A robust treatment option with aspirin—in addition to Indomethacin, Diclofenac, and Celecoxib—in deactivating the inflammasome, and to modulate the overproduction of pro-inflammatory cytokines, has also been proposed by others [26].
A systematic literature search estimated the effect of aspirin on COVID-19 mortality. Results showed that low-dose aspirin use was associated with reduced COVID-19 mortality, with no bleeding risk [133]. A further study showed that the combination of prophylactic anticoagulant therapy and aspirin resulted in a significantly lower risk of mortality in these patients, compared to patients treated with anticoagulant alone [134].
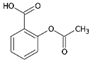
4.4. A Gastric Protector with Possible Antiviral Action: Omeprazole
Together with indomethacin, taking a gastroprotective to protect the gastrointestinal mucosa from the possible adverse effects of anti-inflammatories, including stress ulcers and gastrointestinal bleeding, is recommended [135,136]. Of these, omeprazole was preferred because, in addition to its known ability to protect against gastro-injurious events from NSAIDs at the gastro-duodenal level, it was proposed as a molecule capable of inhibiting the Mpro of SARS-CoV-2, binding to its C-terminal domain [137].
Another interesting study reported that omeprazole suppressed ACE2 expression in a dose-dependent manner, along with the induction of CYP1A1, a gene of the cytochrome P450 super-family of enzymes, involved in the metabolism of xenobiotics and drugs [138]. Finally, a thorough bioinformatics analysis of potential new drug targets useful in COVID-19 identified 21 drug candidates, including omeprazole and aspirin, which interact with CXCL8 (a cytokine with chemotactic activity), IL1beta (pro-inflammatory), and CSF2 (leukocyte growth factor, causes leukocytosis) [139].
To all of this, we must add that some experimental works suggest that quercetin also has protective effects on the gastric [140] and intestinal mucosa during treatment with indomethacin [141,142]. Therefore, omeprazole and quercetin could also synergize in mucosal protection.
5. Discussion
Although the rationale for the use of non-steroidal anti-inflammatory drugs in COVID-19 was clear from the beginning of the pandemic, in Italy and most of the rest of the Western world, no indications were given for their immediate use to halt or, at least, limit the damage caused by the disease. On the contrary, the first guidelines, issued by the Ministry of Health and AIFA [10,143], suggested the use of paracetamol, which has no pathophysiological rationale for use in COVID-19 other than to reduce the extent of certain symptoms such as fever and pain, and to wait the first 72 h in watchful waiting in the hope that the disease would self-limit. This did not take into account the fact that an infectious disease such as this, which can produce severe inflammation in certain vital organs and thrombosis in the days that follow, must be addressed with drugs that are not only symptomatic but also have an action mechanism that makes them capable of interfering with the pathophysiological mechanisms that the virus uses to produce aggravation of the disease and lead to hospitalization and death. A simple, rapid intervention with NSAIDs would probably have led to a major reduction in the number of hospitalizations and deaths. Unfortunately, however, this has not been demonstrated with appropriate, randomized, and controlled studies as EBM would like today. In fact, due to considerable organizational difficulties, mainly retrospective observational studies were carried out, which showed that prompt home intervention with NSAIDs in the treatment of patients with COVID-19 resulted in a more rapid resolution of symptoms, and a significant reduction in the number of hospitalizations and deaths [11,12,13,14,15].
But there was certainly little interest on the part of the major pharmaceutical companies in sponsoring randomized controlled trials with these old drugs, whereas there was considerable interest in some considerably expensive antiviral drugs. Governments, including the Italian one, also showed little interest in investing in studies on these old drugs. Perhaps, they feared that if it turned out that some old anti-inflammatory drugs were effective in treating the disease, the population would not be sufficiently stimulated to take advantage of vaccination to protect themselves. This would have undermined the whole political approach, especially by Western countries that had invested many billions in vaccination as the major and, perhaps, only pharmacological means to eradicate the pandemic.
Yet, published retrospective observational studies on the efficacy of NSAIDs in the treatment of COVID-19 have all pointed in the direction of good efficacy. On the other hand, there are significant limitations affecting the use of RCTs for public health purposes such as in the case of pandemic phase infectious diseases, for which public health decisions must be made quickly based on limited and often imperfect available data. In these cases, results from observational studies remain the most important source of data, but other examples include the analysis of aggregated clinical and epidemiological data [144]. Thus, observational studies published on the subject could certainly have been given more prominence. Probably, vaccination and early at-home treatment with NSAIDs, which were brought together in the fighting of the pandemic, could have produced better results.
Only recently, more than 3 years after the start of the pandemic, did the Italian Federation of General Practitioners (IFMMG), which until now had only pushed in the direction of vaccines, in collaboration with the Infectious Disease Unit of Tor Vergata Polyclinic in Rome, draw up a flow-chart, for General Practitioners, for at home early treatment of patients with COVID-19 [145]. This document states that early at-home treatment of SARS-CoV-2 infection is now possible due to the availability of specific antiviral drugs to be used in at-risk patients, and that non-steroidal anti-inflammatory drugs (NSAIDs) have a very important function in combating the virus right from the early stage of infection. Therefore, the use of NSAIDs is not only rational but also effective in cases, still in the majority, intractable with antivirals. This document also suggests creating a better home control system for patients with COVID-19, sending to the hospital only patients at risk and those who worsen, in order to try to avoid overcrowding of hospitals. These seemingly simple concepts have been applied in Italy since the beginning of the pandemic by doctors who belong to the “Early at home therapy group for COVID-19” (www.terapiadomiciliarecovid19.org (accessed on 19 April 2020)). This group was founded by the lawyer Erich Grimaldi, to provide early help to COVID-19 patients at home at a time marked by organizational difficulties in Italian health institutions and the government. However, this group was largely boycotted by both the Italian Ministry of Health and medical institutions, which mainly suggested the use of paracetamol as symptomatic, and a wait-and-watch approach in the first three days after the beginning of symptoms. Greater cooperation from government and greater dialogue between the parties would certainly have produced better results. In fact, applying these concepts from the beginning could have reduced the very high lethality of the disease during the first two years of the pandemic and prevented hospital overload.
6. Conclusions
While the use of corticosteroids could be contraindicated at the beginning of COVID-19 due to their immune-suppressive mechanism, based on their well-known mechanisms and the supporting literature, NSAIDs can be beneficially used to prevent the worsening of the disease. Furthermore, we also believe that the best therapeutic approach from the beginning of the pandemic should have been based on early at home treatment with NSAIDs, in association with other compounds that act synergistically to counteract the viral load and oxidative stress. Of the various NSAIDs, the choice should fall to indomethacin due to the characteristics we have described. Thus, we hope that a robust randomized-controlled study will be started as soon as possible to verify the efficacy of NSAIDs, with or without supplements, based on a non-inferiority design compared to the authorized antivirals for COVID-19.
Author Contributions
P.B. and S.F. contributed equally to conceptualization, investigation, and writing—review and editing the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors have the bibliography of this review available.
Conflicts of Interest
P.B. has a consultation with Vanda Omeopatici s.r.l. (Roma, Frascati), a company that produces food supplements but had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. S.F. has no potential conflicts of interest to declare.
References
- Evidence-Based Medicine Working Group. Evidence-Based medicine. A new approach to teaching the practice of medicine. JAMA 1992, 268, 2420–2425. [Google Scholar] [CrossRef] [PubMed]
- Straus, S.E.; Glasziou, P.; Richardson, W.S.; Haynes, R.B. Evidence-Based Medicine: How to Practice and Teach It, 4th ed.; Churchill Livingstone: London, UK; Elsevier: Edimburg, UK, 2011. [Google Scholar]
- Adil, T.; Rahman, R.; Whitelaw, D.; Jain, V.; Al-Taan, O.; Rashid, F.; Munasinghe, A.; Jambulingam, P. SARS-CoV-2 and the pandemic of COVID-19. Postgrad. Med. J. 2021, 97, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Scotto Di Vetta, M.; Morrone, M.; Fazio, S. COVID-19: Off-label therapies based on mechanism of action while waiting for evidence-based medicine recommendations. World J. Meta Anal. 2020, 8, 173–177. [Google Scholar] [CrossRef]
- Fazio, S.; Cosentino, M.; Marino, F.; Pandolfi, S.; Zanolin, E.; Bellavite, P. The Problem of Home Therapy during COVID-19 Pandemic in Italy: Government Guidelines versus Freedom of Cure? J. Pharm. Pharmacol. Res. 2022, 6, 100–114. [Google Scholar] [CrossRef]
- Franco-Paredes, C. Transmissibility of SARS-CoV-2 among fully vaccinated individuals. Lancet Infect. Dis. 2022, 22, 16. [Google Scholar] [CrossRef] [PubMed]
- Bonaventura, A.; Vecchié, A.; Dagna, L.; Martinod, K.; Dixon, D.L.; Van Tassell, B.W.; Dentali, F.; Montecucco, F.; Massberg, S.; Levi, M.; et al. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat. Rev. Immunol. 2021, 21, 319–329. [Google Scholar] [CrossRef]
- Chen, R.; Lan, Z.; Ye, J.; Pang, L.; Liu, Y.; Wu, W.; Qin, X.; Guo, Y.; Zhang, P. Cytokine Storm: The Primary Determinant for the Pathophysiological Evolution of COVID-19 Deterioration. Front. Immunol. 2021, 12, 589095. [Google Scholar] [CrossRef]
- Day, M. COVID-19: Ibuprofen should not be used for managing symptoms, say doctors and scientists. BMJ 2020, 368, m1086. [Google Scholar] [CrossRef]
- Rezza, G.; Urbani, A. Circolare Recante “Gestione Domiciliare dei Pazienti con Infezione da SARS-CoV-2”; Ditrezioni Generali Programmazione Sanitaria e Prevenzione Sanitaria; Ministero della Salute: Roma, Italy, 2020. [Google Scholar]
- Suter, F.; Consolaro, E.; Pedroni, S.; Moroni, C.; Pastò, E.; Paganini, M.V.; Pravettoni, G.; Cantarelli, U.; Rubis, N.; Perico, N.; et al. A simple, home-therapy algorithm to prevent hospitalisation for COVID-19 patients: A retrospective observational matched-cohort study. EClinicalMedicine 2021, 37, 100941. [Google Scholar] [CrossRef]
- Fazio, S.; Bellavite, P.; Zanolin, E.; McCullough, P.A.; Pandolfi, S.; Affuso, F. Retrospective Study of Outcomes and Hospitalization Rates of Patients in Italy with a Confirmed Diagnosis of Early COVID-19 and Treated at Home within 3 Days or after 3 Days of Symptom Onset with Prescribed and Non-Prescribed Treatments between November 2020 and August 2021. Med. Sci. Monit. 2021, 27, e935379. [Google Scholar] [CrossRef]
- Consolaro, E.; Suter, F.; Rubis, N.; Pedroni, S.; Moroni, C.; Pasto, E.; Paganini, M.V.; Pravettoni, G.; Cantarelli, U.; Perico, N.; et al. A Home-Treatment Algorithm Based on Anti-inflammatory Drugs to Prevent Hospitalization of Patients with Early COVID-19: A Matched-Cohort Study (COVER 2). Front. Med. 2022, 9, 785785. [Google Scholar] [CrossRef]
- Cosentino, M.; Vernocchi, V.; Martini, S.; Marino, F.; Allasino, B.; Bàlzola, M.A.; Burigana, F.; Dallari, A.; Pagano, C.S.F.; Palma, A.; et al. Early Outpatient Treatment of COVID-19: A Retrospective Analysis of 392 Cases in Italy. J. Clin. Med. 2022, 11, 6138. [Google Scholar] [CrossRef] [PubMed]
- Fazio, S.; Grimaldi, S.; D’Emilio, M.; Mangiagalli, A.; Affuso, F. COVID-19 early treatment with non-steroidal anti-inflammatory drugs reduces hospitalizations and symptom duration. Am. J. Biomed. Sci. Res. 2022, 16, 99–101. [Google Scholar] [CrossRef]
- Guzman-Esquivel, J.; Galvan-Salazar, H.R.; Guzman-Solorzano, H.P.; Cuevas-Velazquez, A.C.; Guzman-Solorzano, J.A.; Mokay-Ramirez, K.A.; Paz-Michel, B.A.; Murillo-Zamora, E.; Delgado-Enciso, J.; Melnikov, V.; et al. Efficacy of the use of mefenamic acid combined with standard medical care vs. standard medical care alone for the treatment of COVID-19: A randomized double-blind placebo-controlled trial. Int. J. Mol. Med. 2022, 49, 29. [Google Scholar] [CrossRef]
- Kelleni, M.T. Early use of non-steroidal anti-inflammatory drugs in COVID-19 might reverse pathogenesis, prevent complications and improve clinical outcomes. Biomed. Pharmacother. 2021, 133, 110982. [Google Scholar] [CrossRef]
- Perico, N.; Cortinovis, M.; Suter, F.; Remuzzi, G. Home as the new frontier for the treatment of COVID-19: The case for anti-inflammatory agents. Lancet Infect. Dis. 2023, 23, e22–e33. [Google Scholar] [CrossRef] [PubMed]
- Maisch, B.; Seferović, P.M.; Ristić, A.D.; Erbel, R.; Rienmüller, R.; Adler, Y.; Tomkowski, W.Z.; Thiene, G.; Yacoub, M.H.; Priori, S.G.; et al. Guidelines on the diagnosis and management of pericardial diseases executive summary; The Task force on the diagnosis and management of pericardial diseases of the European society of cardiology. Eur. Heart J. 2004, 25, 587–610. [Google Scholar] [CrossRef]
- Baerts, W.; van Bel, F.; Thewissen, L.; Derks, J.B.; Lemmers, P.M. Tocolytic indomethacin: Effects on neonatal haemodynamics and cerebral autoregulation in the preterm newborn. Arch. Dis. Child. Fetal Neonatal Ed. 2013, 98, F419–F423. [Google Scholar] [CrossRef]
- Aminpour, M.; Delgado, W.E.M.; Wacker, S.; Noskov, S.; Houghton, M.; Tyrrell, D.L.J.; Tuszynski, J.A. Computational determination of toxicity risks associated with a selection of approved drugs having demonstrated activity against COVID-19. BMC Pharmacol. Toxicol. 2021, 22, 61. [Google Scholar] [CrossRef]
- Desantis, J.; Mercorelli, B.; Celegato, M.; Croci, F.; Bazzacco, A.; Baroni, M.; Siragusa, L.; Cruciani, G.; Loregian, A.; Goracci, L. Indomethacin-based PROTACs as pan-coronavirus antiviral agents. Eur. J. Med. Chem. 2021, 226, 113814. [Google Scholar] [CrossRef]
- Gomeni, R.; Xu, T.; Gao, X.; Bressolle-Gomeni, F. Model based approach for estimating the dosage regimen of indomethacin a potential antiviral treatment of patients infected with SARS-CoV-2. J. Pharmacokinet. Pharmacodyn. 2020, 47, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Krymchantowski, A.V.; Silva-Néto, R.P.; Jevoux, C.; Krymchantowski, A.G. Indomethacin for refractory COVID or post-COVID headache: A retrospective study. Acta Neurol. Belg. 2021, 122, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, R.; Mohan, S.K.; Sukumaran, S.K.; Kamaraj, D.; Daivasuga, S.S.; Ravi, S.O.A.S.; Vijayaraghavalu, S.; Kumar, R.K. An open label randomized clinical trial of Indomethacin for mild and moderate hospitalised COVID-19 patients. Sci. Rep. 2022, 12, 6413. [Google Scholar] [CrossRef] [PubMed]
- Prasher, P.; Sharma, M.; Gunupuru, R. Targeting cyclooxygenase enzyme for the adjuvant COVID-19 therapy. Drug Dev. Res. 2021, 82, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.K.; Adnan, M.; Cho, D.H. Network pharmacology approach to decipher signaling pathways associated with target proteins of NSAIDs against COVID-19. Sci. Rep. 2021, 11, 9606. [Google Scholar] [CrossRef]
- Bellavite, P. Renin-Angiotensin System, SARS-CoV-2 and Hypotheses about Adverse Effects Following Vaccination. EC Pharmacol. Toxicol. 2021, 9, 1–10. [Google Scholar]
- Garvin, M.R.; Alvarez, C.; Miller, J.I.; Prates, E.T.; Walker, A.M.; Amos, B.K.; Mast, A.E.; Justice, A.; Aronow, B.; Jacobson, D. A mechanistic model and therapeutic interventions for COVID-19 involving a RAS-mediated bradykinin storm. eLife 2020, 9, e59177. [Google Scholar]
- Haybar, H.; Maniati, M.; Saki, N.; Zayeri, Z.D. COVID-19: Imbalance of multiple systems during infection and importance of therapeutic choice and dosing of cardiac and anti-coagulant therapies. Mol. Biol. Rep. 2021, 48, 2917–2928. [Google Scholar] [CrossRef]
- Karamyan, V.T. Between two storms, vasoactive peptides or bradykinin underlie severity of COVID-19? Physiol. Rep. 2021, 9, e14796. [Google Scholar] [CrossRef]
- McCarthy, C.G.; Wilczynski, S.; Wenceslau, C.F.; Webb, R.C. A new storm on the horizon in COVID-19: Bradykinin-induced vascular complications. Vasc. Pharmacol. 2020, 137, 106826. [Google Scholar] [CrossRef]
- Rodriguez-Portales, J.A.; Lopez-Moreno, J.M.; Mahana, D. Inhibition of the kallikrein-kinin system and vascular reactivity in Bartter’s syndrome. Hypertension 1985, 7, 1017–1022. [Google Scholar] [CrossRef] [PubMed]
- Alkotaji, M.; Al-Zidan, R.N. Indomethacin: Can It Counteract Bradykinin Effects in COVID-19 Patients? Curr. Pharmacol. Rep. 2021, 7, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.E.; Enquist, L.W. Biological interactions between herpesviruses and cyclooxygenase enzymes. Rev. Med. Virol. 2006, 16, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Schroer, J.; Shenk, T. Inhibition of cyclooxygenase activity blocks cell-to-cell spread of human cytomegalovirus. Proc. Natl. Acad. Sci. USA 2008, 105, 19468–19473. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, H.; Daryani, N.E.; Haghpanah, B.; Moayyeri, A.; Moghadam, K.F.; Mirmomen, S.; Kamangar, F. Effects of indomethacin on viral replication markers in asymptomatic carriers of hepatitis B: A randomized, placebo-controlled trial. Am. J. Gastroenterol. 2005, 100, 856–861. [Google Scholar] [CrossRef] [PubMed]
- Amici, C.; Di, C.A.; Ciucci, A.; Chiappa, L.; Castilletti, C.; Martella, V.; Decaro, N.; Buonavoglia, C.; Capobianchi, M.R.; Santoro, M.G. Indomethacin has a potent antiviral activity against SARS coronavirus. Antivir. Ther. 2006, 11, 1021–1030. [Google Scholar] [CrossRef]
- Kiani, P.; Scholey, A.; Dahl, T.; McMann, L.; Iversen, J.; Verster, J. In Vitro Assessment of the Antiviral Activity of Ketotifen, Indomethacin and Naproxen, Alone and in Combination, against SARS-CoV-2. Viruses 2021, 13, 558. [Google Scholar] [CrossRef]
- Xu, T.; Gao, X.; Wu, Z.; Selinger, D.W.; Zhou, Z. Indomethacin has a potent antiviral activity against SARS-CoV-2 in vitro and canine coronavirus in vivo. BioRxiv 2020. [Google Scholar] [CrossRef]
- Napolitano, F.; Gennaro, G.; Carrella, D.; Gao, X.; di Bernardo, D. Computational Drug Repositioning and Elucidation of Mechanism of Action of Compounds against SARSCoV-2. arXiv 2020, arXiv:2004.07697. [Google Scholar]
- Abo Elmaaty, A.; Hamed, M.; Ismail, M.; Elkaeed, E.B.; Abulkhair, H.S.; Khattab, M.; Al-Karmalawy, A. Computational Insights on the Potential of Some NSAIDs for Treating COVID-19: Priority Set and Lead Optimization. Molecules 2021, 26, 3772. [Google Scholar] [CrossRef]
- Mortezaei, Z.; Mohammadian, A.; Tavallaei, M. Variations of SARS-CoV-2 in the Iranian population and candidate putative drug-like compounds to inhibit the mutated proteins. Heliyon 2022, 8, e09910. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Song, X.; Ma, T.; Pan, X.; Zhou, Y.; Hou, Y.; Zhang, Z.; Li, K.; Karypis, G.; Cheng, F. Repurpose Open Data to Discover Therapeutics for COVID-19 Using Deep Learning. J. Proteome Res. 2020, 19, 4624–4636. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, R.; Bhattacharje, G.; Barak, J.; Manna, B.; Mullick, J.; Mathapati, B.S.; Abraham, P.; Madhumathi, J.; Hasiga, Y.; Ghosh, A.; et al. In-silico screening and in-vitro assay show the antiviral effect of indomethacin against SARS-CoV-2. Comput. Biol. Med. 2022, 147, 105788. [Google Scholar] [CrossRef]
- Shekhar, N.; Kaur, H.; Sarma, P.; Prakash, A.; Medhi, B. Indomethacin: An exploratory study of antiviral mechanism and host-pathogen interaction in COVID-19. Expert Rev. Anti Infect. Ther. 2022, 20, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Fazio, S.; Affuso, F.; Bellavite, P. A Review of the Potential Roles of Antioxidant and Anti-Inflammatory Pharmacological Approaches for the Management of Mild-to-Moderate Symptomatic COVID-19. Med. Sci. Monit. 2022, 28, e936292. [Google Scholar] [CrossRef]
- Divani, A.A.; Andalib, S.; Di, N.M.; Lattanzi, S.; Hussain, M.S.; Biller, J.; McCullough, L.D.; Azarpazhooh, M.R.; Seletska, A.; Mayer, S.A.; et al. Coronavirus Disease 2019 and Stroke: Clinical Manifestations and Pathophysiological Insights. J. Stroke Cerebrovasc. Dis. 2020, 29, 104941. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Y.; Wang, X.; Yang, L.; Li, H.; Wang, Y.; Liu, M.; Zhao, X.; Xie, Y.; Yang, Y.; et al. SARS-CoV-2 binds platelet ACE2 to enhance thrombosis in COVID-19. J. Hematol. Oncol. 2020, 13, 120. [Google Scholar] [CrossRef]
- Liu, P.P.; Blet, A.; Smyth, D.; Li, H. The Science Underlying COVID-19: Implications for the Cardiovascular System. Circulation 2020, 142, 68–78. [Google Scholar] [CrossRef]
- McFadyen, J.D.; Stevens, H.; Peter, K. The Emerging Threat of (Micro)Thrombosis in COVID-19 and Its Therapeutic Implications. Circ. Res 2020, 127, 571–587. [Google Scholar] [CrossRef]
- Zhu, H.; Cong, J.P.; Yu, D.; Bresnahan, W.A.; Shenk, T.E. Inhibition of cyclooxygenase 2 blocks human cytomegalovirus replication. Proc. Natl. Acad. Sci. USA 2002, 99, 3932–3937. [Google Scholar] [CrossRef]
- Homolak, J.; Kodvanj, I. Widely available lysosome targeting agents should be considered as potential therapy for COVID-19. Int. J. Antimicrob. Agents 2020, 56, 106044. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.K.; Brindisi, M.; Shahabi, D.; Chapman, M.; Mesecar, A.D. Drug Development and Medicinal Chemistry Efforts toward SARS-Coronavirus and COVID-19 Therapeutics. ChemMedChem 2020, 15, 907–932. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Sk, M.F.; Sonawane, A.; Kar, P.; Sadhukhan, S. Plant-derived natural polyphenols as potential antiviral drugs against SARS-CoV-2 via RNA-dependent RNA polymerase (RdRp) inhibition: An in-silico analysis. J. Biomol. Struct. Dyn. 2020, 39, 1796810. [Google Scholar] [CrossRef]
- Russo, M.; Moccia, S.; Spagnuolo, C.; Tedesco, I.; Russo, G.L. Roles of flavonoids against coronavirus infection. Chem. Biol. Interact. 2020, 328, 109211. [Google Scholar] [CrossRef] [PubMed]
- Narkhede, R.R.; Pise, A.V.; Cheke, R.S.; Shinde, S.D. Recognition of Natural Products as Potential Inhibitors of COVID-19 Main Protease (Mpro): In-Silico Evidences. Nat. Prod. Bioprospect. 2020, 10, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, B.; Marasini, B.P.; Rayamajhee, B.; Bhattarai, B.R.; Lamichhane, G.; Khadayat, K.; Adhikari, A.; Khanal, S.; Parajuli, N. Potential roles of medicinal plants for the treatment of viral diseases focusing on COVID-19: A review. Phytother. Res. 2020, 35, 1298–1312. [Google Scholar] [CrossRef]
- Di Pierro, F.; Derosa, G.; Maffioli, P.; Bertuccioli, A.; Togni, S.; Riva, A.; Allegrini, P.; Khan, A.; Khan, S.; Khan, B.A.; et al. Possible Therapeutic Effects of Adjuvant Quercetin Supplementation against Early-Stage COVID-19 Infection: A Prospective, Randomized, Controlled, and Open-Label Study. Int. J. Gen. Med. 2021, 14, 2359–2366. [Google Scholar] [CrossRef]
- Ho, P.; Zheng, J.Q.; Wu, C.C.; Hou, Y.C.; Liu, W.C.; Lu, C.L.; Zheng, C.M.; Lu, K.C.; Chao, Y.C. Perspective Adjunctive Therapies for COVID-19: Beyond Antiviral Therapy. Int. J. Med. Sci. 2021, 18, 314–324. [Google Scholar] [CrossRef]
- Brahmi, F.; Vejux, A.; Ghzaiel, I.; Ksila, M.; Zarrouk, A.; Ghrairi, T.; Essadek, S.; Mandard, S.; Leoni, V.; Poli, G.; et al. Role of Diet and Nutrients in SARS-CoV-2 Infection: Incidence on Oxidative Stress, Inflammatory Status and Viral Production. Nutrients 2022, 14, 2194. [Google Scholar] [CrossRef]
- Montenegro-Landívar, M.F.; Tapia-Quirós, P.; Vecino, X.; Reig, M.; Valderrama, C.; Granados, M.; Cortina, J.L.; Saurina, J. Polyphenols and their potential role to fight viral diseases: An overview. Sci. Total. Environ. 2021, 801, 149719. [Google Scholar] [CrossRef]
- Pawar, A.; Russo, M.; Rani, I.; Goswami, K.; Russo, G.L.; Pal, A. A critical evaluation of risk to reward ratio of quercetin supplementation for COVID-19 and associated comorbid conditions. Phytother. Res. 2022, 36, 2394–2415. [Google Scholar] [CrossRef] [PubMed]
- Shohan, M.; Nashibi, R.; Mahmoudian-Sani, M.R.; Abolnezhadian, F.; Ghafourian, M.; Alavi, S.M.; Sharhani, A.; Khodadadi, A. The therapeutic efficacy of quercetin in combination with antiviral drugs in hospitalized COVID-19 patients: A randomized controlled trial. Eur. J. Pharmacol. 2022, 914, 174615. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Iqtadar, S.; Mumtaz, S.U.; Heinrich, M.; Pascual-Figal, D.A.; Livingstone, S.; Abaidullah, S. Oral Co-Supplementation of Curcumin, Quercetin, and Vitamin D3 as an Adjuvant Therapy for Mild to Moderate Symptoms of COVID-19-Results from a Pilot Open-Label, Randomized Controlled Trial. Front. Pharmacol. 2022, 13, 898062. [Google Scholar] [CrossRef] [PubMed]
- Rondanelli, M.; Perna, S.; Gasparri, C.; Petrangolini, G.; Allegrini, P.; Cavioni, A.; Faliva, M.A.; Mansueto, F.; Patelli, Z.; Peroni, G.; et al. Promising Effects of 3-Month Period of Quercetin Phytosome® Supplementation in the Prevention of Symptomatic COVID-19 Disease in Healthcare Workers: A Pilot Study. Life 2022, 12, 66. [Google Scholar] [CrossRef] [PubMed]
- Silvagno, F.; Vernone, A.; Pescarmona, G.P. The Role of Glutathione in Protecting against the Severe Inflammatory Response Triggered by COVID-19. Antioxidants 2020, 9, 624. [Google Scholar] [CrossRef] [PubMed]
- Suhail, S.; Zajac, J.; Fossum, C.; Lowater, H.; McCracken, C.; Severson, N.; Laatsch, B.; Narkiewicz-Jodko, A.; Johnson, B.; Liebau, J.; et al. Role of Oxidative Stress on SARS-CoV (SARS) and SARS-CoV-2 (COVID-19) Infection: A Review. Protein J. 2020, 39, 644–656. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.S.; Czajkowsky, D.M. SARS-CoV-2 infection and oxidative stress: Pathophysiological insight into thrombosis and therapeutic opportunities. Cytokine Growth Factor Rev. 2022, 63, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Erlich, J.R.; To, E.E.; Liong, S.; Brooks, R.; Vlahos, R.; O’Leary, J.J.; Brooks, D.A.; Selemidis, S. Targeting Evolutionary Conserved Oxidative Stress and Immunometabolic Pathways for the Treatment of Respiratory Infectious Diseases. Antioxid. Redox Signal. 2020, 32, 993–1013. [Google Scholar] [CrossRef]
- Potus, F.; Mai, V.; Lebret, M.; Malenfant, S.; Breton-Gagnon, E.; Lajoie, A.C.; Boucherat, O.; Bonnet, S.; Provencher, S. Novel insights on the pulmonary vascular consequences of COVID-19. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 319, L277–L288. [Google Scholar] [CrossRef]
- Fratta Pasini, A.M.; Stranieri, C.; Cominacini, L.; Mozzini, C. Potential Role of Antioxidant and Anti-Inflammatory Therapies to Prevent Severe SARS-CoV-2 Complications. Antioxidants 2021, 10, 272. [Google Scholar] [CrossRef]
- Checconi, P.; De, A.M.; Marcocci, M.E.; Fraternale, A.; Magnani, M.; Palamara, A.T.; Nencioni, L. Redox-Modulating Agents in the Treatment of Viral Infections. Int. J. Mol. Sci. 2020, 21, 4084. [Google Scholar] [CrossRef] [PubMed]
- Bellavite, P.; Donzelli, A. Hesperidin and SARS-CoV-2: New Light on the Healthy Function of Citrus Fruits. Antioxidants 2020, 9, 742. [Google Scholar] [CrossRef] [PubMed]
- Iddir, M.; Brito, A.; Dingeo, G.; Fernandez Del Campo, S.S.; Samouda, H.; La Frano, M.R.; Bohn, T. Strengthening the Immune System and Reducing Inflammation and Oxidative Stress through Diet and Nutrition: Considerations during the COVID-19 Crisis. Nutrients 2020, 12, 1562. [Google Scholar] [CrossRef]
- Filardo, S.; Di, P.M.; Mastromarino, P.; Sessa, R. Therapeutic potential of resveratrol against emerging respiratory viral infections. Pharmacol. Ther. 2020, 214, 107613. [Google Scholar] [CrossRef]
- Marinella, M.A. Indomethacin and resveratrol as potential treatment adjuncts for SARS-CoV-2/COVID-19. Int. J. Clin. Pract. 2020, 74, e13535. [Google Scholar] [CrossRef]
- Mrityunjaya, M.; Pavithra, V.; Neelam, R.; Janhavi, P.; Halami, P.M.; Ravindra, P.V. Immune-Boosting, Antioxidant and Anti-inflammatory Food Supplements Targeting Pathogenesis of COVID-19. Front. Immunol. 2020, 11, 570122. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Liu, Y.; Yang, Y.; Zhang, P.; Zhong, W.; Wang, Y.; Wang, Q.; Xu, Y.; Li, M.; Li, X.; et al. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm. Sin. B 2020, 10, 766–788. [Google Scholar] [CrossRef]
- Chen, Y.W.; Yiu, C.-P.B.; Wong, K.Y. Prediction of the SARS-CoV-2 (2019-nCoV) 3C-like protease (3CLpro) structure: Virtual screening reveals velpatasvir, ledipasvir, and other drug repurposing candidates. F1000Research 2020, 9, 129. [Google Scholar] [CrossRef]
- Adem, S.; Eyupoglu, V.; Sarfraz, I.; Rasul, A.; Ali, M. Identification of Potent COVID-19 Main Protease (Mpro) Inhibitors from Natural Polyphenols: An In Silico Strategy Unveils a Hope against CORONA. Preprints 2020, 2020030333. [Google Scholar] [CrossRef]
- Utomo, R.Y.; Ikawati, M.; Meiyanto, E. Revealing the potency of citrus and galangal constituents to halt SARS-CoV-2 infection. Preprints 2020, 202003021. [Google Scholar] [CrossRef]
- Das, S.; Sarmah, S.; Lyndem, S.; Roy, A.S. An investigation into the identification of potential inhibitors of SARS-CoV-2 main protease using molecular docking study. J. Biomol. Struct. Dyn. 2020, 39, 3347–3357. [Google Scholar] [CrossRef]
- Joshi, R.S.; Jagdale, S.S.; Bansode, S.B.; Shankar, S.S.; Tellis, M.B.; Pandya, V.K.; Chugh, A.; Giri, A.P.; Kulkarni, M.J. Discovery of potential multi-target-directed ligands by targeting host-specific SARS-CoV-2 structurally conserved main protease. J. Biomol. Struct. Dyn. 2020, 39, 3099–3114. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Sarkar, A.; Maulik, U. Molecular docking study of potential phytochemicals and their effects on the complex of SARS-CoV2 spike protein and human ACE2. Sci. Rep. 2020, 10, 17699. [Google Scholar] [CrossRef] [PubMed]
- Bellavite, P. Reappraisal of Dietary Phytochemicals for Coronavirus Infection: Focus on Hesperidin and Quercetin. In Antioxidants: Benefits, Sources, Mechanisms of Action; Waisundara, V.Y., Ed.; IntechOpen: London, UK, 2021; pp. 473–487. [Google Scholar] [CrossRef]
- Zannella, C.; Giugliano, R.; Chianese, A.; Buonocore, C.; Vitale, G.; Sanna, G.; Sarno, F.; Manzin, A.; Nebbioso, A.; Termolino, P.; et al. Antiviral Activity of Vitis vinifera Leaf Extract against SARS-CoV-2 and HSV-1. Viruses 2021, 13, 1263. [Google Scholar] [CrossRef] [PubMed]
- Messina, G.; Polito, R.; Monda, V.; Cipolloni, L.; Di, N.N.; Di, M.G.; Murabito, P.; Carotenuto, M.; Messina, A.; Pisanelli, D.; et al. Functional Role of Dietary Intervention to Improve the Outcome of COVID-19: A Hypothesis of Work. Int. J. Mol. Sci. 2020, 21, 3104. [Google Scholar] [CrossRef]
- Meneguzzo, F.; Ciriminna, R.; Zabini, F.; Pagliaro, M. Review of Evidence Available on Hesperidin-Rich Products as Potential Tools against COVID-19 and Hydrodynamic Cavitation-Based Extraction as a Method of Increasing Their Production. Processes 2020, 8, 549. [Google Scholar] [CrossRef]
- Bhowmik, D.; Nandi, R.; Prakash, A.; Kumar, D. Evaluation of flavonoids as 2019-nCoV cell entry inhibitor through molecular docking and pharmacological analysis. Heliyon 2021, 7, e06515. [Google Scholar] [CrossRef]
- Junior, A.G.; Tolouei, S.E.L.; Dos Reis Lívero, F.A.; Gasparotto, F.; Boeing, T.; de Souza, P. Natural agents modulating ACE-2: A review of compounds with potential against SARS-CoV-2 infections. Curr. Pharm. Des. 2021, 27, 1588–1596. [Google Scholar] [CrossRef]
- Alesci, A.; Aragona, M.; Cicero, N.; Lauriano, E.R. Can nutraceuticals assist treatment and improve COVID-19 symptoms? Nat. Prod. Res. 2021, 36, 2672–2691. [Google Scholar] [CrossRef]
- Harwansh, R.K.; Bahadur, S. Herbal Medicine in Fighting Against COVID-19: New Battle with an Old Weapon. Curr. Pharm. Biotechnol. 2021, 23, 235–260. [Google Scholar] [CrossRef]
- Gour, A.; Manhas, D.; Bag, S.; Gorain, B.; Nandi, U. Flavonoids as potential phytotherapeutics to combat cytokine storm in SARS-CoV-2. Phytother. Res. 2021, 35, 4258–4283. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.; Rane, J.S.; Chatterjee, A.; Kumar, A.; Khan, R.; Prakash, A.; Ray, S. Targeting SARS-CoV-2 spike protein of COVID-19 with naturally occurring phytochemicals: An in silico study for drug development. J. Biomol. Struct. Dyn. 2020, 39, 1796811. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, B.G.; Ramesh, D.; Joji, A.; Jayachandra, P.J.; Kannan, T. In silico pharmacokinetic and molecular docking studies of natural flavonoids and synthetic indole chalcones against essential proteins of SARS-CoV-2. Eur. J Pharmacol. 2020, 886, 173448. [Google Scholar] [CrossRef]
- Tallei, T.E.; Tumilaar, S.G.; Niode, N.J.; Kepel, B.; Idroes, R.; Effendi, Y.; Sakib, S.A.; Emran, T.B. Potential of Plant Bioactive Compounds as SARS-CoV-2 Main Protease (Mpro) and Spike (S) Glycoprotein Inhibitors: A Molecular Docking Study. Scientifica 2020, 2020, 6307457. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.H.; Woo, H.J.; Kang, H.K.; Nguyen, V.D.; Kim, Y.M.; Kim, D.W.; Ahn, S.A.; Xia, Y.; Kim, D. Flavonoid-mediated inhibition of SARS coronavirus 3C-like protease expressed in Pichia pastoris. Biotechnol. Lett. 2012, 34, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Yuk, H.J.; Ryu, H.W.; Lim, S.H.; Kim, K.S.; Park, K.H.; Ryu, Y.B.; Lee, W.S. Evaluation of polyphenols from Broussonetia papyrifera as coronavirus protease inhibitors. J. Enzym. Inhib. Med. Chem. 2017, 32, 504–515. [Google Scholar] [CrossRef]
- Khaerunnisa, S.; Kurniawan, H.; Awaluddin, R.; Suhartati, S.; Soetjipto, S. Potential Inhibitor of COVID-19 Main Protease (Mpro) From Several Medicinal Plant Compounds by Molecular Docking Study. Preprints 2020, 2020030226. [Google Scholar] [CrossRef]
- Abian, O.; Ortega-Alarcon, D.; Jimenez-Alesanco, A.; Ceballos-Laita, L.; Vega, S.; Reyburn, H.T.; Rizzuti, B.; Velazquez-Campoy, A. Structural stability of SARS-CoV-2 3CLpro and identification of quercetin as an inhibitor by experimental screening. Int. J. Biol. Macromol. 2020, 164, 1693–1703. [Google Scholar] [CrossRef]
- Gogoi, N.; Chowdhury, P.; Goswami, A.K.; Das, A.; Chetia, D.; Gogoi, B. Computational guided identification of a citrus flavonoid as potential inhibitor of SARS-CoV-2 main protease. Mol. Divers. 2020, 25, 1745–1759. [Google Scholar] [CrossRef]
- Shaik, Y.B.; Castellani, M.L.; Perrella, A.; Conti, F.; Salini, V.; Tete, S.; Madhappan, B.; Vecchiet, J.; De Lutiis, M.A.; Caraffa, A.; et al. Role of quercetin (a natural herbal compound) in allergy and inflammation. J. Biol. Regul. Homeost. Agents 2006, 20, 47–52. [Google Scholar]
- Saeedi-Boroujeni, A.; Mahmoudian-Sani, M.R. Anti-inflammatory potential of Quercetin in COVID-19 treatment. J. Inflamm. 2021, 18, 3. [Google Scholar] [CrossRef] [PubMed]
- Kimata, M.; Shichijo, M.; Miura, T.; Serizawa, I.; Inagaki, N.; Nagai, H. Effects of luteolin, quercetin and baicalein on immunoglobulin E-mediated mediator release from human cultured mast cells. Clin. Exp. Allergy 2000, 30, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Min, Y.D.; Choi, C.H.; Bark, H.; Son, H.Y.; Park, H.H.; Lee, S.; Park, J.W.; Park, E.K.; Shin, H.I.; Kim, S.H. Quercetin inhibits expression of inflammatory cytokines through attenuation of NF-kappaB and p38 MAPK in HMC-1 human mast cell line. Inflamm. Res. 2007, 56, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Kalogeromitros, D.; Makris, M.; Chliva, C.; Aggelides, X.; Kempuraj, D.; Theoharides, T.C. A quercetin containing supplement reduces niacin-induced flush in humans. Int. J. Immunopathol. Pharmacol. 2008, 21, 509–514. [Google Scholar] [CrossRef]
- Park, S.J.; Chung, H.Y.; Lee, J.H. Rapid in vivo screening system for anti-oxidant activity using bacterial redox sensor strains. J. Appl. Microbiol. 2009, 108, 1217–1225. [Google Scholar] [CrossRef]
- Lee, E.J.; Ji, G.E.; Sung, M.K. Quercetin and kaempferol suppress immunoglobulin E-mediated allergic inflammation in RBL-2H3 and Caco-2 cells. Inflamm. Res. 2010, 59, 847–854. [Google Scholar] [CrossRef]
- Chirumbolo, S.; Conforti, A.; Ortolani, R.; Vella, A.; Marzotto, M.; Bellavite, P. Stimulus-specific regulation of CD63 and CD203c membrane expression in human basophils by the flavonoid quercetin. Int. Immunopharmacol. 2010, 10, 183–192. [Google Scholar] [CrossRef]
- Chirumbolo, S.; Marzotto, M.; Conforti, A.; Vella, A.; Ortolani, R.; Bellavite, P. Bimodal action of the flavonoid quercetin on basophil function: An investigation of the putative biochemical targets. Clin. Mol. Allergy 2010, 8, 13. [Google Scholar] [CrossRef]
- Theoharides, T.C. COVID-19, pulmonary mast cells, cytokine storms, and beneficial actions of luteolin. Biofactors 2020, 46, 306–308. [Google Scholar] [CrossRef]
- Wu, M.L.; Liu, F.L.; Sun, J.; Li, X.; He, X.Y.; Zheng, H.Y.; Zhou, Y.H.; Yan, Q.; Chen, L.; Yu, G.Y.; et al. SARS-CoV-2-triggered mast cell rapid degranulation induces alveolar epithelial inflammation and lung injury. Signal Transduct. Target. Ther. 2021, 6, 428. [Google Scholar] [CrossRef]
- Conti, P.; Caraffa, A.; Tete, G.; Gallenga, C.E.; Ross, R.; Kritas, S.K.; Frydas, I.; Younes, A.; Di Emidio, P.; Ronconi, G. Mast cells activated by SARS-CoV-2 release histamine which increases IL-1 levels causing cytokine storm and inflammatory reaction in COVID-19. J. Biol. Regul. Homeost. Agents 2020, 34, 1629–1632. [Google Scholar] [CrossRef] [PubMed]
- Murota, K.; Nakamura, Y.; Uehara, M. Flavonoid metabolism: The interaction of metabolites and gut microbiota. Biosci. Biotechnol. Biochem. 2018, 82, 600–610. [Google Scholar] [CrossRef]
- Xiang, Z.; Koo, H.; Chen, Q.; Zhou, X.; Liu, Y.; Simon-Soro, A. Potential implications of SARS-CoV-2 oral infection in the host microbiota. J. Oral Microbiol. 2020, 13, 1853451. [Google Scholar] [CrossRef] [PubMed]
- Lamers, M.M.; Beumer, J.; van der Vaart, J.; Knoops, K.; Puschhof, J.; Breugem, T.I.; Ravelli, R.B.G.; van Schayck, J.P.; Mykytyn, A.Z.; Duimel, H.Q.; et al. SARS-CoV-2 productively infects human gut enterocytes. Science 2020, 369, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Burgueño, J.F.; Reich, A.; Hazime, H.; Quintero, M.A.; Fernandez, I.; Fritsch, J.; Santander, A.M.; Brito, N.; Damas, O.M.; Deshpande, A.; et al. Expression of SARS-CoV-2 Entry Molecules ACE2 and TMPRSS2 in the Gut of Patients With IBD. Inflamm. Bowel Dis. 2020, 26, 797–808. [Google Scholar] [CrossRef]
- Zuo, T.; Liu, Q.; Zhang, F.; Lui, G.C.; Tso, E.Y.; Yeoh, Y.K.; Chen, Z.; Boon, S.S.; Chan, F.K.; Chan, P.K.; et al. Depicting SARS-CoV-2 faecal viral activity in association with gut microbiota composition in patients with COVID-19. Gut 2021, 70, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Yeoh, Y.K.; Zuo, T.; Lui, G.C.; Zhang, F.; Liu, Q.; Li, A.Y.; Chung, A.C.; Cheung, C.P.; Tso, E.Y.; Fung, K.S.; et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut 2021, 70, 698–706. [Google Scholar] [CrossRef]
- Augusti, P.R.; Conterato, G.M.M.; Denardin, C.C.; Prazeres, I.D.; Serra, A.T.; Bronze, M.R.; Emanuelli, T. Bioactivity, bioavailability, and gut microbiota transformations of dietary phenolic compounds: Implications for COVID-19. J. Nutr. Biochem. 2021, 97, 108787. [Google Scholar] [CrossRef]
- Licciardello, F.; Arena, E.; Rizzo, V.; Fallico, B. Contribution of Blood Orange-Based Beverages to Bioactive Compounds Intake. Front. Chem. 2018, 6, 374. [Google Scholar] [CrossRef]
- Grosso, G.; Galvano, F.; Mistretta, A.; Marventano, S.; Nolfo, F.; Calabrese, G.; Buscemi, S.; Drago, F.; Veronesi, U.; Scuderi, A. Red orange: Experimental models and epidemiological evidence of its benefits on human health. Oxid. Med. Cell. Longev. 2013, 2013, 157240. [Google Scholar] [CrossRef]
- Carr, A.C.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, H.; Bae, S.; Choi, J.; Lim, S.Y.; Lee, N.; Kong, J.M.; Hwang, Y.I.; Kang, J.S.; Lee, W.J. Vitamin C Is an Essential Factor on the Anti-viral Immune Responses through the Production of Interferon-alpha/beta at the Initial Stage of Influenza A Virus (H3N2) Infection. Immune Netw. 2013, 13, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Jang, M.; Kim, Y.; Choi, J.; Jeon, J.; Kim, J.; Hwang, Y.I.; Kang, J.S.; Lee, W.J. Red ginseng and vitamin C increase immune cell activity and decrease lung inflammation induced by influenza A virus/H1N1 infection. J. Pharm. Pharmacol. 2016, 68, 406–420. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Ward, S.A.; Kalantar-Zadeh, K.; El-Omar, E. Considering the Effects of Microbiome and Diet on SARS-CoV-2 Infection: Nanotechnology Roles. ACS Nano 2020, 14, 5179–5182. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Moore, L.W. Impact of Nutrition and Diet on COVID-19 Infection and Implications for Kidney Health and Kidney Disease Management. J. Ren. Nutr. 2020, 30, 179–181. [Google Scholar] [CrossRef] [PubMed]
- Lehene, M.; Fischer-Fodor, E.; Scurtu, F.; Hădade, N.D.; Gal, E.; Mot, A.C.; Matei, A.; Silaghi-Dumitrescu, R. Excess Ascorbate is a Chemical Stress Agent against Proteins and Cells. Pharmaceuticals 2020, 13, 107. [Google Scholar] [CrossRef]
- Colunga Biancatelli, R.M.L.; Berrill, M.; Catravas, J.D.; Marik, P.E. Quercetin and Vitamin C: An Experimental, Synergistic Therapy for the Prevention and Treatment of SARS-CoV-2 Related Disease (COVID-19). Front. Immunol. 2020, 11, 1451. [Google Scholar] [CrossRef]
- Li, G.; Wei, W.; Suo, L.; Zhang, C.; Yu, H.; Liu, H.; Guo, Q.; Zhen, X.; Yu, Y. Low-Dose Aspirin Prevents Kidney Damage in LPS-Induced Preeclampsia by Inhibiting the WNT5A and NF-kappaB Signaling Pathways. Front. Endocrinol. 2021, 12, 639592. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, N.; Li, A.; Zhou, Y.; Liang, L.; Song, X.; Yang, Z.; Zhou, X. Effect of low-dose aspirin on mortality and viral duration of the hospitalized adults with COVID-19. Medicine 2021, 100, e24544. [Google Scholar] [CrossRef]
- Ma, S.; Su, W.; Sun, C.; Lowe, S.; Zhou, Z.; Liu, H.; Qu, G.; Xia, W.; Xie, P.; Wu, B.; et al. Does aspirin have an effect on risk of death in patients with COVID-19? A meta-analysis. Eur. J. Clin. Pharmacol. 2022, 78, 1403–1420. [Google Scholar] [CrossRef]
- Santoro, F.; Núñez-Gil, I.J.; Vitale, E.; Viana-Llamas, M.C.; Romero, R.; Maroun Eid, C.; Feltes Guzman, G.; Becerra-Muñoz, V.M.; Fernandez Rozas, I.; Uribarri, A.; et al. Aspirin Therapy on Prophylactic Anticoagulation for Patients Hospitalized With COVID-19: A Propensity Score-Matched Cohort Analysis of the HOPE-COVID-19 Registry. J. Am. Heart Assoc. 2022, 11, e024530. [Google Scholar] [CrossRef] [PubMed]
- Massimo Claar, G.; Monaco, S.; Del Veccho Blanco, C.; Capurso, L.; Fusillo, M.; Annibale, B. Omeprazole 20 or 40 mg daily for healing gastroduodenal ulcers in patients receiving non-steroidal anti-inflammatory drugs. Aliment. Pharmacol. Ther. 1998, 12, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Bianchi Porro, G.; Lazzaroni, M.; Petrillo, M.; Manzionna, G.; Montrone, F.; Caruso, I. Prevention of gastroduodenal damage with omeprazole in patients receiving continuous NSAIDs treatment. A double blind placebo controlled study. Ital. J. Gastroenterol. Hepatol. 1998, 30, 43–47. [Google Scholar]
- Gao, J.; Zhang, L.; Liu, X.; Li, F.; Ma, R.; Zhu, Z.; Zhang, J.; Wu, J.; Shi, Y.; Pan, Y.; et al. Repurposing Low-Molecular-Weight Drugs against the Main Protease of Severe Acute Respiratory Syndrome Coronavirus 2. J. Phys. Chem. Lett. 2020, 11, 7267–7272. [Google Scholar] [CrossRef] [PubMed]
- Tanimoto, K.; Hirota, K.; Fukazawa, T.; Matsuo, Y.; Nomura, T.; Tanuza, N.; Hirohashi, N.; Bono, H.; Sakaguchi, T. Inhibiting SARS-CoV-2 infection in vitro by suppressing its receptor, angiotensin-converting enzyme 2, via aryl-hydrocarbon receptor signal. Sci. Rep. 2021, 11, 16629. [Google Scholar] [CrossRef] [PubMed]
- El-Aarag, S.A.; Mahmoud, A.; ElHefnawi, M. Identifying potential novel insights for COVID-19 pathogenesis and therapeutics using an integrated bioinformatics analysis of host transcriptome. Int. J. Biol. Macromol. 2022, 194, 770–780. [Google Scholar] [CrossRef] [PubMed]
- Min, Y.S.; Lee, S.E.; Hong, S.T.; Kim, H.S.; Choi, B.C.; Sim, S.S.; Whang, W.K.; Sohn, U.D. The Inhibitory Effect of Quercetin-3-O-beta-D-Glucuronopyranoside on Gastritis and Reflux Esophagitis in Rats. Korean J. Physiol. Pharmacol. 2009, 13, 295–300. [Google Scholar] [CrossRef]
- Fuentes, J.; de Camargo, A.C.; Atala, E.; Gotteland, M.; Olea-Azar, C.; Speisky, H. Quercetin Oxidation Metabolite Present in Onion Peel Protects Caco-2 Cells against the Oxidative Stress, NF-kB Activation, and Loss of Epithelial Barrier Function Induced by NSAIDs. J. Agric. Food Chem. 2021, 69, 2157–2167. [Google Scholar] [CrossRef]
- Fan, J.; Li, B.R.; Zhang, Q.; Zhao, X.H.; Wang, L. Pretreatment of IEC-6 cells with quercetin and myricetin resists the indomethacin-induced barrier dysfunction via attenuating the calcium-mediated JNK/Src activation. Food Chem. Toxicol. 2021, 147, 111896. [Google Scholar] [CrossRef]
- Rezza, G.; Urbani, A. Circolare Recante “Gestione Domiciliare Dei Pazienti Con Infezione Da SARS-CoV-2 Aggiornata al 26 Aprile 2021”; Ditrezioni Generali Programmazione Sanitaria e Prevenzione Sanitaria; Ministero della Salute: Roma, Italy, 2021. [Google Scholar]
- Frieden, T.R. Evidence for Health Decision Making—Beyond Randomized, Controlled Trials. N. Engl. J. Med. 2017, 377, 465–475. [Google Scholar] [CrossRef]
- Andreoni, M.; Bartoletti, P.L. CORONAVIRUS: Dalla FIMMG Roma una Flow Chart per il Trattamento Farmacologico Dell’infezione da SARS-CoV-2 in (FIMMG Sezione Provinciale di Roma, iccPTV, ed.); FIMMG, Sezione di Roma: Roma, Italy, 2023. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).